Abstract
CD4+ T helper (Th) cells play central roles in immunity in health and disease. While much is known about the effector function of Th cells in combating pathogens and promoting autoimmune diseases, the roles and biology of memory CD4+ Th cells are complex and less well understood. In human autoimmune diseases such as multiple sclerosis (MS), there is a critical need to better understand the function and biology of memory T cells. In this review article we summarize current concepts in the field of CD4+ T cell memory, including natural history, developmental pathways, subsets, and functions. Furthermore, we discuss advancements in the field of the newly-described CD4+ tissue-resident memory T cells and of CD4+ memory T cells in autoimmune diseases, two major areas of important unresolved questions in need of answering to advance new vaccine design and development of novel treatments for CD4+ T cell-mediated autoimmune diseases.
1. Introduction
CD4+ T helper (Th) cells play a central role in the immune system and carry out multiple functions including activation, coordination, modulation, and regulation of innate and adaptive immune responses. These various functions of Th cells are necessary to attain effective immune responses against a variety of different pathogens, while maintaining self-tolerance and avoiding undesired attacks against self-tissues [1,2,3]. The regulation of immune responses by Th cells is accomplished through the secretion of specific cytokines, which, together with a “master” regulatory transcription factor, define the respective Th cell subset and its specialized functions and attributes [1,2,3,4]. While much is known about the effector function of Th cells, the roles and biology of memory CD4+ T cells are more complex and less well understood. Moreover, the function of memory CD4+ T cells in mounting an immune response can only partially be defined by the precursor Th subset from which the primary immune response originated. An additional layer of complexity is added by the different memory CD4+ T cell subsets generated during the primary immune response [3,5]. Memory T cells are generally subdivided into three main populations: central memory T cells (TCM), effector memory T cells (TEM), and tissue-resident memory T cells (TRM). At present, these memory T cell subsets are primarily characterized by their phenotype, migratory properties, and tissue homing patterns, which in many instances imply unique functional attributes [5,6,7].
Antigen (Ag)-specific naïve CD4+ T cells are first activated in lymphatic tissues by professional Ag-presenting cells (APCs) presenting their specific antigen on major histocompatibility complex (MHC) class II molecules and providing costimulatory signals, for example in the context of an infection with microbial pathogens. Activated CD4+ T cells proliferate and differentiate into specific Th subsets, which will mount distinct immune responses directed against specific pathogens [8]. After the infectious pathogen has been cleared, the majority of effector Th cells will undergo apoptosis, while the remaining cells contribute to the CD4+ memory T cell pool [9]. The importance of memory T cell generation centers on the ability to provide a faster and augmented immune response upon secondary exposure to previously encountered microbial pathogens. The ability of memory T cells to respond faster and more efficiently is based on several essential characteristics, which render them superior in their ability to alter the outcome of infections. First, memory T cells have a lower activation threshold and are less co-stimulation dependent [10]. Therefore, upon re-challenge, memory T cells will generate robust effector responses more effectively and faster as compared with a primary T cell response [11,12,13]. Similar to the effector response generated by recently-activated naive T cells, the effector response of memory T cells is dependent on the nature and context of their encounter with their cognate antigen, for example provided by cues such as cytokines in the microenvironment [14]. Second, the frequencies of Ag-experienced memory T cells are much higher than those of naïve T cells during a primary immune response [14,15,16]. The higher frequencies of Ag-specific memory T cells increase their likelihood to encounter their cognate antigen faster upon re-infection, and to more rapidly generate a larger effector T cell pool [17,18]. Third, unlike naïve T cells, which circulate between secondary lymphatic tissues (i.e., lymph nodes) and blood, memory T cells circulate between lymphatic, blood, and peripheral tissues (e.g., lungs, gut, or skin) [19,20]. This allows memory T cells to directly and rapidly respond to the presence of pathogens in peripheral tissues. More recently a subset of non-circulating and tissue-homing memory T cells was identified and termed TRM [21,22,23]. Both CD8+ and CD4+ TRM have been described [reviewed in 24]. TRM cells migrate to specific peripheral tissues locations, including the skin, liver, and lungs, and take up permanent residency. Importantly, the strategic location of TRM at many barrier sites such as the skin and mucosal tissues, where pathogens preferentially seek access, enhances the likelihood that they rapidly encounter pathogens upon infection. CD8+ TRM have been reviewed elsewhere [24,25,26] and this review will focus on CD4+ TRM.
Taken together, the unique characteristics of memory T cells enhance their ability for in situ immune surveillance, increase their likelihood for faster encounter of pathogens at the site of infection, and facilitate the generation of more rapid and generally superior effector responses.
2. Memory T Cell Development
2.1. Development Pathways
The developmental pathways of memory T cells are still not fully understood, and there are some controversies as to the different models proposed (reviewed in [27]), and compelling evidence exists for each of the models. The models are shown in Figure 1 and are summarized below.
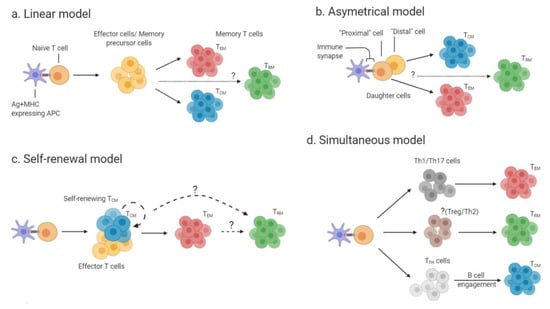
Figure 1.
Developmental models of memory T cells: (a) In the linear model, effector T cells or memory precursor cells (yellow) are generated following activation of naïve T cell by antigen-presenting cells (APCs) presenting peptide on major histocompatibility complex (MHC) molecules. The intermediate effectors or memory precursors give rise to mature effector memory T cells (TEM) (red) and central memory T cells (TCM) (blue). It remains to be answered if the intermediate effectors/precursors also give rise to tissue-resident memory T cells (TRM) (b) In the asymmetrical model, the proximal daughter cells to the immune-synapse (naïve T cell- T cell receptor (TCR) + peptide and MHC-APC) develop into TEM, while the distal daughter cells develop into TCM. It is currently unknown which cells give rise to TRM (green). (c) In the self-renewal model self-renewing effector T cells or TCM are generated from naïve T cells. These self-renewing cells can then give rise to TEM cells. It is unresolved if TRM are generated from self-renewing TCM/effector cells or from TEM. (d) In the simultaneous model naïve T cells first differentiate into different T cell subsets. T cell subsets give rise to different memory subsets as follows: Th1 and Th17 cells (dark gray) generate TEM, while TFH cells (light gray) generate TCM. The T helper cell subset(s) that generate TRM has not yet been identified.
The linear model of memory T cell development (Figure 1a) suggests that during the contraction phase of a primary T cell immune response, the surviving effector T cells differentiate into TEM, which will then give rise to TCM [27,28]. In contrast, the asymmetrical model of memory T cell development (Figure 1b), also called the bifurcative model, suggests that two daughter cells of the same T cell clone can undergo different fates: the daughter cell proximal to the immunological synapse can give rise to both terminally-differentiated effector cells and TEM, while the distal daughter cell gives rise to TCM [29]. A third model (Figure 1c, self-renewal model) proposes that naïve T cells first give rise to either self-renewing TCM or effector T cells, and that those can further differentiate to TEM, which give rise to terminally-differentiated effector cells in non-lymphoid tissues [27]. Pepper and Jenkins suggested an additional model for the generation of memory CD4+ T cells. In this “simultaneous” model (Figure 1d), the effector T cell subset (e.g., Th1 or Th2) determines the fate of the generated memory T cell subset. For instance, Th1 cell or Th17 cell subsets will give rise to TEM, whereas T follicular helper (TFH) cell subset will generate TCM upon help from B cells [30].
2.2. Role of T Cell Receptor Signaling Strength and Precursor Frequencies for Memory T Cell Development
Central to memory T cell development are the roles of T cell receptor (TCR) signaling strength and T cell precursor frequencies. The TCR signaling strength is determined by the affinity of the TCR for Ag and MHC molecules, the density of antigen presented on APCs, and the duration of the TCR interaction with peptide-loaded MHC [31,32]. The degree of these parameters affects TCR-dependent biochemical pathways which result in changes to T cell transcriptional profiles, to either promote or inhibit memory T cell generation [33,34]. This appears to be regulated via differences in the ratios of several transcription factors, including Bcl-6, Blimp-1, Eomes, and T-bet [33,35,36], their upstream regulators nuclear factor of activated T-cells (NFAT) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), or by directly affecting the expression of cytokines and cytokine receptors, such as IL-2 and the IL-2 receptor alpha chain (CD25), which synergize with TCR signaling to affect memory-cell development [35,37,38]. For memory CD8+ T cells, it is generally accepted that TCR signaling strength inversely correlates with memory-cell formation [33]. However, for the generation of memory CD4+ T cells the data are less clear, as described below [38,39,40,41].
CD4+ T cells have been shown in several systems to compete for Ag, and a higher TCR affinity for Ag may affect the access of T cells to Ag [42,43,44]. Thus, TCRs with higher affinity for Ag are likely to promote greater expansion of Ag-specific T cells, and thereby increase the reservoir of T cells that could potentially enter the memory T cell pool. Furthermore, stable and sustained interaction with antigens is a critical determining factor for promoting the differentiation of memory CD4+ T cells during acute viral infection [45]. In contrast, low affinity TCRs for MHC plus Ag results in T cell memory with shorter lifespan and with impaired secondary responses upon re-challenge [46,47]. Not only may TCR affinity for MHC and Ag influence the generation of memory T cells, but it may also affect their recruitment into different memory T cell subsets and their lifespan. McKinstry et al. reported that CD4+ T cells require signals from MHC class-II molecules and CD70 during the effector phase of an immune response, and are dependent on IL-2 signaling in order to generate long-lived memory cells [48]. It has been proposed that TCM may require relatively stronger TCR stimuli for their generation, while relatively weaker antigenic stimuli may generate TEM. Consistent with this notion, naïve CD4+ T cells require prolonged exposure to antigen during the expansion phase to generate T cell memory [39].
However, memory T cell formation is not only dependent on TCR affinity/antigen accessibility, but also on the frequencies of naïve T cell precursors [49]. The naïve T cell precursor frequency is defined by the fraction of naive T cells capable of responding to a specific antigenic epitope and to enter the effector/memory Ag-specific T cell pool. For example, excessively high precursor frequencies of Ag-specific T cells seem to have a negative impact on memory T cell formation. Accordingly, high precursor frequencies of adoptively-transferred TCR-transgenic CD4+ T cells reduced the proliferation and differentiation of these cells upon infection, and thereby resulted in impaired memory T cell formation [50]. This observation is further supported by the inverse relationship between T cell precursor frequencies and the survival of both naïve and memory CD4+ T cells [51]. Similarly, Blair and Lefrançois showed that transfer of high precursor frequencies of TCR-transgenic naïve CD4+ T cells resulted in a lack of T cell memory, which was linked to impaired effector T cell induction, reduced proliferation, and cytokine production [52]. Importantly, this phenomenon was independent of IL-7R expression by the responding memory T cells. Additionally, they showed that competition for antigen during CD4+ T cell priming is a major confounding factor for the development of the memory T cell pool [52]. However, in these studies, during the initial priming of naïve CD4+ T cells, the availability of Ag rather than the frequency of precursor cells per se appeared to be pivotal for the formation of CD4+ T cell memory. Nevertheless, Ag persistence negatively affects the function of CD4+ memory T lymphocytes and impairs their ability to produce effector cytokines, perhaps by promoting the generation of TCM rather than TEM [53].
2.3. Role of Transcription Factors and Cytokine Signaling for Memory T Cell Development
T-bet, the master-regulator of Th1 cells, acts in an expression-level-dependent manner to regulate formation of memory and effector CD4+ T cells, such that high expression of T-bet promotes terminal effector cells, while intermediate to low expression promotes memory development [36]. Furthermore, T-bet appears to regulate the generation of memory T cell subsets from effector cells: T-betlow effector cells express the chemokine receptor (CCR) 7 and give rise to TCM cells, whereas T-bethigh effector cells rapidly produce interferon (IFN)-γ, lack CCR7 expression and give rise to TEM cells [54]. Similar to CD4+ memory T cells, high levels of T-bet expression by CD8+ effector T cells generate short-lived memory cells and favor terminally-differentiated effector cells, while low expression levels generate long-lived memory cells [55]. Along these lines, the cytokine IFN-γ, which induces T-bet expression in a signal transducer and activator of transcription (STAT) 1-dependent manner, has been shown to enhance the development of memory CD4+ T cells generated from both naïve and effector cells [47,56,57]. Additionally, IFN-γ producing effector cells give rise to long-lived TCM and TEM [58]. Thus, it appears that effector cytokines present during the primary response, and in particular IFN-γ, may tip the balance in favor of the generation of specific memory T cell subsets. Moreover, since TEM cells are generated from progenitor cells which express a master regulator (e.g., T-bet for Th1), it appears that TEM cells maintain lineage integrity, whereas TCM cells that are generated from progenitor cells that lack (or express at low levels) a master regulator show more lineage plasticity and can generate different effector responses upon re-challenge [30,54]. Similarly, IL-12 induces the expression of T-bet in a STAT4-dependent manner in CD4+ T cells [59], and IL-12 promotes development of hepatitis B virus (HBV)-specific CD8+ TEM cells [60]. Along these lines, although no data exists for the role of IL-12 in development of CD4+ memory T cells, mice lacking both STAT4 and T-bet have marginally reduced virus-specific CD4+ memory T cell frequencies as compared with mice lacking either one of these transcription factors (STAT4 or T-bet) [61]. These data suggest that early activation of STAT4 and T-bet may not be required for development of CD4+ T cell memory; however, their expression may affect the quality of memory responses during recall and the type (subset) of memory generated. Although CD4+ TRM were reported to express low levels of T-bet [62], suggesting that this pathway is dispensable for CD4 TRM maintenance, future work should investigate the role of T-bet-promoting cytokines for regulating CD4+ TRM development, since this has not yet been fully resolved.
IL-2, via regulation of Bcl-6 expression, also plays fundamental roles in memory CD4 T cell development [63,64,65]. Current dogma suggests that naïve CD4+ T cells require high levels of IL-2 to differentiate into memory precursor cells; however, these precursor cells are then dependent on low levels of IL-2 to develop into long-lived memory cells. IL-2 signals sustain the expression of STAT5 and Bcl-6, and promote expression of IL-7 receptor, thereby enabling the survival and maturation of CD4+ memory T cells [48,66]. Interestingly, however, IL-2-deficient memory CD4+ T cells generate a more vigorous and effective recall response against influenza virus than wild-type memory cells that produce IL-2 [67], suggesting that IL-2 and Bcl-6 may have regulatory or inhibitory roles once Ag-specific T cell memory has established, perhaps to prevent immunopathology during chronic antigen exposure. Of note, IL-2, IL-7, and IL-15, also known as common-gamma chain receptor cytokines, play key roles in memory (as well as in naïve and effector) CD4+ and CD8+ T cell development, homeostasis, and maintenance [68,69,70]. The functions of these cytokines in CD4+ T cell memory remain somewhat controversial, and this has been reviewed elsewhere [10,68].
Shown in Figure 2 is the integration of TCR signaling- and inflammation-dependent factors influencing memory T cell development.
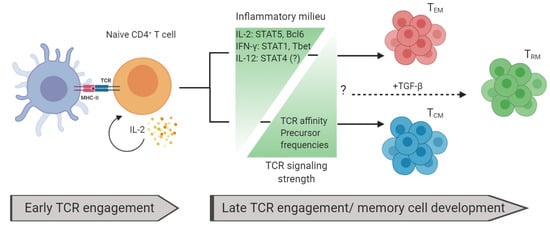
Figure 2.
Factors influencing memory T cell development: Naïve T cells are activated by peptide and MHC via TCR. High levels of interleukin (IL)-2 are critical during early TCR engagement for memory-cell development. The memory T cell development fate is dependent on the inflammatory milieu and TCR signaling strength. Increased levels of inflammatory signals favor TEM generation and decreased levels of these signals favor TCM. Conversely, increased levels of TCR affinity and precursor frequencies favor TCM development while decreased levels favor TEM. TGF-β is important for generating TRM. How other inflammatory signals and TCR signaling strength affect TRM generation remains unresolved.
Naturally, models of T cell memory development became more complex with the discovery of TRM, and there are still many open questions, in particular for the generation of CD4+ TRM [15,71]. Nevertheless, it is widely accepted that TRM require transforming growth factor (TGF)-β for their generation, which can be produced by a variety of cells in tissues, including fibroblasts, epithelial cells, keratinocytes, and enterocytes [72,73]. While CD8+ TRM have been at the center of research in this area, as much as 20% of CD4+ T cells in certain tissues, such as the intestinal epithelium, also express CD103, the prototypical marker for tissue-resident memory cells [74]. TGF-β induces the expression of CD103 by TRM precursors and promotes their entry and retention in epithelial sites [72,73]. Moreover, a sizable fraction of CD4+ TRM express Foxp3+, a key transcription factor of regulatory T cells, which is induced by TGF-β signaling [75]. Therefore, these data suggest that TGF-β is a pivotal factor in CD4+ TRM generation and that CD4+ TRM may assume regulatory functions [76]. Future investigations will elucidate the mechanisms guiding the generation of CD4+ TRM, as well as characterize their functions.
Taken together, several models have been proposed for the generation of memory T lymphocytes. It is conceivable that more than one of these models contribute to memory T cell development in vivo, and may be influenced by factors such as TCR signal strength and inflammatory conditions in the microenvironment (cytokines), and in addition, costimulatory signals (e.g., CD28 family) [77] and other environmental cues, such as chemokines and even the microbiota from the tissue in which a T cell is activated [78,79,80].
3. Memory T Cell Subsets and Function
3.1. Memory T Cell Phenotype
Memory T cells are generally subdivided into three main populations: TCM, TEM, and TRM. Human memory T cells share many phenotypic characteristics with other species such as mouse. The phenotype of the different memory T cell subsets is discussed below and summarized in Figure 3.
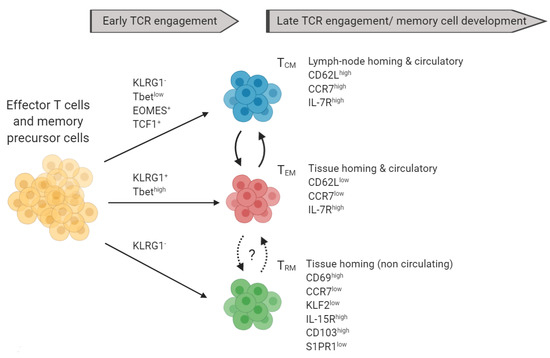
Figure 3.
Markers of memory T cell subsets and their precursors: Terminally differentiated TCM (blue) and TEM (red) can be distinguished based on the expression of CD62L, CCR7, IL7R, and other markers not shown. TRM can be further characterized based on the expression of KLF2, IL-15R, CD103, and S1PR1. TCM can give rise to TEM and vice versa. *Recent data suggested that TRM can re-enter the circulation.
Human memory T cells can be distinguished from naïve T cells by expression changes of the CD45 isoforms and increased capacity for effector cytokine production upon antigen recall [81,82,83]. Human memory T cells were initially distinguished from naïve T cells by being CD45RO+ and CD45RA−, whereas naïve T cells show the CD45RO− CD45RA+ phenotype [84,85,86]. Sallusto et al. subsequently showed that CD45RA− memory T cells could be further divided into two subpopulations based on the expression of the chemokine receptor CCR7 [28]. They proposed a model in which CD45RA− CCR7+ T cells recirculated to lymphatic tissues and termed these cells TCM, whereas CD45RA− CCR7− T cells, which remained in the immune periphery, were termed TEM [28,87].
For mice, Bradley et al. showed that mouse naïve and memory T cells can be phenotypically distinguished based on the expression of CD62L (L-selectin) and CD44, and increased expression of effector cytokines upon re-challenge. Naïve cells are CD62L+ and CD44low, whereas memory cells (TEM) are CD62L− and CD44high [88,89]. Additional differences in cell surface marker expression in mouse (and human) T cells have emerged to allow classification of naïve vs. memory T cells, such as CD69 and chemokine receptors [90,91,92]. Reinhardt et al. showed that the CD4+ TCM and TEM paradigm also translates to mice, and that these memory-cell subsets can be distinguished based on the expression of CD62L and CCR7 [87,93,94]. Thus, both mouse and human TCM are CD62L+ CCR7+, while TEM are CD62L− CCR7− [95]. Therefore, in addition to classifying memory vs. naïve T cells based on CD45 isoforms, both human and mouse naïve T cells can be characterized by a CD44low CCR7+ CD62Lhigh phenotype, whereas TCM are CD44high CCR7+ CD62Lhigh, and TEM cells are CD44high CCR7− CD62Llow [96,97]. Unlike human T cells, mouse naïve and memory T cells cannot be distinguished based on the expression of CD45 isoforms [98].
Recently, Lefrançois and colleagues described an additional memory T cell subset, termed TRM [99]. This subset resides in peripheral tissues during or after infectious encounters and does not recirculate between blood, lymphatics, or other peripheral tissues, as do TCM or TEM [100,101,102]. TRM constitutively express CD69, CD103 (integrin alpha E, ITGAE; most prominent in CD8+ TRM), and S1PR1 (sphingosine-1-phosphate receptor 1), but they do not express CCR7 or CD62L [23,24,102]. The expression of CD69, CD103, and S1PR1 by TRM, together with the absence of CCR7 expression, promotes their tissue homing and impedes their tissue egress [24,100,101]. Interestingly, the expression of CD69 and CD103 by TRM is independent of TCR signaling and Ag persistence, and may be dependent on constitutive signaling of “alarm cytokines” such as IL-33 and type I interferons [103,104]. Human TRM are also CD45RO+ CD45RA− [105]. Interestingly, Kumar et al. recently identified a core transcriptional profile for human CD4+ and CD8+ TRM at various sites that shows increased expression of specific adhesion molecules (such as CD103) and production of both pro-inflammatory and regulatory cytokines and chemokines (such as IL-2 and IL-10) [106].
Recently, the dogma of TRM as a non-circulating subset was challenged by identifying a population of circulating CD4+ TRM in human blood, which was designated as “ex-TRM” [107]. These CD4+ ex-TRM express the skin homing and retention glycan cutaneous lymphocyte-associated antigen (CLA), have similar phenotypic and transcriptional attributes as skin resident CD4+ CD103+ CLA+ TRM, and share a clonal origin with CD4+ CD103+ CLA+ TRM in the skin, based on TCR sequencing [108]. Thus, these new data suggest that CD4+ TRM may reside in tissues for prolonged periods of time, but possibly not for all of their lifespan.
3.2. Memory T Cell Subset Function
All memory CD4+ T cell subsets play a pivotal role in defending against pathogens [109,110,111]. However, their individual contributions vary, which is to some degree a function of their respective migratory properties and tissue homing [112]. Along these lines, the expression of CD62L and CCR7 by TCM endows them with the ability to migrate and home to secondary lymphatic tissues, and thereby facilitates immune surveillance of antigens collected via lymphatic drainage or dendritic cells (DCs) from peripheral tissues [113,114]. The frequencies of Ag-specific TCM are as much as thousand-fold increased as compared with Ag-specific naïve T cells, and thereby they are able to rapidly generate a robust effector T cell pool upon secondary encounter of cognate antigens [16]. Moreover, while TCM show a lower capacity for the production of some cytokines, such as IFN-γ and IL-4, as compared with TEM, they produce more IL-2 and have an overall greater capacity for proliferation [93,115]. Thus, TCM have a higher likelihood to become activated by APCs in lymphatic tissues upon re-infection with a previously encountered pathogen to provide a stronger and more rapid response and proliferate rapidly to generate a large pool of pathogen-specific effector T cells. Furthermore, CD4+ TCM provide superior B cell help, which results in faster B cell expansion, more rapid class switching, and increased antibody production [89,116]. Indeed, CD4+ TCM with TFH cell phenotype persist in germinal centers of draining lymph nodes following vaccination to regulate memory B cell development and maintenance and support rapid generation of long-lived plasma cells upon re-exposure to antigen [117,118].
In contrast to TCM, TEM preferentially recirculate between blood and peripheral tissues. As indicated by their designation, TEM rapidly exhibit effector functions, such as the production of cytokines upon activation. Interestingly, IL-1β promotes effector-cytokine production, such as IL-17 and IFN-γ, by CD4 TEM cells by stabilizing the cytokine transcripts upon Ag-encounter [119]. TEM have a longer lifespan as compared with effector T cells and provide a readily-available pool for effector T cells in the immune periphery [113]. Thus, TEM can quickly supply Ag-specific effector T cells in peripheral tissues when the need arises, as compared with the longer time required for the differentiation of naïve T cells into effector T cells.
3.3. CD4+ TRM Subset Function
CD4+ TRM, which reside in peripheral tissues, function as the first line of defense at these sites together with TEM. However, TRM exhibit some unique functions different from those of TEM, and in some cases, show more vigorous responses during secondary Ag-encounter [120,121,122,123]. For instance, lung-resident memory CD4+ TRM cells provide optimal protection against secondary respiratory viral challenge with influenza virus, whereas protection provided by influenza-specific circulating memory CD4+ T cells is weaker despite their ability to expand and migrate to the lungs upon infection with the same pathogens [121]. Likewise, optimal protection against Chlamydia infection is dependent on the generation of mucosa (genital tract) homing CD4+ TRM, while protection provided by circulating memory T cells is less effective [124] suggesting that mucosal CD4+ TRM are critical for optimal protection against pathogens entering via the mucosal entry sites. Of note, tumor-homing CD4+ TRM are more potent producers of TNF and IFN-γ compared with other tumor infiltrating T cells [125]. Additionally, CD4+ TRM directed against certain pathogens emerge and persist in peripheral tissues following infection, such as influenza-virus-specific CD4+ TRM in the lungs and Leishmania-specific memory CD4+ TRM in the skin [126,127]. These CD4+ TRM cells rapidly produce effector cytokines such as IFN-γ and IL-17 upon re-challenge [126,127]. Using a new strategy for mucosal vaccination, Stary et al. showed that IFN-γ producing CD4+ TRM are pivotal for protection against Chlamydia trachomatis [124,128]. Similar findings have been reported for genital tract herpes simplex virus (HSV) vaccination [120], and gastric subserous vaccination with Helicobacter pylori vaccine [129]. Therefore, identification of mechanisms which promote the generation and retention of CD4+ TRM should be further explored for development of more effective vaccines against a range of human pathogens [130]. Of note, female lower genital tract CD4+ TRM were identified to serve as primary targets of HIV infection and persistence, thus providing an HIV cellular sanctuary [131]. Thus, HIV treatment strategies and vaccines may consider targeting TRM [131].
The mechanisms by which CD4+ TRM provide enhanced protection is an area of intense research, and some evidence suggests that they may differ from those used by circulating effector/memory CD4+ T cells. Along this line, CD4+ TRM provide rapid protection by promoting the recruitment of immune cells into the affected tissues [121,122,132,133,134]. In addition, CD4+ TRM are important for the maintenance, distribution, and homing of CD8+ TRM in situ [135,136]. Since it was shown that CD4+ T cells can foster the development of lung CD8+ TRM cells during infection with influenza virus [137], it is conceivable that CD4+ TRM may also contribute to the generation of CD8+ TRM. Interestingly, CD4+ TRM outnumber CD8+ TRM in many tissues [23,123], suggesting a critical role for CD4+ TRM in tissue-specific immunity and barrier function. For instance, approximately 70% to 85% of total TRM in the human skin are CD4+ cells.
Mechanistically, CD4+ TRM cells in the skin proliferate more extensively and produce significantly higher levels of IFN-γ, TNF, and IL-22 (and to a lesser extent IL-17 and IL-4) as compared with circulating memory CD4+ T cells [123]. In fact, immunosurveillance of non-lymphoid tissues is orchestrated by CD4+ TRM cells rather than by CD8+ TRM [138]. Notably, CD4+ TRM share overlapping transcriptional, phenotypic, and location-specific functional properties with CD8+ TRM and orchestrate local recall responses [138]. In contrast to CD8+ TRM, the human skin is populated with CD4+ TRM which are either CD103+ and reside primarily in the epidermis, or CD103- which mainly reside in the dermis [123]. Interestingly, CD103+ CD4+ TRM in skin show lower proliferative capacity but increased effector function as compared with CD103- CD4+ TRM, independent of their location in the dermis or epidermis [123]. These data suggest that CD103+ and CD103- CD4+ TRM cells encompass unique functional attributes in which CD103+ TRM cells provide robust effector responses (cytokine production), while CD103- TRM cells proliferate extensively to supply the Ag-specific CD4+ TRM cell pool. Future studies should investigate the cross-regulation between these two populations and whether CD103− CD4+ TRM can give rise to CD103+ TRM cells or vice versa. Finally, the generation and retention of skin CD4+ TRM was shown to be dependent on skin-resident CD8+ T cells or CD11b+ skin-resident macrophages [139], adding to the complexity in this system.
In addition to providing enhanced tissue protection, CD4+ TRM have also been implicated in undesired immunopathology of inflammatory diseases and they may contribute to the persistence of inflammatory cells and chronic inflammation in the affected tissues [140,141,142]. Nevertheless, approximately 10% of CD4+ TRM express the transcription factor Foxp3 and are thought to have regulatory functions [143]. In this context, Foxp3+ CD4+ T cells enter and reside in the skin during the neonatal period and mediate tolerance to commensal, non-pathogenic microbes [144]. Therefore, it will be critical to elucidate the mechanisms of CD4+ TRM developmental pathways, generation and maintenance, and their intersection with anti-microbial, regulatory, or pathologic functions to elicit optimal protection, while avoiding tissue damage.
In summary, memory CD4+ T cell responses and their unique functional attributes provide critical contributions to protection against microbial pathogens [111]. These include increased cytokine production, regulation of innate immune cell functions, mobilization of immune cells to sites of infection, providing B cell help, and enhancing cytotoxic T cell responses [111]. Robust and rapid local responses are provided at entry sites of infection by CD4+ TRM, which tailor immune-responses to specific tissues and the local microenvironment by providing local cues, mediating the rapid recruitment of other immune cells, and by regulating and maintaining other tissue-resident cells, including CD8+ TRM.
4. Memory CD4+ T Cells in Autoimmunity
Memory CD4+ T cells are of great interest in the context of autoimmune diseases because of their long-lived nature, efficient responses to antigens, and unique potential to mediate recurring autoimmune responses. However, until now, T cell memory has been more extensively investigated in the context of infectious diseases and its role in autoimmune diseases is not fully elucidated. Here, we will summarize and discuss some of the most pertinent findings on memory CD4+ T cells in autoimmune diseases, with special focus on multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE).
4.1. Persistence of Autoreactive Memory T Cells in Autoimmune Diseases and the Role of Immunoscenescence
Autoreactive memory CD4+ T cells have been studied in patients in several autoimmune disease conditions [145,146]. For instance, patients with MS and psoriasis show increased numbers of memory CD4+ T cells as compared with healthy individuals, suggesting that memory CD4+ T cell are critical mediators of autoimmune disease [147]. Subsequent work in animal models of autoimmune diseases further highlighted the roles which memory CD4+ T cell play in promoting autoimmunity. Along these lines, adoptive transfer of autoreactive memory CD4+ T cells is sufficient to induce disease, for example in animal models of MS, diabetes, and uveoretinitis [148,149,150]. However, while autoreactive CD4+ memory T cells are sufficient to induce autoimmune pathology, the context that these cells are transferred into is important. For example, in an elegant study in EAE, neonatal mice were injected with myelin basic protein (MBP)-specific CD4+ T cells to allow the generation of MBP-specific memory CD4+ T cells prior to adulthood [151]. Importantly, these animals remained healthy despite the presence of memory CD4+ T cells in lymphoid tissues when they reached adulthood. However, mice that had received MBP-specific CD4+ T cells developed earlier and had more severe EAE disease when they were immunized with MBP in complete Freud’s adjuvant (CFA) as compared with mice that received ovalbumin (OVA)-specific CD4+ T cells. These data suggest that memory CD4+ T cells that develop at early ages (prior to adulthood) may have regulatory functions, or that autoreactive memory T cells can form and persist in healthy individuals but may require additional events in order to become activated and induce disease. Nevertheless, these data demonstrate that autoreactive CD4+ memory T cells generate a more vigorous response upon exposure to autoantigen as compared with Ag-inexperienced naïve autoreactive T cells. These data also suggest that the re-activation of autoimmune memory T cells requires a lower Ag threshold than that of naïve T cells, similar to pathogen-reactive memory T cells.
Along these lines, a critical unresolved question for future studies is how T cell senescence and aging affects memory T cell functions. The effect of aging on CD4+ naïve and memory T cells in the context of infectious diseases has been studied and extensively reviewed elsewhere [152,153,154,155]. Immunoscenescence (i.e., ageing of the immune system) is characterized by a decline of adoptive and innate immune cell functions, including of CD4+ T cells [152,156]. However, aging is also associated with autoimmune phenomena, and certain autoimmune disease conditions are more frequently observed in elderly individuals [157]. Moreover, aging and T cell immunoscenescence has been associated with MS and EAE [158]), as well as with other autoimmune diseases [159,160]). Furthermore, treatment efficacy and development of progressive multifocal leukoencephalopathy in MS patients has been linked and associated with immunoscenescence [161,162,163]. As the role of the aging immune system in infectious diseases and autoimmunity appears somewhat contradictory [160], an interesting question is how does the inflammatory environment during aging/immunoscenescence contribute to and modulate autoimmune memory? Along these lines, a recent interesting study showed that aging promotes the development and accumulation of extreme pro-inflammatory cytotoxic CD4+ T cells, as well as anti-inflammatory regulatory T cells (Tregs) (~30% of the total T cell pool) [164]. How these findings affect autoreactive memory T cells will be an interesting question for further research. Furthermore, many pressing questions remain to be further explored, for instance, which antigens these cells recognize and/or their clonotypes both in healthy individuals as well as in autoimmune disease, what are the mechanisms that maintain or revoke the activation/quiescence of these cells, and what is their relationship to memory T cells? Taken together, immunoscenescence may have important implications for the approach to treating autoimmune diseases in the elderly.
4.2. Role of Autoantigen for Memory T Cells
A question central to autoimmune memory is which autoantigens the memory CD4+ T cells recognize in human autoimmune diseases. The answer to this question could provide insights into mechanisms that promote escape from immune tolerance. For instance, rheumatoid arthritis (RA) patients exhibit memory CD4+ T cells specific for various citrullinated antigens, including citrullinated aggrecan and citrullinated vimentin, which correspond to autoantibodies directed against citrullinated antigens and proteins in these patients [165,166,167]. Furthermore, CD4+ memory cells with a Th17 phenotype and specific for a citrullinated vimentin epitope expanded more significantly in RA patients with active disease and significantly decreased upon anti-TNF treatment [168]. Additionally, memory CD4+ T cells recognizing glycosylated type II collagen peptides have been identified in RA patients [169]. Together, these data suggest that post-translationally modified autoantigens, and particularly citrullinated autoantigens, may be critical to drive RA progression and increase the number of autoreactive memory T cell clones. Memory CD4+ T cells from MS patients have been reported to recognize several neuroantigens, particularly myelin antigens, including myelin oligodendrocyte glycoprotein (MOG) and MBP [170,171], and T cell responses against these autoantigens are pathogenic in its animal model EAE [151]. It remains to be determined whether modifications of myelin antigens also play a role in MS patients, similar to RA, and whether this results in generation of autoreactive memory CD4+ T cells. This may provide important clues for the role of autoreactive memory T cells in disease etiology and progression. Taken together, strong evidence supports a key role for memory CD4+ T cells in the pathogenesis of autoimmune diseases. Important remaining questions will center on the mechanisms that activate, sustain, and regulate these autoreactive T cells.
4.3. Autoreactive Memory T Cells with Th17 Cell Phenotype
Th17 cells are key drivers of many chronic autoimmune disease conditions, including MS, RA, and psoriasis [2,172,173]. Thus, memory T cells with a Th17 cell phenotype have been reported in many of these conditions. Th17 cells can give rise to self-renewing CD4+ TEM that maintain their Th17 cell phenotype [174]. Moreover, CD4+ TCM with Th1 and Th17 phenotype were reported as selectively increased in blood of MS patients and to correlate with disease severity. Interestingly, the transcriptional profile of blood Th1 TCM and Th17 TCM strongly resembled conventional effector Th17 cells (and not Th1 cells) with more pathogenic features. The cerebrospinal fluid (CSF) of these patients contained mainly CXCR3-expressing Th1/Th17 TCM cells. However, CSF Th17 TCM cells of MS patients reacted strongly to myelin-derived self-antigens (including MOG and MBP), while Th1 cells responded consistently only to virus antigens (such as Epstein–Barr virus). Additionally, the CSF Th1 TCM and Th17 TCM from MS patients had the capacity to produce high levels of pathogenic cytokines upon activation, including IFN-γ, IL-17, GM-CSF, and IL-22 [170]. Moreover, IL-23, which is essential for terminal differentiation of pathogenic Th17 cells [175], has been proposed to regulate memory Th17 cell generation and function. In support of this view, IL-23 drives the proliferation and expansion of memory Th17 cells from MS patients and promotes expression of IL-17 and IFN-γ in these cells [176]. Similarly, IL-23 signaling is critical for development and proliferation of memory Th17 cells in EAE [177], and memory CD4+ T cells in EAE mice proliferate more and produce more IFN-γ but less IL-17 as compared with effector T cells [148]. Furthermore, a population of gut resident CD161+ Th17 cells was identified in patients with Crohn’s disease. These gut-homing memory Th17 cells expressed high levels of pro-inflammatory cytokines, including IL-17, IL-22, and IFN-γ upon re-activation with αCD3 and αCD28 in presence of IL-1β and IL-23 [178]. Moreover, in an animal model of colitis, IL-23 promotes the proliferation of memory CD4+ T cells and increases the expression of IFN-γ and IL-17 to promote inflammation [179]. Thought-provokingly, IL-21, a cytokine that is expressed by Th17 cells and enhances their development and expansion in an autocrine fashion [180,181], was reported to inhibit de novo generation of pathogenic Th17 (and Th1) effector T cells from IL-21-expressing TCM cells. These data indicate a potentially protective role for IL-21+ TCM in the context of autoimmunity [182].
4.4. Autoreactive TCM and TEM Subsets and Disease-Modifying Therapies
A critical remaining question centers on the functional attributes of the different memory CD4+ T cell subsets (i.e., TCM, TEM, and TRM) in promoting autoimmune disease. Although both autoantigen- specific CD4+ TCM and TEM cells have been reported, the generation of CD4+ TEM appears more common [183]. This phenomenon is attributed to the presence of chronic autoantigen exposure, which appears to favor the development of CD4+ TEM while hindering CD4+ TCM cell formation, similar to chronic infection settings [174,178,183]. Along these lines, MS and systemic lupus erythematosus (SLE) patients show higher frequencies of autoreactive CD4+ TEM and lower CD4+ TCM in peripheral blood compared with healthy controls [183]. Similar results were reported in patients with colitis, type 1 diabetes, and other autoimmune diseases [174,178,183]. Interestingly, unlike pathogen-specific memory T cells, which are long-lived and highly proliferative, memory CD4+ T cells from autoimmune disease patients are more likely to undergo apoptosis and are less likely to proliferate, most notably for CD4+ TCM [183]. These data suggest that chronic autoimmune disease conditions promote memory CD4+ T cell death and inhibit their proliferation and survival. Determining how differentiation and survival of different subsets of memory CD4+ T cells in autoimmune disease conditions are affected by factors such as cytokine milieu and the presence of autoantigens may lead to potential new avenues for treatment of disease progression and relapses.
Further implicating memory T cells in MS are the results of treating MS patients with fingolimod (also known as FTY720), an S1P receptor (S1PR) antagonist which is thought to act by downregulating the expression of S1PR1 on lymphocytes and is now approved for the treatment of relapsing MS [184,185]. Responsiveness to S1P (via S1PRs) and S1P-dependent tissue trafficking from lymphoid tissues to inflamed tissues are complex and reviewed elsewhere [186,187]. Briefly, S1P acts to promote lymph node egress by overcoming retention signals mediated by factors such as CCR7 [188]. Additionally, TCM largely depend on the S1P/S1PR-axis to traffic/exit from lymphoid tissues to the blood circulation, while TEM which are CCR7low, have already egressed to the circulation and do no-longer rely on S1P/S1PR1 [187,188]. Thus, fingolimod prevents the circulation of TCM but not TEM to the CNS and promotes (CCR7-mediated) TCM retention in secondary lymphoid tissues [189]. TRM cells do not express S1PR1 as they do not express its transcription factor KLF2 (Figure 3) [24]. Along these lines, fingolimod treatment of MS patients showed a marked reduction in blood-circulating CD4+ TCM but not in TEM [189,190]. Subsequent studies showed that fingolimod affected primarily IL-17-producing CD4+ TCM [191]. Interestingly, fingolimod-treated relapsed MS patients showed greater percentages of CD4+ TCM (and naïve cells) but not TEM, suggesting that CD4+ TCM may be involved in promoting relapses following fingolimod treatment in MS patients [192]. Furthermore, fingolimod treatment was associated with elevated frequencies of CD56+ memory T cells, and increased granzyme (GZM) B, perforin, and Fas ligand expression in memory T cells in MS patients, and interestingly, this T cell phenotype was also associated with clinical relapses [192]. Additionally, Herich et al. demonstrated that CD4+ TEM expressing high levels of CCR5 and GZMK are involved in CNS immune surveillance in healthy individuals, but that this subset was dominant in peripheral blood mononuclear cells of MS patients, and that natalizumab (anti-α4-integrin) treatment significantly decreased these cells [193]. Furthermore, the CCR5high GZMK+ CD4+ TEM subset shares many transcriptional features with TRM and Th17 cells, suggesting that it could play a central role in CNS pathology [193].
Therapies aimed at modulating the function of autoreactive memory T cells should take advantage of the current understanding of mechanism that regulate effector T cells (Table 1). For example, are immune checkpoints similarly effective in memory T cells as compared to effector T cells? Furthermore, what is the impact of regulatory T cells on memory T cell pool and function? At least some of the mechanisms known to regulate effector T cells act differently on memory T cells, which could potentially be explored therapeutically. A better understanding of these critical mechanisms may have major implications for therapeutic intervention for autoimmune diseases, for instance by targeting the common gamma-chain-receptor cytokines (e.g., IL-7, IL-15), which greatly affect naïve and memory CD4+ T cell development and homeostasis in healthy individuals and infectious diseases [194,195].Thus, future work should focus on further unraveling the different mechanisms by which memory T cell subsets contribute to autoimmune inflammatory diseases, and elucidate mechanisms by which regulatory mechanisms and therapeutic drugs may affect memory T cell subset effector functions and migratory capacities.

Table 1.
Regulation of memory and effector CD4+ T cells. Abbreviations used: programmed cell death protein 1 (PD-1), B-and T-lymphocyte attenuator (BTLA), lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin, mucin-domain containing-3 (TIM-3), and dendritic cell (DC).
5. Concluding Remarks
A better understanding of the immunobiology of CD4+ memory T cells in chronic autoimmune diseases is critical to develop better treatments. While it is understood that there are differences in memory T cell populations and subpopulations in autoimmune disease conditions, there are important gaps in the current understanding of how these cells develop and how the host microenvironment, including antigen exposure and cytokine milieu, affect the function and maintenance of these cells. Additionally, it remains incompletely understood if and how these cells differ from memory T cells directed against infectious pathogens in terms of activation thresholds, cytokine secretion, and long-term survival, and how regulatory mechanism apply to these cells as compared with naïve/effector T cells.
CD8+ and CD4+ TRM cells were identified in human brain and in lesions of MS patients [212,213], and recent research primarily focused on the role of CD8+ TRM cells and their contribution to autoimmune pathology in MS and EAE [212,213,214,215,216]. However, autoimmune CD4+ TRM are not as well studied, and many critical questions remain as to their potential contribution to autoimmune disease pathology. Moreover, a better understanding of the role of autoreactive, pathogenic CD4+ T cells in relapses and progression of autoimmune diseases could have major therapeutic implications. Addressing these questions will be paramount to develop better treatments for CD4+ T cell-driven autoimmune diseases.
Funding
This research was supported by grants G12MD007591 and NS084201 from the National Institute of Health (TGF) and grants RG5501 and RG1602 from the National Multiple Sclerosis Society (TGF).
Acknowledgments
We would like to thank Saisha Nalawade and Carol Chase for reading the manuscript. Figures created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, S.; Dong, C. A complex issue on CD4(+) T-cell subsets. Immunol. Rev. 2013, 252, 5–11. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Forsthuber, T.G. Stability of T-cell lineages in autoimmune diseases. Expert Rev. Clin. Immunol. 2012, 8, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Baaten, B.J.; Cooper, A.M.; Swain, S.L.; Bradley, L.M. Location, location, location: The impact of migratory heterogeneity on T cell function. Front. Immunol. 2013, 4, 311. [Google Scholar] [CrossRef]
- Mackay, C.R. T-cell memory: The connection between function, phenotype and migration pathways. Immunol Today 1991, 12, 189–192. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Khoruts, A.; Ingulli, E.; Mueller, D.L.; McSorley, S.J.; Reinhardt, R.L.; Itano, A.; Pape, K.A. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 2001, 19, 23–45. [Google Scholar] [CrossRef]
- Sprent, J.; Surh, C.D. T cell memory. Annu Rev Immunol 2002, 20, 551–579. [Google Scholar] [CrossRef]
- MacLeod, M.K.; Kappler, J.W.; Marrack, P. Memory CD4 T cells: Generation, reactivation and re-assignment. Immunology 2010, 130, 10–15. [Google Scholar] [CrossRef]
- Croft, M. Activation of naive, memory and effector T cells. Curr. Opin. Immunol. 1994, 6, 431–437. [Google Scholar] [CrossRef]
- Cho, B.K.; Wang, C.; Sugawa, S.; Eisen, H.N.; Chen, J. Functional differences between memory and naive CD8 T cells. Proc. Natl. Acad. Sci. USA 1999, 96, 2976–2981. [Google Scholar] [CrossRef] [PubMed]
- Mishima, T.; Toda, S.; Ando, Y.; Matsunaga, T.; Inobe, M. Rapid proliferation of activated lymph node CD4(+) T cells is achieved by greatly curtailing the duration of gap phases in cell cycle progression. Cell Mol. Biol. Lett. 2014, 19, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Berard, M.; Tough, D.F. Qualitative differences between naive and memory T cells. Immunology 2002, 106, 127–138. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef]
- Blattman, J.N.; Antia, R.; Sourdive, D.J.; Wang, X.; Kaech, S.M.; Murali-Krishna, K.; Altman, J.D.; Ahmed, R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002, 195, 657–664. [Google Scholar] [CrossRef]
- Mackay, C.R. Dual personality of memory T cells. Nature 1999, 401, 659–660. [Google Scholar] [CrossRef]
- MacLeod, M.K.; Clambey, E.T.; Kappler, J.W.; Marrack, P. CD4 memory T cells: What are they and what can they do? Semin. Immunol. 2009, 21, 53–61. [Google Scholar] [CrossRef]
- Mora, J.R.; von Andrian, U.H. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006, 27, 235–243. [Google Scholar] [CrossRef]
- Woodland, D.L.; Kohlmeier, J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009, 9, 153–161. [Google Scholar] [CrossRef]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol 2009, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Gordon, C.L.; Farber, D.L. Tissue-resident T cells, in situ immunity and transplantation. Immunol. Rev. 2014, 258, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M.; et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Topham, D.J.; Reilly, E.C. Tissue-Resident Memory CD8(+) T Cells: From Phenotype to Function. Front Immunol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Wu, X.; Wu, P.; Shen, Y.; Jiang, X.; Xu, F. CD8(+) Resident Memory T Cells and Viral Infection. Front Immunol. 2018, 9, 2093. [Google Scholar] [CrossRef]
- Ahmed, R.; Bevan, M.J.; Reiner, S.L.; Fearon, D.T. The precursors of memory: Models and controversies. Nat. Rev. Immunol. 2009, 9, 662–668. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Chang, J.T.; Palanivel, V.R.; Kinjyo, I.; Schambach, F.; Intlekofer, A.M.; Banerjee, A.; Longworth, S.A.; Vinup, K.E.; Mrass, P.; Oliaro, J.; et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 2007, 315, 1687–1691. [Google Scholar] [CrossRef]
- Pepper, M.; Jenkins, M.K. Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar] [CrossRef]
- Corse, E.; Gottschalk, R.A.; Allison, J.P. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J. Immunol. 2011, 186, 5039–5045. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.K.; Yagita, H.; Malherbe, L.P. A TCR affinity threshold regulates memory CD4 T cell differentiation following vaccination. J. Immunol. 2012, 189, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.A.; Teixeiro, E. TCR Signaling in T Cell Memory. Front. Immunol. 2015, 6, 617. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, M.S.; Davis, M.M. TCR Signaling Emerges from the Sum of Many Parts. Front. Immunol. 2012, 3, 159. [Google Scholar] [CrossRef]
- Pepper, M.; Pagan, A.J.; Igyarto, B.Z.; Taylor, J.J.; Jenkins, M.K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 2011, 35, 583–595. [Google Scholar] [CrossRef]
- Marshall, H.D.; Chandele, A.; Jung, Y.W.; Meng, H.; Poholek, A.C.; Parish, I.A.; Rutishauser, R.; Cui, W.; Kleinstein, S.H.; Craft, J.; et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity 2011, 35, 633–646. [Google Scholar] [CrossRef]
- Richer, M.J.; Nolz, J.C.; Harty, J.T. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity 2013, 38, 140–152. [Google Scholar] [CrossRef]
- Snook, J.P.; Kim, C.; Williams, M.A. TCR signal strength controls the differentiation of CD4(+) effector and memory T cells. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Obst, R.; van Santen, H.M.; Mathis, D.; Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2005, 201, 1555–1565. [Google Scholar] [CrossRef]
- Jelley-Gibbs, D.M.; Brown, D.M.; Dibble, J.P.; Haynes, L.; Eaton, S.M.; Swain, S.L. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 2005, 202, 697–706. [Google Scholar] [CrossRef]
- Gasper, D.J.; Tejera, M.M.; Suresh, M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 2014, 34, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Wikstrom, M.E.; Fazekas de St Groth, B. Visualizing T cell competition for peptide/MHC complexes: A specific mechanism to minimize the effect of precursor frequency. Immunity 2000, 13, 783–794. [Google Scholar] [CrossRef]
- Butz, E.A.; Bevan, M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 1998, 8, 167–175. [Google Scholar] [CrossRef]
- Busch, D.H.; Pamer, E.G. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 1999, 189, 701–710. [Google Scholar] [CrossRef]
- Kim, C.; Wilson, T.; Fischer, K.F.; Williams, M.A. Sustained interactions between T cell receptors and antigens promote the differentiation of CD4(+) memory T cells. Immunity 2013, 39, 508–520. [Google Scholar] [CrossRef]
- Williams, M.A.; Ravkov, E.V.; Bevan, M.J. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 2008, 28, 533–545. [Google Scholar] [CrossRef]
- Whitmire, J.K.; Benning, N.; Eam, B.; Whitton, J.L. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J. Immunol. 2008, 180, 6777–6785. [Google Scholar] [CrossRef]
- McKinstry, K.K.; Strutt, T.M.; Bautista, B.; Zhang, W.; Kuang, Y.; Cooper, A.M.; Swain, S.L. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat Commun. 2014, 5, 5377. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Moon, J.J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012, 188, 4135–4140. [Google Scholar] [CrossRef]
- Foulds, K.E.; Shen, H. Clonal competition inhibits the proliferation and differentiation of adoptively transferred TCR transgenic CD4 T cells in response to infection. J. Immunol. 2006, 176, 3037–3043. [Google Scholar] [CrossRef]
- Hataye, J.; Moon, J.J.; Khoruts, A.; Reilly, C.; Jenkins, M.K. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 2006, 312, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.A.; Lefrancois, L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15045–15050. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Asoyan, A.; Rabenstein, H.; Nakano, N.; Obst, R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc. Natl. Acad. Sci. USA 2010, 107, 20453–20458. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Linehan, J.L.; Pagan, A.J.; Zell, T.; Dileepan, T.; Cleary, P.P.; Jenkins, M.K. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010, 11, 83–89. [Google Scholar] [CrossRef]
- Joshi, N.S.; Cui, W.; Chandele, A.; Lee, H.K.; Urso, D.R.; Hagman, J.; Gapin, L.; Kaech, S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 2007, 27, 281–295. [Google Scholar] [CrossRef]
- Whitmire, J.K.; Eam, B.; Benning, N.; Whitton, J.L. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J. Immunol. 2007, 179, 1190–1197. [Google Scholar] [CrossRef]
- Afkarian, M.; Sedy, J.R.; Yang, J.; Jacobson, N.G.; Cereb, N.; Yang, S.Y.; Murphy, T.L.; Murphy, K.M. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002, 3, 549–557. [Google Scholar] [CrossRef]
- Harrington, L.E.; Janowski, K.M.; Oliver, J.R.; Zajac, A.J.; Weaver, C.T. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 2008, 452, 356–360. [Google Scholar] [CrossRef]
- Thieu, V.T.; Yu, Q.; Chang, H.C.; Yeh, N.; Nguyen, E.T.; Sehra, S.; Kaplan, M.H. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity 2008, 29, 679–690. [Google Scholar] [CrossRef]
- Xiong, S.Q.; Lin, B.L.; Gao, X.; Tang, H.; Wu, C.Y. IL-12 promotes HBV-specific central memory CD8+ T cell responses by PBMCs from chronic hepatitis B virus carriers. Int. Immunopharmacol. 2007, 7, 578–587. [Google Scholar] [CrossRef]
- Mollo, S.B.; Ingram, J.T.; Kress, R.L.; Zajac, A.J.; Harrington, L.E. Virus-specific CD4 and CD8 T cell responses in the absence of Th1-associated transcription factors. J. Leukoc. Biol. 2014, 95, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Oja, A.E.; Piet, B.; Helbig, C.; Stark, R.; van der Zwan, D.; Blaauwgeers, H.; Remmerswaal, E.B.M.; Amsen, D.; Jonkers, R.E.; Moerland, P.D.; et al. Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol. 2018, 11, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.T.; Masson, F. Interleukin-2 tickles T cell memory. Immunity 2010, 32, 7–9. [Google Scholar] [CrossRef][Green Version]
- Dhume, K.; McKinstry, K.K. Early programming and late-acting checkpoints governing the development of CD4 T-cell memory. Immunology 2018, 155, 53–62. [Google Scholar] [CrossRef]
- Crotty, S.; Johnston, R.J.; Schoenberger, S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 2010, 11, 114–120. [Google Scholar] [CrossRef]
- McKinstry, K.K.; Alam, F.; Flores-Malavet, V.; Nagy, M.Z.; Sell, S.; Cooper, A.M.; Swain, S.L.; Strutt, T.M. Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. Plos Pathog 2019, 15, e1007989. [Google Scholar] [CrossRef]
- Prlic, M.; Lefrancois, L.; Jameson, S.C. Multiple choices: Regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 2002, 195, F49–F52. [Google Scholar] [CrossRef]
- Cui, W.; Kaech, S.M. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol. Rev. 2010, 236, 151–166. [Google Scholar] [CrossRef]
- Schluns, K.S.; Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003, 3, 269–279. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Masopust, D. Identification of a resident T-cell memory core transcriptional signature. Immunol. Cell Biol. 2014, 92, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, R.; Feng, Y.; El-Asady, R.; Farber, D.L.; Gress, R.E.; Lucas, P.J.; Hadley, G.A. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J. Immunol. 2004, 172, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Mackay, L.K. Local immunity by tissue-resident CD8(+) memory T cells. Front. Immunol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, L.; Barrett, T.A.; Havran, W.L.; Puddington, L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur. J. Immunol. 1994, 24, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kitani, A.; Strober, W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010, 3, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Zhu, J.; Huang, X.; Yang, Y. The development and function of memory regulatory T cells after acute viral infections. J. Immunol. 2012, 189, 2805–2814. [Google Scholar] [CrossRef]
- Duttagupta, P.A.; Boesteanu, A.C.; Katsikis, P.D. Costimulation signals for memory CD8+ T cells during viral infections. Crit. Rev. Immunol. 2009, 29, 469–486. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Luster, A.D. Chemokines: Critical Regulators of Memory T Cell Development, Maintenance, and Function. Adv. Immunol. 2018, 138, 71–98. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential Role of Gut Microbiota in Induction and Regulation of Innate Immune Memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Thomas, M.L.; Lefrancois, L. Differential expression of the leucocyte-common antigen family. Immunol. Today 1988, 9, 320–326. [Google Scholar] [CrossRef]
- Akbar, A.N.; Salmon, M.; Ivory, K.; Taki, S.; Pilling, D.; Janossy, G. Human CD4+CD45R0+ and CD4+CD45RA+ T cells synergize in response to alloantigens. Eur. J. Immunol. 1991, 21, 2517–2522. [Google Scholar] [CrossRef]
- Lee, W.T.; Vitetta, E.S. Changes in expression of CD45R during the development of Th1 and Th2 cell lines. Eur. J. Immunol. 1992, 22, 1455–1459. [Google Scholar] [CrossRef]
- Merkenschlager, M.; Terry, L.; Edwards, R.; Beverley, P.C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: Implications for differential CD45 expression in T cell memory formation. Eur. J. Immunol. 1988, 18, 1653–1661. [Google Scholar] [CrossRef]
- Sanders, M.E.; Makgoba, M.W.; Sharrow, S.O.; Stephany, D.; Springer, T.A.; Young, H.A.; Shaw, S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J. Immunol. 1988, 140, 1401–1407. [Google Scholar]
- Akbar, A.N.; Timms, A.; Janossy, G. Cellular events during memory T-cell activation in vitro: The UCHL1 (180,000 MW) determinant is newly synthesized after mitosis. Immunology 1989, 66, 213–218. [Google Scholar]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Bradley, L.M.; Atkins, G.G.; Swain, S.L. Long-term CD4+ memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J. Immunol. 1992, 148, 324–331. [Google Scholar]
- Bradley, L.M.; Duncan, D.D.; Yoshimoto, K.; Swain, S.L. Memory effectors: A potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J. Immunol. 1993, 150, 3119–3130. [Google Scholar]
- Budd, R.C.; Cerottini, J.C.; Horvath, C.; Bron, C.; Pedrazzini, T.; Howe, R.C.; MacDonald, H.R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 1987, 138, 3120–3129. [Google Scholar]
- Springer, T.A.; Dustin, M.L.; Kishimoto, T.K.; Marlin, S.D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: Cell adhesion receptors of the immune system. Annu Rev. Immunol. 1987, 5, 223–252. [Google Scholar] [CrossRef]
- Birkeland, M.L.; Johnson, P.; Trowbridge, I.S.; Pure, E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc. Natl. Acad. Sci. USA 1989, 86, 6734–6738. [Google Scholar] [CrossRef]
- Reinhardt, R.L.; Khoruts, A.; Merica, R.; Zell, T.; Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 2001, 410, 101–105. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Hussain, S.F.; Farber, D.L. Effector CD4 T cells are biochemically distinct from the memory subset: Evidence for long-term persistence of effectors in vivo. J. Immunol. 1999, 163, 3053–3063. [Google Scholar]
- Unsoeld, H.; Pircher, H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J. Virol. 2005, 79, 4510–4513. [Google Scholar] [CrossRef]
- Tough, D.F.; Sprent, J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994, 179, 1127–1135. [Google Scholar] [CrossRef]
- Yu, X.Z.; Anasetti, C. Memory stem cells sustain disease. Nat. Med. 2005, 11, 1282–1283. [Google Scholar] [CrossRef]
- Rogers, P.R.; Pilapil, S.; Hayakawa, K.; Romain, P.L.; Parker, D.C. CD45 alternative exon expression in murine and human CD4+ T cell subsets. J. Immunol. 1992, 148, 4054–4065. [Google Scholar]
- Klonowski, K.D.; Williams, K.J.; Marzo, A.L.; Blair, D.A.; Lingenheld, E.G.; Lefrancois, L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 2004, 20, 551–562. [Google Scholar] [CrossRef]
- Jiang, X.; Clark, R.A.; Liu, L.; Wagers, A.J.; Fuhlbrigge, R.C.; Kupper, T.S. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012, 483, 227–231. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Lefrancois, L. Regional and mucosal memory T cells. Nat. Immunol. 2011, 12, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Fraser, K.A.; Schenkel, J.M.; Moran, A.; Abt, M.C.; Beura, L.K.; Lucas, P.J.; Artis, D.; Wherry, E.J.; Hogquist, K.; et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012, 188, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Cookenham, T.; Roberts, A.D.; Miller, S.C.; Woodland, D.L. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity 2010, 33, 96–105. [Google Scholar] [CrossRef]
- Skon, C.N.; Lee, J.Y.; Anderson, K.G.; Masopust, D.; Hogquist, K.A.; Jameson, S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 1285–1293. [Google Scholar] [CrossRef]
- Clark, R.A.; Chong, B.F.; Mirchandani, N.; Yamanaka, K.; Murphy, G.F.; Dowgiert, R.K.; Kupper, T.S. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Invest. Derm. 2006, 126, 1059–1070. [Google Scholar] [CrossRef]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef]
- Carbone, F.R.; Gebhardt, T. Should I stay or should I go-Reconciling clashing perspectives on CD4(+) tissue-resident memory T cells. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Klicznik, M.M.; Morawski, P.A.; Hollbacher, B.; Varkhande, S.R.; Motley, S.J.; Kuri-Cervantes, L.; Goodwin, E.; Rosenblum, M.D.; Long, S.A.; Brachtl, G.; et al. Human CD4(+)CD103(+) cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Geginat, J.; Sallusto, F.; Lanzavecchia, A. Cytokine-driven proliferation and differentiation of human naive, central memory and effector memory CD4+ T cells. Pathol. Biol. (Paris) 2003, 51, 64–66. [Google Scholar] [CrossRef]
- Park, C.O.; Kupper, T.S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015, 21, 688–697. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- von Andrian, U.H.; Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000, 343, 1020–1034. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Hengel, R.L.; Thaker, V.; Pavlick, M.V.; Metcalf, J.A.; Dennis, G., Jr.; Yang, J.; Lempicki, R.A.; Sereti, I.; Lane, H.C. Cutting edge: L-selectin (CD62L) expression distinguishes small resting memory CD4+ T cells that preferentially respond to recall antigen. J. Immunol. 2003, 170, 28–32. [Google Scholar] [CrossRef]
- Wang, A.; Chandran, S.; Shah, S.A.; Chiu, Y.; Paria, B.C.; Aghamolla, T.; Alvarez-Downing, M.M.; Lee, C.C.; Singh, S.; Li, T.; et al. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci. Transl. Med. 2012. [Google Scholar] [CrossRef]
- MacLeod, M.K.; David, A.; McKee, A.S.; Crawford, F.; Kappler, J.W.; Marrack, P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 2011, 186, 2889–2896. [Google Scholar] [CrossRef]
- Fazilleau, N.; Eisenbraun, M.D.; Malherbe, L.; Ebright, J.N.; Pogue-Caley, R.R.; McHeyzer-Williams, L.J.; McHeyzer-Williams, M.G. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat. Immunol. 2007, 8, 753–761. [Google Scholar] [CrossRef]
- Hale, J.S.; Ahmed, R. Memory T follicular helper CD4 T cells. Front. Immunol. 2015, 6, 16. [Google Scholar] [CrossRef]
- Jain, A.; Song, R.; Wakeland, E.K.; Pasare, C. T cell-intrinsic IL-1R signaling licenses effector cytokine production by memory CD4 T cells. Nat. Commun. 2018, 9, 3185. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrancois, L.; Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef]
- Sakai, S.; Kauffman, K.D.; Schenkel, J.M.; McBerry, C.C.; Mayer-Barber, K.D.; Masopust, D.; Barber, D.L. Cutting edge: Control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 2014, 192, 2965–2969. [Google Scholar] [CrossRef]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015, 7, 279ra239. [Google Scholar] [CrossRef]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef]
- Oja, A.E.; Piet, B.; van der Zwan, D.; Blaauwgeers, H.; Mensink, M.; de Kivit, S.; Borst, J.; Nolte, M.A.; van Lier, R.A.W.; Stark, R.; et al. Functional Heterogeneity of CD4(+) Tumor-Infiltrating Lymphocytes With a Resident Memory Phenotype in NSCLC. Front. Immunol. 2018, 9, 2654. [Google Scholar] [CrossRef]
- Glennie, N.D.; Yeramilli, V.A.; Beiting, D.P.; Volk, S.W.; Weaver, C.T.; Scott, P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015, 212, 1405–1414. [Google Scholar] [CrossRef]
- Chapman, T.J.; Topham, D.J. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J. Immunol. 2010, 184, 3841–3849. [Google Scholar] [CrossRef]
- Nogueira, C.V.; Zhang, X.; Giovannone, N.; Sennott, E.L.; Starnbach, M.N. Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae. J. Immunol. 2015, 194, 2319–2329. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Z.; Luo, S.; Hu, C.; Xu, N.; Huang, A.; Zheng, L.; Sundberg, E.J.; Xi, T.; Xing, Y. Gastric Subserous Vaccination with Helicobacter pylori Vaccine: An Attempt to Establish Tissue-Resident CD4+ Memory T Cells and Induce Prolonged Protection. Front. Immunol. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Wilk, M.M.; Mills, K.H.G. CD4 TRM Cells Following Infection and Immunization: Implications for More Effective Vaccine Design. Front. Immunol. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Cantero-Perez, J.; Grau-Exposito, J.; Serra-Peinado, C.; Rosero, D.A.; Luque-Ballesteros, L.; Astorga-Gamaza, A.; Castellvi, J.; Sanhueza, T.; Tapia, G.; Lloveras, B.; et al. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat. Commun. 2019, 10, 4739. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Lu, B.; Gerard, C.; Iwasaki, A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 2009, 462, 510–513. [Google Scholar] [CrossRef]
- McKinstry, K.K.; Dutton, R.W.; Swain, S.L.; Strutt, T.M. Memory CD4 T cell-mediated immunity against influenza A virus: More than a little helpful. Arch. Immunol. Exp. (Warsz) 2013, 61, 341–353. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. Tissue-resident memory T cells. Immunol. Rev. 2013, 255, 165–181. [Google Scholar] [CrossRef]
- Sun, J.C.; Williams, M.A.; Bevan, M.J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004, 5, 927–933. [Google Scholar] [CrossRef]
- Azadniv, M.; Bowers, W.J.; Topham, D.J.; Crispe, I.N. CD4+ T cell effects on CD8+ T cell location defined using bioluminescence. PLoS ONE 2011, 6, e16222. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef]
- Beura, L.K.; Fares-Frederickson, N.J.; Steinert, E.M.; Scott, M.C.; Thompson, E.A.; Fraser, K.A.; Schenkel, J.M.; Vezys, V.; Masopust, D. CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019, 216, 1214–1229. [Google Scholar] [CrossRef]
- Collins, N.; Jiang, X.; Zaid, A.; Macleod, B.L.; Li, J.; Park, C.O.; Haque, A.; Bedoui, S.; Heath, W.R.; Mueller, S.N.; et al. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun. 2016, 7, 11514. [Google Scholar] [CrossRef]
- Turner, D.L.; Farber, D.L. Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol. 2014, 5, 331. [Google Scholar] [CrossRef]
- Clark, R.A. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015, 7, 269rv261. [Google Scholar] [CrossRef]
- Turner, D.L.; Goldklang, M.; Cvetkovski, F.; Paik, D.; Trischler, J.; Barahona, J.; Cao, M.; Dave, R.; Tanna, N.; D’Armiento, J.M.; et al. Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J. Immunol. 2018, 200, 1561–1569. [Google Scholar] [CrossRef]
- Seneschal, J.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 43, 1011–1021. [Google Scholar] [CrossRef]
- Burns, J.; Bartholomew, B.; Lobo, S. Isolation of myelin basic protein-specific T cells predominantly from the memory T-cell compartment in multiple sclerosis. Ann. Neurol. 1999, 45, 33–39. [Google Scholar] [CrossRef]
- Allegretta, M.; Nicklas, J.A.; Sriram, S.; Albertini, R.J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science 1990, 247, 718–721. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Ratzer, R.; Bornsen, L.; von Essen, M.R.; Christensen, J.R.; Sellebjerg, F. Characterization of naive, memory and effector T cells in progressive multiple sclerosis. J. Neuroimmunol. 2017, 310, 17–25. [Google Scholar] [CrossRef]
- Elyaman, W.; Kivisakk, P.; Reddy, J.; Chitnis, T.; Raddassi, K.; Imitola, J.; Bradshaw, E.; Kuchroo, V.K.; Yagita, H.; Sayegh, M.H.; et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am. J. Pathol. 2008, 173, 411–422. [Google Scholar] [CrossRef]
- McKeever, U.; Mordes, J.P.; Greiner, D.L.; Appel, M.C.; Rozing, J.; Handler, E.S.; Rossini, A.A. Adoptive transfer of autoimmune diabetes and thyroiditis to athymic rats. Proc. Natl. Acad. Sci. USA 1990, 87, 7618–7622. [Google Scholar] [CrossRef]
- Mochizuki, M.; Kuwabara, T.; McAllister, C.; Nussenblatt, R.B.; Gery, I. Adoptive transfer of experimental autoimmune uveoretinitis in rats. Immunopathogenic mechanisms and histologic features. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1–9. [Google Scholar]
- Kawakami, N.; Odoardi, F.; Ziemssen, T.; Bradl, M.; Ritter, T.; Neuhaus, O.; Lassmann, H.; Wekerle, H.; Flugel, A. Autoimmune CD4+ T cell memory: Lifelong persistence of encephalitogenic T cell clones in healthy immune repertoires. J. Immunol. 2005, 175, 69–81. [Google Scholar] [CrossRef]
- Swain, S.; Clise-Dwyer, K.; Haynes, L. Homeostasis and the age-associated defect of CD4 T cells. Semin. Immunol. 2005, 17, 370–377. [Google Scholar] [CrossRef]
- Moro-Garcia, M.A.; Alonso-Arias, R.; Lopez-Larrea, C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front. Immunol. 2013, 4, 107. [Google Scholar] [CrossRef]
- Haynes, L.; Swain, S.L. Why aging T cells fail: Implications for vaccination. Immunity 2006, 24, 663–666. [Google Scholar] [CrossRef]
- Haynes, L.; Eaton, S.M.; Burns, E.M.; Randall, T.D.; Swain, S.L. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl. Acad. Sci. USA 2003, 100, 15053–15058. [Google Scholar] [CrossRef]
- Prelog, M. Aging of the immune system: A risk factor for autoimmunity? Autoimmun. Rev. 2006, 5, 136–139. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Immune aging and autoimmunity. Cell Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef]
- Bolton, C.; Smith, P.A. The influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE). Ageing Res. Rev. 2018, 41, 64–81. [Google Scholar] [CrossRef]
- Fulop, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Invest. Clin. 2016, 68, 84–91. [Google Scholar]
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin Immunol 2018, 196, 59–63. [Google Scholar] [CrossRef]
- Grebenciucova, E.; Berger, J.R. Immunosenescence: The Role of Aging in the Predisposition to Neuro-Infectious Complications Arising from the Treatment of Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2017, 17, 61. [Google Scholar] [CrossRef]
- Mills, E.A.; Mao-Draayer, Y. Aging and lymphocyte changes by immunomodulatory therapies impact PML risk in multiple sclerosis patients. Mult. Scler. 2018, 24, 1014–1022. [Google Scholar] [CrossRef]
- Foley, J.; Christensen, A.; Hoyt, T.; Foley, A.; Metzger, R. Is Aging and Immunosenescence a Risk Factor for Dimethyl Fumarate Induced PML? (P2. 088); AAN Enterprises: Karnataka, India, 2016. [Google Scholar]
- Elyahu, Y.; Hekselman, I.; Eizenberg-Magar, I.; Berner, O.; Strominger, I.; Schiller, M.; Mittal, K.; Nemirovsky, A.; Eremenko, E.; Vital, A.; et al. Aging promotes reorganization of the CD4 T cell landscape toward extreme regulatory and effector phenotypes. Sci. Adv. 2019, 5, eaaw8330. [Google Scholar] [CrossRef]
- von Delwig, A.; Locke, J.; Robinson, J.H.; Ng, W.F. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum 2010, 62, 143–149. [Google Scholar] [CrossRef]
- Law, S.C.; Street, S.; Yu, C.H.; Capini, C.; Ramnoruth, S.; Nel, H.J.; van Gorp, E.; Hyde, C.; Lau, K.; Pahau, H.; et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res. Ther. 2012, 14, R118. [Google Scholar] [CrossRef]
- Feitsma, A.L.; van der Voort, E.I.; Franken, K.L.; el Bannoudi, H.; Elferink, B.G.; Drijfhout, J.W.; Huizinga, T.W.; de Vries, R.R.; Toes, R.E.; Ioan-Facsinay, A. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheumatol. 2010, 62, 117–125. [Google Scholar] [CrossRef]
- Cianciotti, B.C.; Ruggiero, E.; Campochiaro, C.; Oliveira, G.; Magnani, Z.I.; Baldini, M.; Doglio, M.; Tassara, M.; Manfredi, A.A.; Baldissera, E.; et al. CD4(+) memory stem T cells recognizing citrullinated epitopes are expanded in patients with Rheumatoid Arthritis and sensitive to TNF-alpha blockade. Arthritis Rheumatol. 2019. [Google Scholar] [CrossRef]
- Snir, O.; Backlund, J.; Bostrom, J.; Andersson, I.; Kihlberg, J.; Buckner, J.H.; Klareskog, L.; Holmdahl, R.; Malmstrom, V. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheumatol. 2012, 64, 2482–2488. [Google Scholar] [CrossRef]
- Paroni, M.; Maltese, V.; De Simone, M.; Ranzani, V.; Larghi, P.; Fenoglio, C.; Pietroboni, A.M.; De Riz, M.A.; Crosti, M.C.; Maglie, S.; et al. Recognition of viral and self-antigens by TH1 and TH1/TH17 central memory cells in patients with multiple sclerosis reveals distinct roles in immune surveillance and relapses. J. Allergy Clin. Immunol. 2017, 140, 797–808. [Google Scholar] [CrossRef]
- Venken, K.; Hellings, N.; Hensen, K.; Rummens, J.L.; Stinissen, P. Memory CD4+CD127high T cells from patients with multiple sclerosis produce IL-17 in response to myelin antigens. J. Neuroimmunol. 2010, 226, 185–191. [Google Scholar] [CrossRef]
- Yang, J.; Sundrud, M.S.; Skepner, J.; Yamagata, T. Targeting Th17 cells in autoimmune diseases. Trends Pharm. Sci 2014, 35, 493–500. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Kryczek, I.; Zhao, E.; Liu, Y.; Wang, Y.; Vatan, L.; Szeliga, W.; Moyer, J.; Klimczak, A.; Lange, A.; Zou, W. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 2011, 3, 104ra100. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Chen, Y.; Tato, C.M.; Laurence, A.; Joyce-Shaikh, B.; Blumenschein, W.M.; McClanahan, T.K.; O’Shea, J.J.; Cua, D.J. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009, 10, 314–324. [Google Scholar] [CrossRef]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef]
- Haines, C.J.; Chen, Y.; Blumenschein, W.M.; Jain, R.; Chang, C.; Joyce-Shaikh, B.; Porth, K.; Boniface, K.; Mattson, J.; Basham, B.; et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep 2013, 3, 1378–1388. [Google Scholar] [CrossRef]
- Kleinschek, M.A.; Boniface, K.; Sadekova, S.; Grein, J.; Murphy, E.E.; Turner, S.P.; Raskin, L.; Desai, B.; Faubion, W.A.; de Waal Malefyt, R.; et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009, 206, 525–534. [Google Scholar] [CrossRef]
- Yen, D.; Cheung, J.; Scheerens, H.; Poulet, F.; McClanahan, T.; McKenzie, B.; Kleinschek, M.A.; Owyang, A.; Mattson, J.; Blumenschein, W.; et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006, 116, 1310–1316. [Google Scholar] [CrossRef]
- Wei, L.; Laurence, A.; Elias, K.M.; O’Shea, J.J. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007, 282, 34605–34610. [Google Scholar] [CrossRef]
- Tian, Y.; Zajac, A.J. IL-21 and T Cell Differentiation: Consider the Context. Trends Immunol. 2016, 37, 557–568. [Google Scholar] [CrossRef]
- Kastirr, I.; Maglie, S.; Paroni, M.; Alfen, J.S.; Nizzoli, G.; Sugliano, E.; Crosti, M.C.; Moro, M.; Steckel, B.; Steinfelder, S.; et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J. Immunol. 2014, 193, 3322–3331. [Google Scholar] [CrossRef]
- Fritsch, R.D.; Shen, X.; Illei, G.G.; Yarboro, C.H.; Prussin, C.; Hathcock, K.S.; Hodes, R.J.; Lipsky, P.E. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheumatol. 2006, 54, 2184–2197. [Google Scholar] [CrossRef]
- Mao-Draayer, Y.; Sarazin, J.; Fox, D.; Schiopu, E. The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin. Immunol. 2017, 175, 10–15. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Masopust, D.; Schenkel, J.M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013, 13, 309–320. [Google Scholar] [CrossRef]
- Pham, T.H.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef]
- Pinschewer, D.D.; Brinkmann, V.; Merkler, D. Impact of sphingosine 1-phosphate modulation on immune outcomes. Neurology 2011, 76, S15–s19. [Google Scholar] [CrossRef]
- Mehling, M.; Brinkmann, V.; Antel, J.; Bar-Or, A.; Goebels, N.; Vedrine, C.; Kristofic, C.; Kuhle, J.; Lindberg, R.L.; Kappos, L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008, 71, 1261–1267. [Google Scholar] [CrossRef]
- Mehling, M.; Lindberg, R.; Raulf, F.; Kuhle, J.; Hess, C.; Kappos, L.; Brinkmann, V. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology 2010, 75, 403–410. [Google Scholar] [CrossRef]
- Fujii, C.; Kondo, T.; Ochi, H.; Okada, Y.; Hashi, Y.; Adachi, T.; Shin-Ya, M.; Matsumoto, S.; Takahashi, R.; Nakagawa, M.; et al. Altered T cell phenotypes associated with clinical relapse of multiple sclerosis patients receiving fingolimod therapy. Sci. Rep. 2016, 6, 35314. [Google Scholar] [CrossRef]
- Herich, S.; Schneider-Hohendorf, T.; Rohlmann, A.; Khaleghi Ghadiri, M.; Schulte-Mecklenbeck, A.; Zondler, L.; Janoschka, C.; Ostkamp, P.; Richter, J.; Breuer, J.; et al. Human CCR5high effector memory cells perform CNS parenchymal immune surveillance via GZMK-mediated transendothelial diapedesis. Brain 2019, 142, 3411–3427. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Surh, C.D.; Boyman, O.; Purton, J.F.; Sprent, J. Homeostasis of memory T cells. Immunol. Rev. 2006, 211, 154–163. [Google Scholar] [CrossRef]
- Ribas, A.; Shin, D.S.; Zaretsky, J.; Frederiksen, J.; Cornish, A.; Avramis, E.; Seja, E.; Kivork, C.; Siebert, J.; Kaplan-Lefko, P.; et al. PD-1 Blockade Expands Intratumoral Memory T Cells. Cancer Immunol. Res. 2016, 4, 194–203. [Google Scholar] [CrossRef]
- De Sousa Linhares, A.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front Immunol. 2018, 9, 1909. [Google Scholar] [CrossRef]
- Workman, C.J.; Cauley, L.S.; Kim, I.J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004, 172, 5450–5455. [Google Scholar] [CrossRef]
- Avery, L.; Filderman, J.; Szymczak-Workman, A.L.; Kane, L.P. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc. Natl. Acad. Sci. USA 2018, 115, 2455–2460. [Google Scholar] [CrossRef]
- Metz, D.P.; Farber, D.L.; Taylor, T.; Bottomly, K. Differential role of CTLA-4 in regulation of resting memory versus naive CD4 T cell activation. J. Immunol. 1998, 161, 5855–5861. [Google Scholar]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Kim, T.S.; Hufford, M.M.; Sun, J.; Fu, Y.X.; Braciale, T.J. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J. Exp. Med. 2010, 207, 1161–1172. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Matarese, G.; De Rosa, V.; La Cava, A. Regulatory CD4 T cells: Sensing the environment. Trends Immunol. 2008, 29, 12–17. [Google Scholar] [CrossRef]
- Fehervari, Z.; Sakaguchi, S. CD4+ Tregs and immune control. J Clin Invest 2004, 114, 1209–1217. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Li, J.; Huston, G.; Swain, S.L. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003, 198, 1807–1815. [Google Scholar] [CrossRef]
- Chetoui, N.; Boisvert, M.; Gendron, S.; Aoudjit, F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology 2010, 130, 418–426. [Google Scholar] [CrossRef]
- Bradley, L.M.; Haynes, L.; Swain, S.L. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005, 26, 172–176. [Google Scholar] [CrossRef]
- Purton, J.F.; Tan, J.T.; Rubinstein, M.P.; Kim, D.M.; Sprent, J.; Surh, C.D. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 2007, 204, 951–961. [Google Scholar] [CrossRef]
- Picker, L.J.; Reed-Inderbitzin, E.F.; Hagen, S.I.; Edgar, J.B.; Hansen, S.G.; Legasse, A.; Planer, S.; Piatak, M., Jr.; Lifson, J.D.; Maino, V.C.; et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 2006, 116, 1514–1524. [Google Scholar] [CrossRef]
- Smolders, J.; Heutinck, K.M.; Fransen, N.L.; Remmerswaal, E.B.M.; Hombrink, P.; Ten Berge, I.J.M.; van Lier, R.A.W.; Huitinga, I.; Hamann, J. Tissue-resident memory T cells populate the human brain. Nat. Commun. 2018, 9, 4593. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Troscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082. [Google Scholar] [CrossRef]
- Prasad, S.; Hu, S.; Sheng, W.S.; Chauhan, P.; Lokensgard, J.R. Recall Responses from Brain-Resident Memory CD8(+) T Cells (bTRM) Induce Reactive Gliosis. iScience 2019, 20, 512–526. [Google Scholar] [CrossRef]
- Scholler, A.S.; Fonnes, M.; Nazerai, L.; Christensen, J.P.; Thomsen, A.R. Local Antigen Encounter Is Essential for Establishing Persistent CD8(+) T-Cell Memory in the CNS. Front. Immunol. 2019, 10, 351. [Google Scholar] [CrossRef]
- Sabatino, J.J., Jr.; Wilson, M.R.; Calabresi, P.A.; Hauser, S.L.; Schneck, J.P.; Zamvil, S.S. Anti-CD20 therapy depletes activated myelin-specific CD8(+) T cells in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).