A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Proteins and Antibodies
2.3. Affinity Chromatography, Immunoadsorption, and Immunoblotting
2.4. Cytotoxicity Assays
2.5. Mass Spectrometry
2.6. Peptides
2.7. Confocal Microscopy
2.8. Mice
2.9. Histological Studies
2.10. Statistical Analysis
3. Results
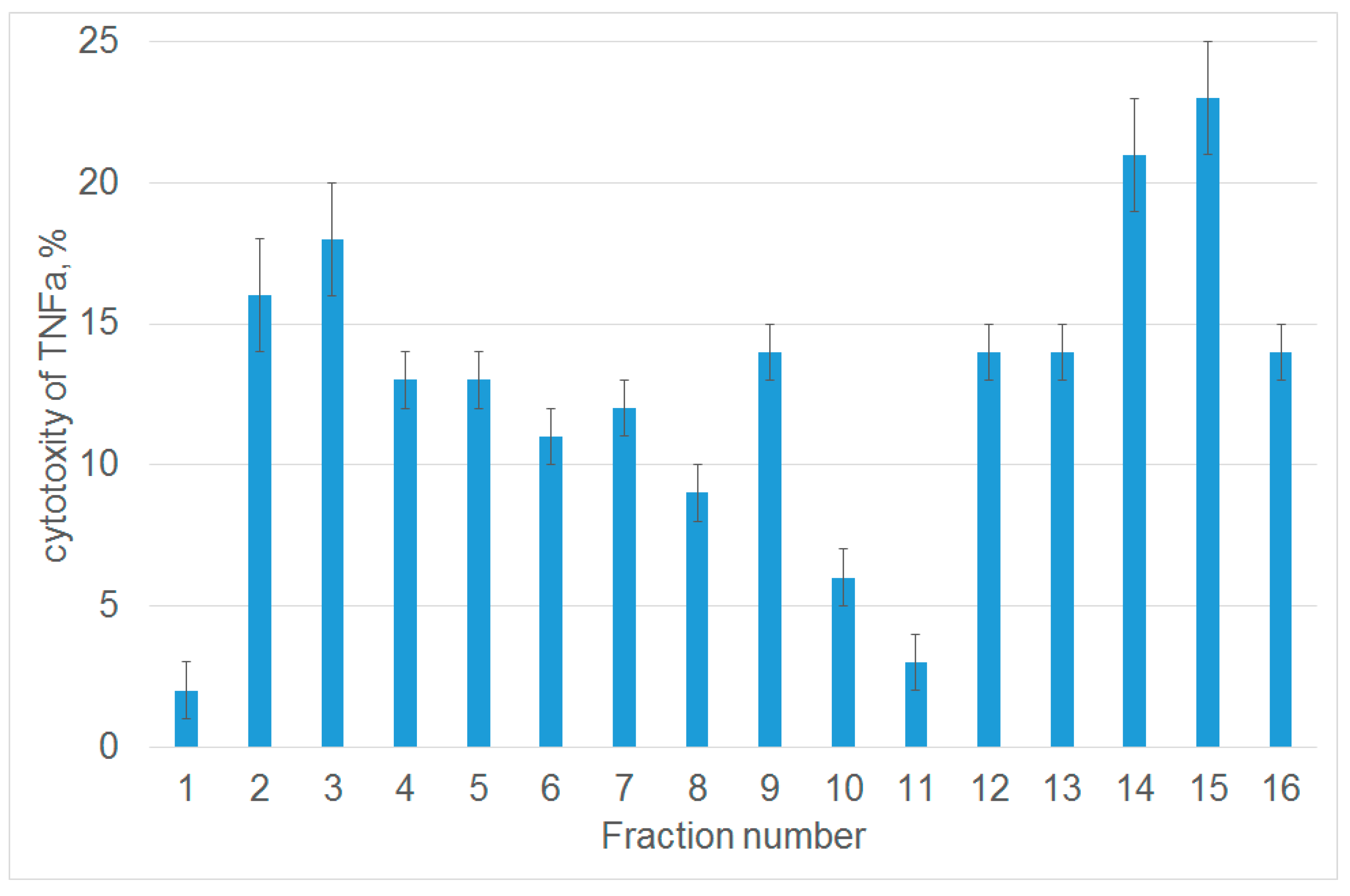
3.1. Tryptic Peptide Derived from Tag7 Protein Inhibits the Cytotoxic Activity of TNFα
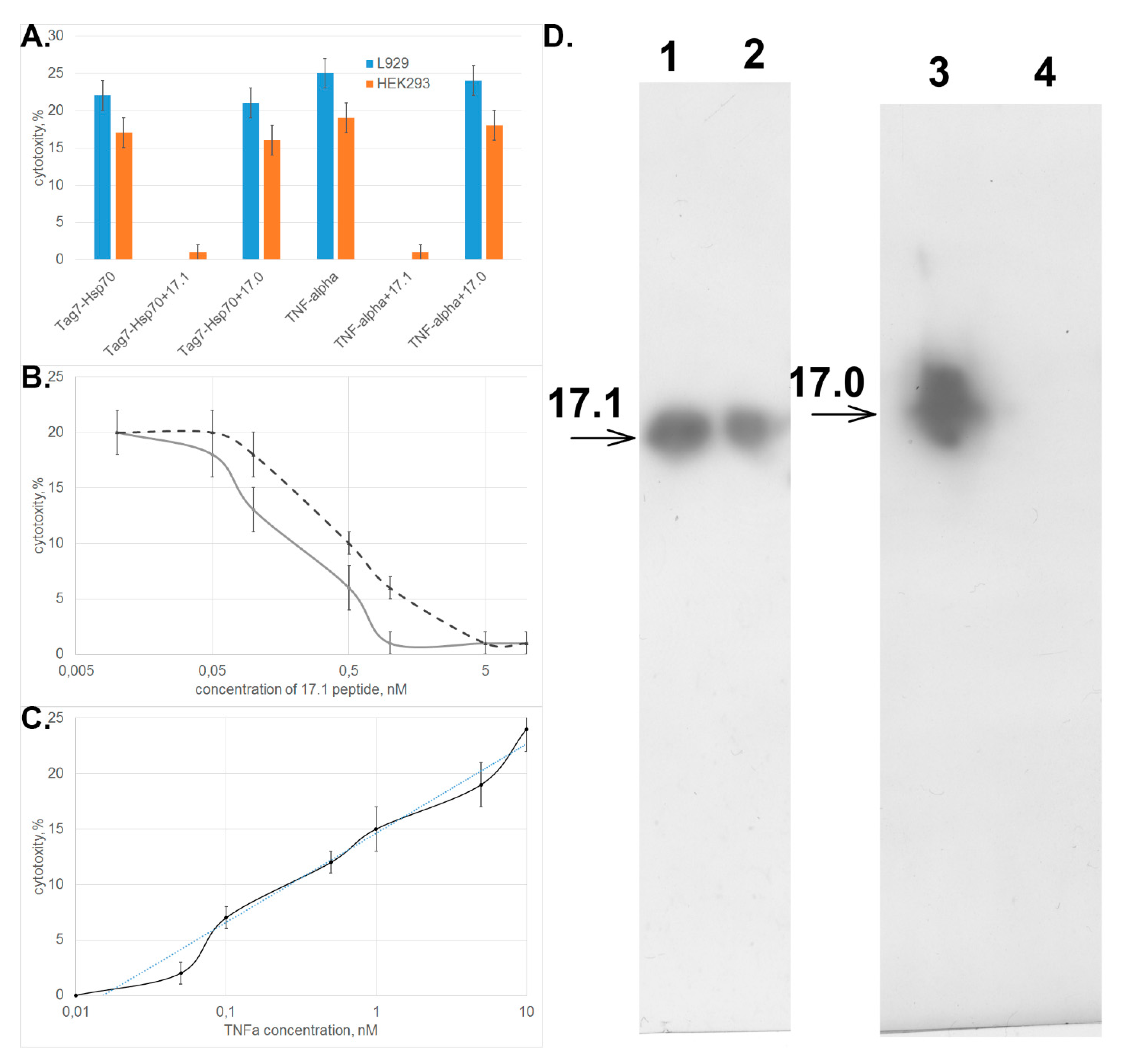
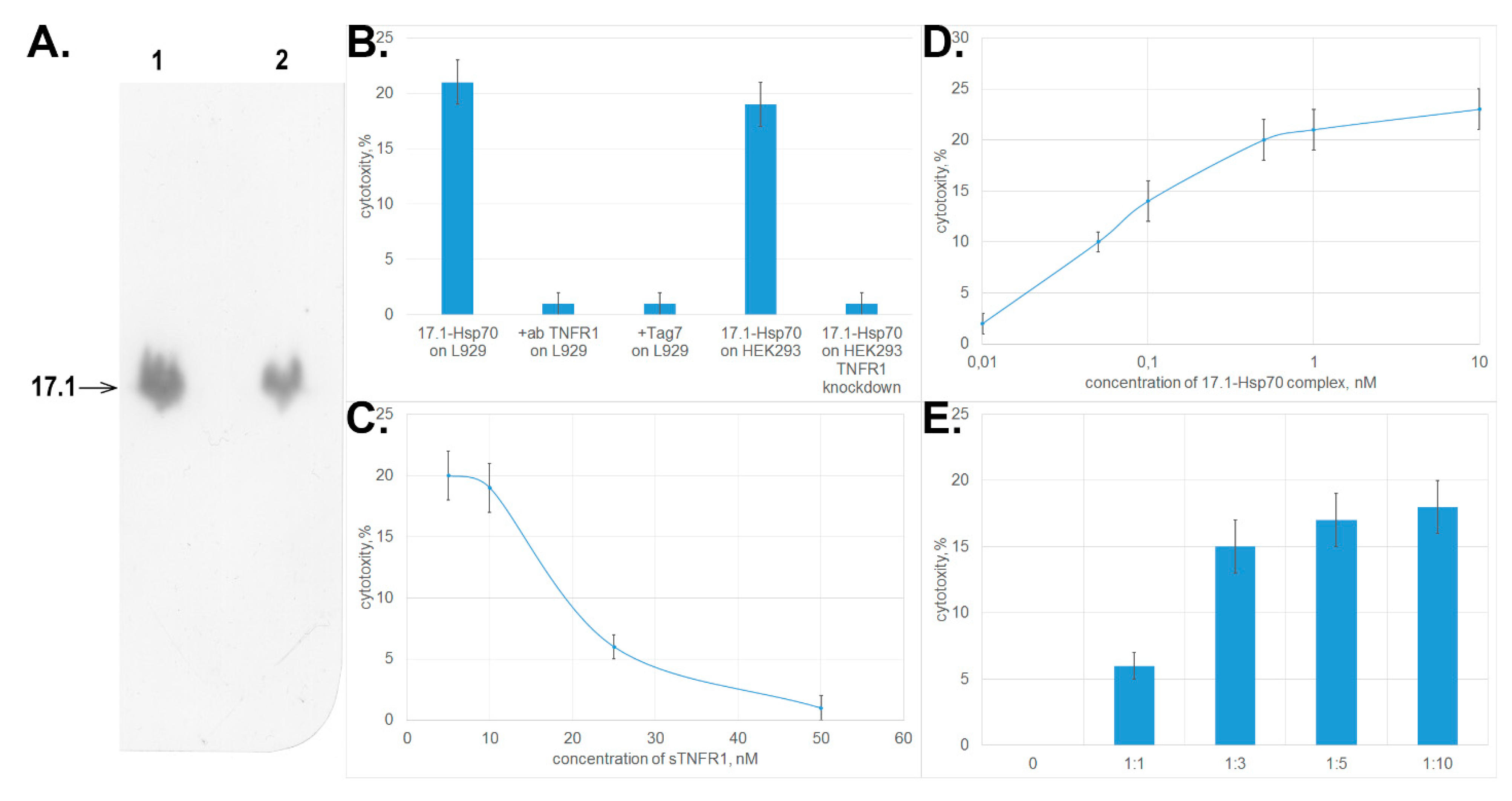
3.2. Peptide 17.1 Binds to TNFRI Receptor and Inhibits Cytotoxic Activity of TNFα and the Tag7–Hsp70 Complex
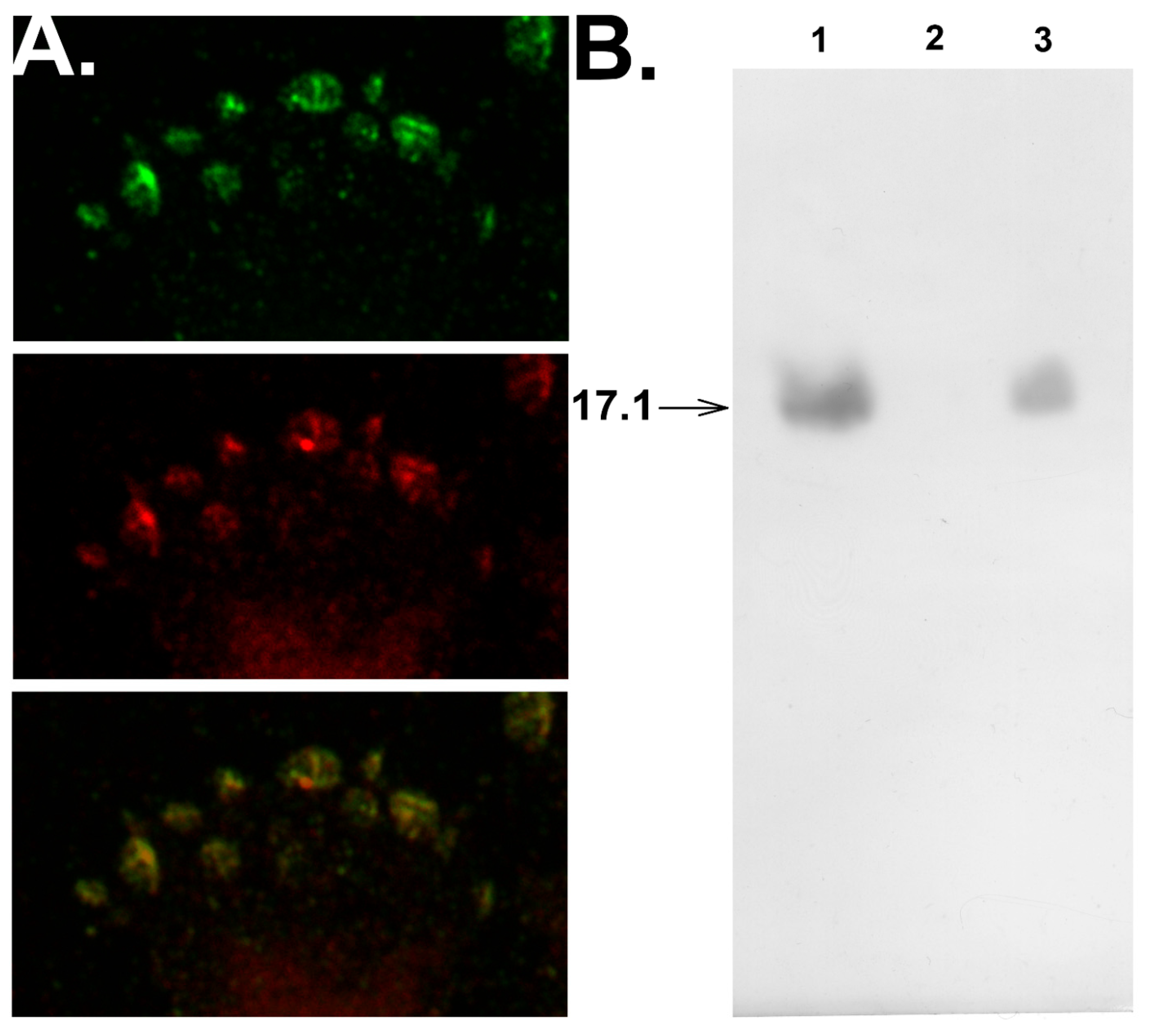
3.3. Peptide 17.1 Forms a Cytotoxic Complex 17.1–Hsp70
3.4. Peptide 17.1 Exhibits an Anti-Inflammatory Activity in the Model of CFA-Induced Arthritis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Scheringa, M.; Marquet, R.L. TNF: A brief review with emphasis on its antitumor activity. Biotherapy 1990, 2, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cahalon, L.; Hershkoviz, R.; Gilat, D.; Miller, A.; Akiyama, S.K.; Yamada, K.M.; Lider, O. Functional interactions of fibronectin and TNF alpha: A paradigm of physiological linkage between cytokines and extracellular matrix moieties. Cell Adhes. Commun. 1994, 2, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Meylan, F.; Siegel, R.M. TNF superfamily cytokines in the promotion of Th9 differentiation and immunopathology. Semin. Immunopathol. 2017, 39, 21–28. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.A.; Ting, A.T. Chronicles of a death foretold: Dual sequential cell death checkpoints in TNF signaling. Cell Cycle 2010, 9, 1065–1071. [Google Scholar] [CrossRef]
- Chan, K.F.; Siegel, M.R.; Lenardo, J.M. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 2000, 13, 419–422. [Google Scholar] [CrossRef]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- McDermott, M.F. TNF and TNFR biology in health and disease. Cell. Mol. Biol. 2001, 47, 619–635. [Google Scholar]
- Mackay, F.; Kalled, S.L. TNF ligands and receptors in autoimmunity: An update. Curr. Opin. Immunol. 2002, 14, 783–790. [Google Scholar] [CrossRef]
- Standiford, T.J.; Strieter, R.M. TNF and IL-1 in sepsis: Good cytokines gone bad. J. Lab. Clin. Med. 1992, 120, 179–180. [Google Scholar] [PubMed]
- Yi, F.; Frazzette, N.; Cruz, A.C.; Klebanoff, C.A.; Siegel, R.M. Beyond Cell Death: New Functions for TNF Family Cytokines in Autoimmunity and Tumor Immunotherapy. Trends Mol. Med. 2018, 24, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, S.; Xian, P.; Yang, L.; Chen, Y.; Mo, X. Effect of Anti-TNF Antibodies on Clinical Response in Rheumatoid Arthritis Patients: A Meta-Analysis. Biomed. Res. Int. 2016, 7185708. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Yashin, D.V.; Ivanova, O.K.; Soshnikova, N.V.; Sheludchenkov, A.A.; Romanova, E.A.; Dukhanina, E.A.; Tonevitsky, A.G.; Gnuchev, N.V.; Gabibov, A.G.; Georgiev, G.P.; et al. Tag7 (PGLYRP1) in Complex with Hsp70 Induces Alternative Cytotoxic Processes in Tumor Cells via TNFR1 Receptor. J. Biol. Chem. 2015, 290, 21724–21731. [Google Scholar] [CrossRef]
- Kustikova, O.S.; Kiselev, S.L.; Borodulina, O.R.; Senin, V.M.; Afanas’eva, A.V.; Kabishev, A.A. Cloning of the tag7 gene expressed in metastatic mouse tumors. Genetika 1996, 32, 621–628. [Google Scholar]
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef]
- Dziarski, R. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 2004, 40, 877–886. [Google Scholar] [CrossRef]
- Liu, C.; Gelius, E.; Liu, G.; Steiner, H.; Dziarski, R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 2000, 275, 24490–24499. [Google Scholar] [CrossRef]
- Dukhanina, E.A.; Lukyanova, T.I.; Romanova, E.A.; Guerriero, V.; Gnuchev, N.V.; Georgiev, G.P.; Yashin, D.V.; Sashchenko, L.P. A new role for PGRP-S (Tag7) in immune defense: Lymphocyte migration is induced by a chemoattractant complex of Tag7 with Mts1. Cell Cycle. 2015, 14, 3635–3643. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Ivanova, O.K.; Soshnikova, N.V.; Romanova, E.A.; Sashchenko, L.P.; Yashin, D.V. Innate Immunity Protein Tag7 Induces 3 Distinct Populations of Cytotoxic Cells That Use Different Mechanisms to Exhibit Their Antitumor Activity on Human Leukocyte Antigen-Deficient Cancer Cells. J. Innate Immun. 2017, 9, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, Q.; Sundberg, E.J.; Mariuzza, R.A. Crystal structure of human peptidoglycan recognition protein S (PGRP-S) at 1.70 A resolution. J. Mol. Biol. 2005, 347, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sashchenko, L.P.; Dukhanina, E.A.; Yashin, D.V.; Shatalov, Y.V.; Romanova, E.A.; Korobko, E.V.; Demin, A.V.; Lukyanova, T.I.; Kabanova, O.D.; Khaidukov, S.V.; et al. Peptidoglycan recognition protein tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J. Biol. Chem. 2004, 279, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Andreev-Andrievskiy, A.A.; Kolosova, N.G.; Stefanova, N.A.; Lovat, M.V.; Egorov, M.V.; Manskikh, V.N.; Zinovkin, R.A.; Galkin, I.I.; Prikhodko, A.S.; Skulachev, M.V.; et al. Efficacy of Mitochondrial Antioxidant Plastoquinonyl-decyl-triphenylphosphonium Bromide (SkQ1) in the Rat Model of Autoimmune Arthritis. Oxid. Med. Cell. Longev. 2016, 2016, 8703645. [Google Scholar] [CrossRef]
- Yashin, D.V.; Romanova, E.A.; Ivanova, O.K.; Sashchenko, L.P. The Tag7-Hsp70 cytotoxic complex induces tumor cell necroptosis via permeabilisation of lysosomes and mitochondria. Biochimie 2016, 123, 32–36. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.S.; Huh, S.H.; Seo, J.S.; Choi, E.J. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001, 20, 446–456. [Google Scholar] [CrossRef]
- Ravagnan, L.; Gurbuxani, S.; Susin, S.A.; Maisse, C.; Daugas, E.; Zamzami, N.; Mak, T.; Jäättelä, M.; Penninger, J.M.; Garrido, C.; et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001, 3, 839–843. [Google Scholar] [CrossRef]
- Gross, C.; Koelch, W.; DeMaio, A.; Arispe, N.; Multhoff, G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J. Biol. Chem. 2003, 278, 41173–41181. [Google Scholar] [CrossRef]
- Komarova, E.Y.; Afanasyeva, E.A.; Bulatova, M.M.; Cheetham, M.E.; Margulis, B.A.; Guzhova, I.V. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones 2004, 9, 265–275. [Google Scholar] [CrossRef]
- Dressel, R.; Grzeszik, C.; Kreiss, M.; Lindemann, D.; Herrmann, T.; Walter, L.; Günther, E. Differential effect of acute and permanent heat shock protein 70 overexpression in tumor cells on lysability by cytotoxic T lymphocytes. Cancer Res. 2003, 63, 8212–8220. [Google Scholar]
- Sashchenko, L.P.; Dukhanina, E.A.; Shatalov, Y.V.; Yashin, D.V.; Lukyanova, T.I.; Kabanova, O.D.; Romanova, E.A.; Khaidukov, S.V.; Galkin, A.V.; Gnuchev, N.V.; et al. Cytotoxic T lymphocytes carrying a pattern recognition protein Tag7 can detect evasive, HLA-negative but Hsp70-exposing tumor cells, thereby ensuring FasL/Fas-mediated contact killing. Blood 2007, 110, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Guzhova, I.V.; Shevtsov, M.A.; Abkin, S.V.; Pankratova, K.M.; Margulis, B.A. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int. J. Hyperthermia 2013, 29, 399–408. [Google Scholar] [CrossRef] [PubMed]
| Number of Days after Inflammation Was Induced | Study Groups | Periarticular Inflammation (Intensity of Infiltration of White Blood Cells into SoftTissues Surrounding the Joint, Score) | Synovitis (Infiltration of WBCs into the Synovial Membrane, Score) | Synovial Hyperplasia (Score) | Articular Cartilage Damage (Score) | Destruction of Bone Tissue (Score) |
|---|---|---|---|---|---|---|
| 2–4 days | NS + NS | 0 | 0 | 0 | 0.25 | 0 |
| CFA + NS | 2 | 0.67 | 0 | 0.33 | 0.33 | |
| CFA + peptide | 2.56 | 0.89 | 0.33 | 0.78 | 0.11 | |
| CFA + Norocarp | 2.25 | 1.75 | 1 | 1.00 | 0.25 | |
| 6–10 days | NS + NS | 0 | 0 | 0 | 0.33 | 0 |
| CFA + NS | 3 | 1.86 | 1.25 | 0.75 | 0.75 | |
| CFA + peptide | 2.33 | 1 | 0.67 | 0.50 | 1.33 | |
| CFA + Norocarp | 2.5 | 1.29 | 0.25 | 0.75 | 1.25 | |
| 21 days | NS + NS | 0 | 0 | 0 | 0.25 | 0 |
| CFA + NS | 2.75 | 0.5 | 0.5 | 1 | 2 | |
| CFA + peptide | 2.75 | 0.75 | 0.5 | 0.5 | 0.75 | |
| CFA + Norocarp | 2.75 | 0 | 0 | 0.75 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, E.A.; Sharapova, T.N.; Telegin, G.B.; Minakov, A.N.; Chernov, A.S.; Ivanova, O.K.; Bychkov, M.L.; Sashchenko, L.P.; Yashin, D.V. A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death. Cells 2020, 9, 488. https://doi.org/10.3390/cells9020488
Romanova EA, Sharapova TN, Telegin GB, Minakov AN, Chernov AS, Ivanova OK, Bychkov ML, Sashchenko LP, Yashin DV. A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death. Cells. 2020; 9(2):488. https://doi.org/10.3390/cells9020488
Chicago/Turabian StyleRomanova, Elena A., Tatiana N. Sharapova, Georgii B. Telegin, Alexei N. Minakov, Alexander S. Chernov, Olga K. Ivanova, Maxim L. Bychkov, Lidia P. Sashchenko, and Denis V. Yashin. 2020. "A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death" Cells 9, no. 2: 488. https://doi.org/10.3390/cells9020488
APA StyleRomanova, E. A., Sharapova, T. N., Telegin, G. B., Minakov, A. N., Chernov, A. S., Ivanova, O. K., Bychkov, M. L., Sashchenko, L. P., & Yashin, D. V. (2020). A 12-mer Peptide of Tag7 (PGLYRP1) Forms a Cytotoxic Complex with Hsp70 and Inhibits TNF-Alpha Induced Cell Death. Cells, 9(2), 488. https://doi.org/10.3390/cells9020488





