The Potential of IgG to Induce Murine and Human Thymic Maturation of IL-10+ B Cells (B10) Revealed in a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Patient Samples
2.3. SPT, Serum Antiallergen IgE Determination, and Collection of Blood Samples
2.4. Determination of Murine and Human Total IgG Subclasses
2.5. Murine Serum Total IgE Levels and Anaphylactic Anti-OVA IgE Titers
2.6. Murine Immunization
2.7. Passive In Vivo Transfer of Purified IgG
2.8. Passive in vivo Transfer of Thymocytes
2.9. Murine Lung Inflammation
2.10. Purification of Mouse and Human IgG
2.11. Spleen, Thymus, Bone Marrow and Lung Cell Suspensions
2.12. Murine Cell Culture
2.13. Murine Flow Cytometry
2.14. Separation of Human Thymocyte Suspensions
2.15. Human Cell Culture
2.16. Human Flow Cytometry
2.17. Statistical Analysis
3. Results
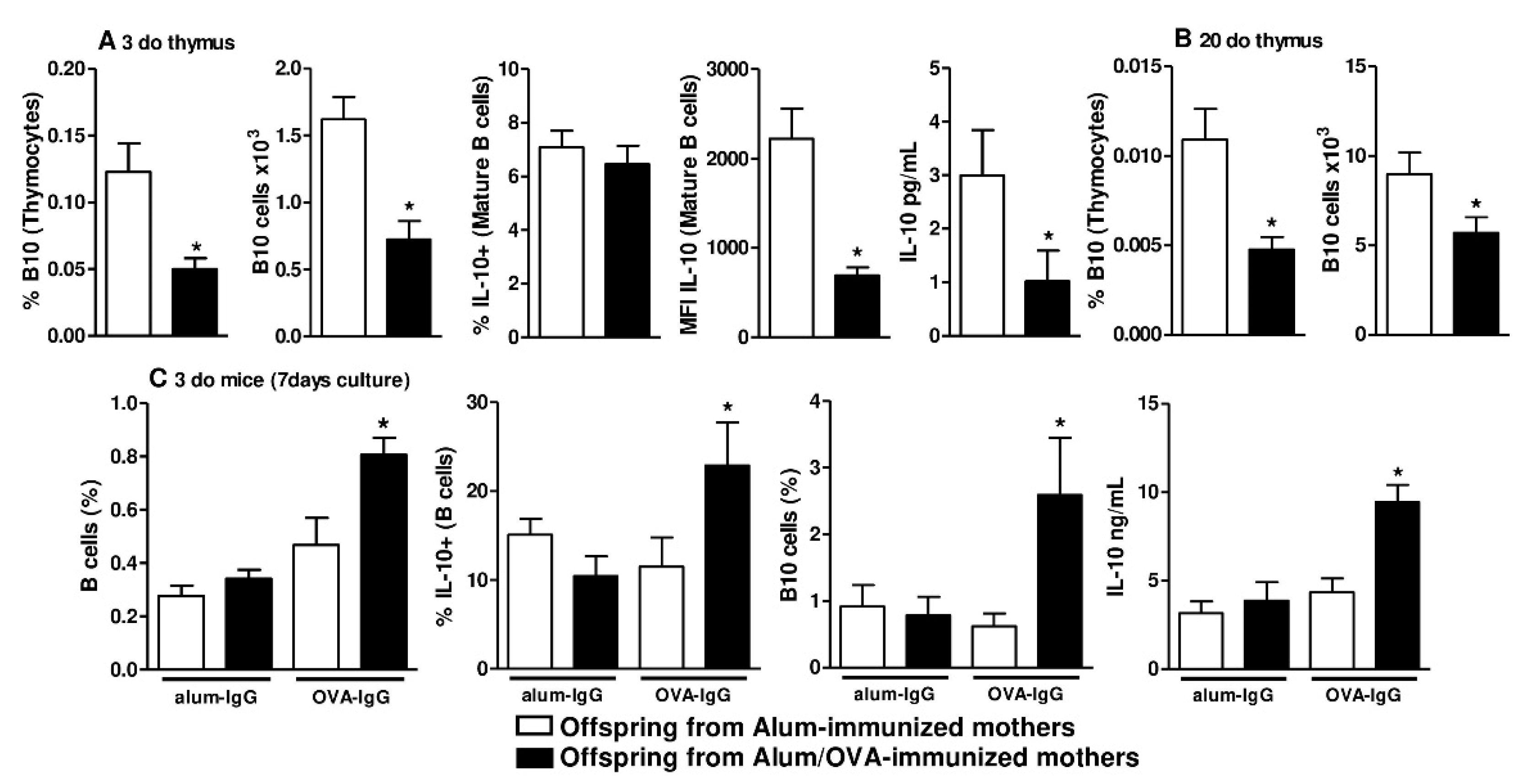
3.1. Identification of B10 Cells in the Thymus and Bone Marrow
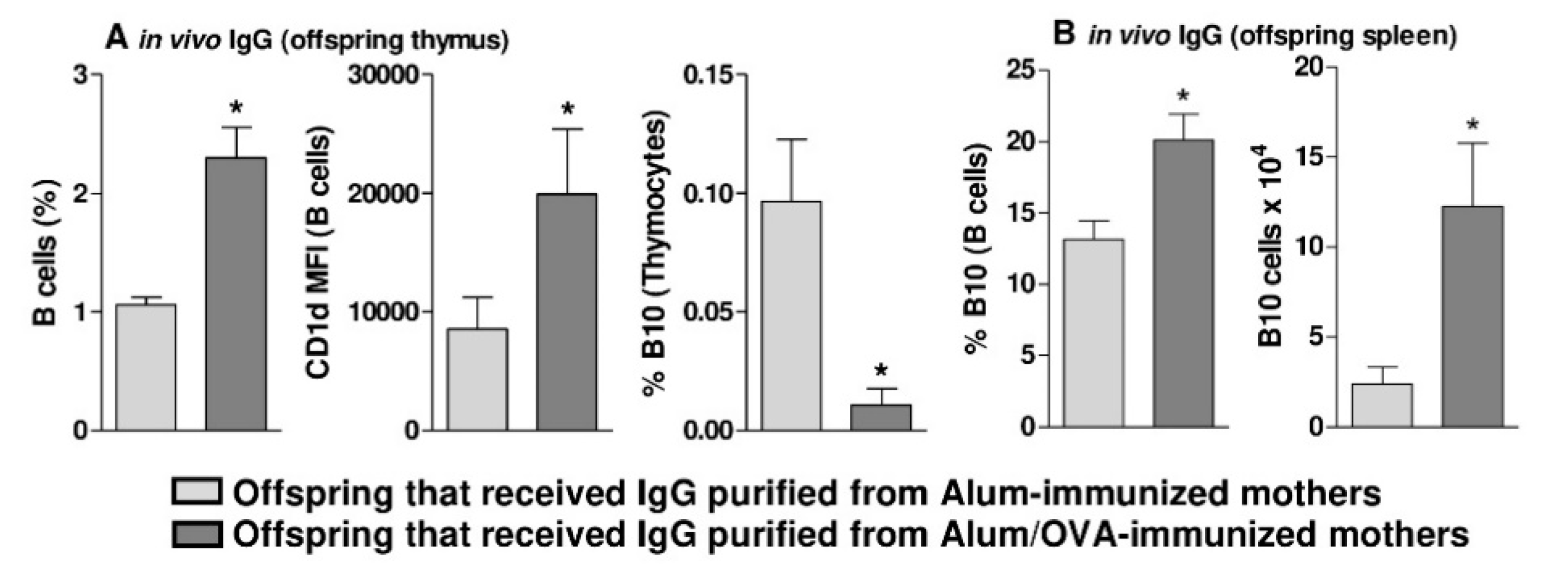
3.2. Functional and In Vivo Evidence of Thymic B10 Cells
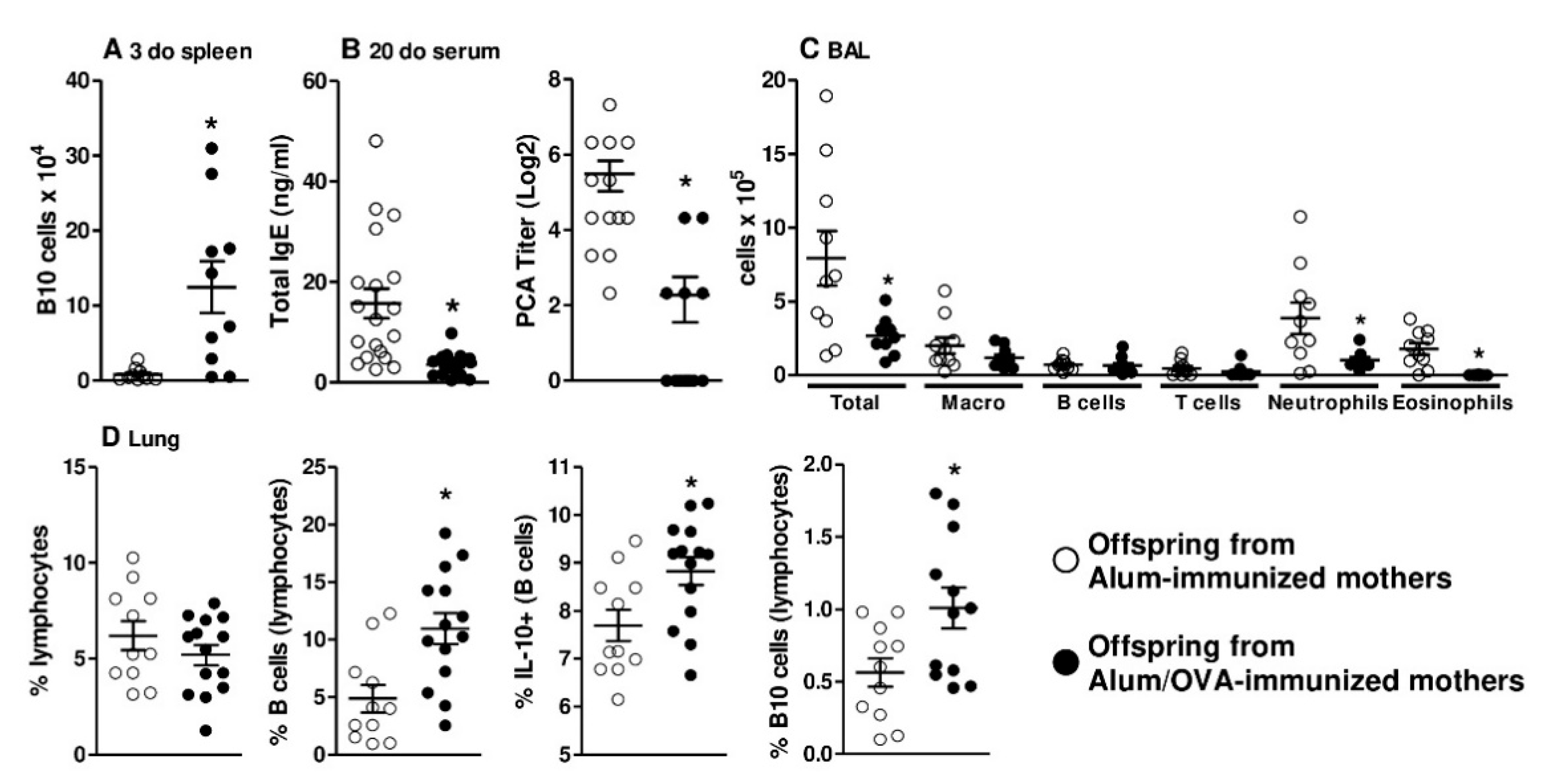
3.3. In Vivo Evidence that Thymic B10 Cells Can Migrate to the Spleen And Inflammatory Sites
3.4. Human Nonatopic Infant Thymic and Adult Peripheral B Cells Can be Modulated to Produce IL-10 by Treatment with Purified IgG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hubeau, C.; Apostolou, I.; Kobzik, L. Adoptively transferred allergen-specific T cells cause maternal transmission of asthma risk. Am. J. Pathol. 2006, 168, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Matson, A.P.; Zhu, L.; Lingenheld, E.G.; Schramm, C.M.; Clark, R.B.; Selander, D.M.; Thrall, R.S.; Breen, E.; Puddington, L. Maternal transmission of resistance to development of allergic airway disease. J. Immunol. 2007, 179, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Verhasselt, V.; Milcent, V.; Cazareth, J.; Kanda, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008, 14, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ellertsen, L.K.; Nygaard, U.C.; Melkild, I.; Løvik, M. Maternal allergen immunisation to prevent sensitisation in offspring: Th2-polarising adjuvants are more efficient than a Th1-polarising adjuvant in mice. BMC Immunol. 2010, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Sgnotto, F.D.R.; Oliveira, M.G.; Lira, A.A.L.; Bento-de-Souza, L.; Duarte, A.J.D.S.; Victor, J.R. Low doses of IgG from atopic individuals can modulate in vitro IFN-γ production by human intra-thymic TCD4 and TCD8 cells: An IVIg comparative approach. Hum. Vaccines Immunother. 2017, 13, 1563–1572. [Google Scholar] [CrossRef]
- Victor, J.R. Influence of maternal immunization with allergens on the thymic maturation of lymphocytes with regulatory potential in children: A broad field for further exploration. J. Immunol. Res. 2014, 2014, 780386. [Google Scholar] [CrossRef]
- Lira, A.A.L.; de-Oliveira, M.G.; Inoue, A.H.S.; Beltrame, G.R.; Duarte, A.J.D.S.; Victor, J.R. Preconceptional allergen immunization can induce offspring IL-17 secreting B cells (B17): Do they share similarities with regulatory B10 cells? Allergol. Immunopathol. 2018, 46, 454–459. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Oliveira, L.M.; Lira, A.A.L.; Sgnotto, F.D.R.; Duarte, A.J.D.S.; Sato, M.N.; Victor, J.R. Preconception allergen sensitization can induce B10 cells in offspring: A potential main role for maternal IgG. Allergy Asthma Clin. Immunol. 2017, 13, 22. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Amu, S.; Saunders, S.P.; Kronenberg, M.; Mangan, N.E.; Atzberger, A.; Fallon, P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 2010, 125, 1114–1124.e1118. [Google Scholar] [CrossRef]
- Jin, G.; Hamaguchi, Y.; Matsushita, T.; Hasegawa, M.; Le Huu, D.; Ishiura, N.; Naka, K.; Hirao, A.; Takehara, K.; Fujimoto, M. B-cell linker protein expression contributes to controlling allergic and autoimmune diseases by mediating IL-10 production in regulatory B cells. J. Allergy Clin. Immunol. 2013, 131, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.A.; Vasconcellos, R.; Rosser, E.C.; Tulone, C.; Muñoz-Suano, A.; Kamanaka, M.; Ehrenstein, M.R.; Flavell, R.A.; Mauri, C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J. Immunol. 2011, 186, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Yeung, M.; Camirand, G.; Zeng, Q.; Akiba, H.; Yagita, H.; Chalasani, G.; Sayegh, M.H.; Najafian, N.; Rothstein, D.M. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Investig. 2011, 121, 3645–3656. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Tedder, T.F.; Sato, S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am. J. Pathol. 2011, 178, 735–743. [Google Scholar] [CrossRef]
- Carter, N.A.; Rosser, E.C.; Mauri, C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res. Ther. 2012, 14, R32. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Liu, Y.; Li, Y.; Sun, L.; Jiao, L.; Jiao, W.; Xiao, J.; Qi, H.; Xu, F.; et al. Impaired Function of CD5+CD19+CD1dhi B10 Cells on IgE Secretion in an Atopic Dermatitis-Like Mouse Model. PLoS ONE 2015, 10, e0132173. [Google Scholar] [CrossRef]
- Padberg, W.M.; Lord, R.H.; Kupiec-Weglinski, J.W.; Williams, J.M.; Di Stefano, R.; Thornburg, L.E.; Araneda, D.; Strom, T.B.; Tilney, N.L. Two phenotypically distinct populations of T cells have suppressor capabilities simultaneously in the maintenance phase of immunologic enhancement. J. Immunol. 1987, 139, 1751–1757. [Google Scholar]
- Daien, C.I.; Gailhac, S.; Mura, T.; Audo, R.; Combe, B.; Hahne, M.; Morel, J. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014, 66, 2037–2046. [Google Scholar] [CrossRef]
- Gao, N.; Dresel, J.; Eckstein, V.; Gellert, R.; Störch, H.; Venigalla, R.K.; Schwenger, V.; Max, R.; Blank, N.; Lorenz, H.M.; et al. Impaired suppressive capacity of activation-induced regulatory B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 2849–2861. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Xu, R.C.; Chen, Y.Y.; Yuan, H.J.; Cao, H.; Zhao, X.Q.; Zheng, J.; Wang, Y.; Pan, M. Impaired function of CD19(+) CD24(hi) CD38(hi) regulatory B cells in patients with pemphigus. Br. J. Derm. 2015, 172, 101–110. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Liaskos, C.; Simopoulou, T.; Bogdanos, D.P.; Sakkas, L.I. IL-10-producing regulatory B cells (B10 cells), IL-17+ T cells and autoantibodies in systemic sclerosis. Clin. Immunol. 2017, 184, 26–32. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Varna, A.; Zafiriou, E.; Liaskos, C.; Alexiou, I.; Roussaki-Schulze, A.; Vlychou, M.; Katsiari, C.; Bogdanos, D.P.; Sakkas, L.I. IL-10 producing Bregs are impaired in psoriatic arthritis and psoriasis and inversely correlate with IL-17- and IFNγ-producing T cells. Clin. Immunol. 2017, 184, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Richie, L.I.; Miyamoto, T.; Carr, W.H.; Weissman, I.L. B lymphopoiesis in the thymus. J. Immunol. 2000, 164, 5221–5226. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Ma, N.; Xiao, H.; Wang, X.; Zheng, M.; Han, G.; Chen, G.; Hou, C.; Shen, B.; Li, Y.; et al. Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. J. Leukoc. Biol. 2015, 97, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test - European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; de Lima Lira, A.A.; da Ressureição Sgnotto, F.; Inoue, A.H.S.; Santos, L.S.; Nakamatsu, B.Y.; Duarte, A.J.D.S.; Leite-de-Moraes, M.; Victor, J.R. Maternal IgG impairs the maturation of offspring intrathymic IL-17-producing γδT cells: Implications for murine and human allergies. Clin. Exp. Allergy 2019. [Google Scholar] [CrossRef] [PubMed]
- de Lima Lira, A.A.; de Oliveira, M.G.; de Oliveira, L.M.; da Silva Duarte, A.J.; Sato, M.N.; Victor, J.R. Maternal immunization with ovalbumin or Dermatophagoides pteronyssinus has opposing effects on Fc gamma RIIb expression on offspring B cells. Allergy Asthma Clin. Immunol. 2014, 10. [Google Scholar] [CrossRef]
- Victor, J.R.; Muniz, B.P.; Fusaro, A.E.; de Brito, C.A.; Taniguchi, E.F.; Duarte, A.J.S.; Sato, M.N. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol. 2010, 11. [Google Scholar] [CrossRef]
- Freer, G.; Rindi, L. Intracellular cytokine detection by fluorescence-activated flow cytometry: Basic principles and recent advances. Methods 2013, 61, 30–38. [Google Scholar] [CrossRef]
- van Rijt, L.S.; Kuipers, H.; Vos, N.; Hijdra, D.; Hoogsteden, H.C.; Lambrecht, B.N. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J. Immunol. Methods 2004, 288, 111–121. [Google Scholar] [CrossRef]
- Sgnotto, F.D.R.; de Oliveira, M.G.; Lira, A.A.L.; Inoue, A.H.S.; Titz, T.O.; Orfali, R.L.; Bento-de-Souza, L.; Sato, M.N.; Aoki, V.; Duarte, A.J.S.; et al. IgG from atopic dermatitis patients induces IL-17 and IL-10 production in infant intrathymic TCD4 and TCD8 cells. Int. J. Derm. 2018. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Taniguchi, E.; Fusaro, A.; Victor, J.; Brito, C.; Duarte, A.; Sato, M. Bystander effect in synergy to anergy in oral tolerance of Blomia tropicalis/ovalbumin murine co-immunization model. J. Clin. Immunol. 2005, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Muniz, B.P.; Victor, J.R.; Oliveira, L.d.M.; de Lima Lira, A.A.; Perini, A.; Olivo, C.R.; Arantes-Costa, F.M.; Martins, M.A.; da Silva Duarte, A.J.; Sato, M.N. Tolerogenic microenvironment in neonatal period induced by maternal immunization with ovalbumin. Immunobiology 2014, 219, 377–384. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.; Lira, A.A.L.; Sgnotto, F.D.R.; Inoue, A.H.S.; Beltrame, G.R.; da Silva, D.; Menghini, R.P.; Duarte, A.J.D.S.; Victor, J.R. Maternal immunization downregulates offspring TCD4 regulatory cells (Tregs) thymic maturation without implications for allergy inhibition. Scand. J. Immunol. 2018, e12721. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.; Lira, A.A.L.; Sgnotto, F.D.R.; Inoue, A.H.S.; Duarte, A.J.D.S.; Victor, J.R. Preconception immunization can modulate intracellular Th2 cytokine profile in offspring. Cent. Eur. J. Immunol. 2018, 43, 378–388. [Google Scholar] [CrossRef]
- Futata, E.; de Brito, C.; Victor, J.; Fusaro, A.; Oliveira, C.; Maciel, M.; Duarte, A.; Sato, M. Long-term anergy in orally tolerized mice is linked to decreased B7.2 expression on B cells. Immunobiology 2006, 211, 157–166. [Google Scholar] [CrossRef]
- Sato, M.; Fusaro, A.; Oliveira, V.; Futata, E.; Maciel, M.; Carvalho, A.; Duarte, A. Oral tolerance induction in Dermatophagoides pteronyssinus - Sensitized mice induces inhibition of IgE response and upregulation of TGF-beta secretion. J. Interferon Cytokine Res. 2001, 21, 827–833. [Google Scholar] [CrossRef]
- Kabuto, M.; Fujimoto, N.; Tanaka, T. Increase of interleukin-10-producing B cells associated with long-term remission after i.v. immunoglobulin treatment for pemphigus. J. Derm. 2016. [Google Scholar] [CrossRef]
- Noh, G.; Lee, J.H. Regulatory B cells and allergic diseases. Allergy Asthma Immunol. Res. 2011, 3, 168–177. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Matsushita, T.; Tedder, T.F. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010, 1183, 38–57. [Google Scholar] [CrossRef]
- Morlacchi, S.; Soldani, C.; Viola, A.; Sarukhan, A. Self-antigen presentation by mouse B cells results in regulatory T-cell induction rather than anergy or clonal deletion. Blood 2011, 118, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.N.; Webster, K.E.; Daley, S.; Grey, S.T. A role for intrathymic B cells in the generation of natural regulatory T cells. J. Immunol. 2014, 193, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, A.; Miyagaki, T.; DiLillo, D.J.; Matsushita, T.; Horikawa, M.; Kountikov, E.I.; Spolski, R.; Poe, J.C.; Leonard, W.J.; Tedder, T.F. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012, 491, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.S.; Sgnotto, F.D.R.; Sousa, T.R.; Orfali, R.L.; Aoki, V.; Duarte, A.J.D.S.; Victor, J.R. IgG from atopic dermatitis patients induces non-atopic infant thymic invariant natural killer T (iNKT) cells to produce IL-4, IL-17, and IL-10. Int. J. Derm. 2019. [Google Scholar] [CrossRef]
- da Ressureição Sgnotto, F.; Souza Santos, L.; Rodrigues de Sousa, T.; Feitosa de Lima, J.; Mara da Silva Oliveira, L.; Saeed Sanabani, S.; José da Silva Duarte, A.; Russo Victor, J. IgG From HIV-1-Exposed Seronegative and HIV-1-Infected Subjects Differently Modulates IFN-γ Production by Thymic T and B Cells. J. Acquir. Immune Defic. Syndr. 2019, 82, e56–e60. [Google Scholar] [CrossRef]
- Victor, J.R. Allergen-specific IgG as a mediator of allergy inhibition: Lessons from mother to child. Hum. Vaccines Immunother. 2017, 13, 507–513. [Google Scholar] [CrossRef]
- Santos, L.S.; Sgnotto, F.D.R.; Inoue, A.H.S.; Padreca, A.F.; Menghini, R.P.; Duarte, A.J.D.S.; Victor, J.R. IgG from Non-atopic Individuals Induces In Vitro IFN-γ and IL-10 Production by Human Intra-thymic γδT Cells: A Comparison with Atopic IgG and IVIg. Arch. Immunol. Ther. Exp. (Warsz) 2019. [Google Scholar] [CrossRef]
- Victor, J.R. Do different IgG repertoires play a role in B- and T-cell functional modulation during ontogeny? The “hooks without bait” theory. Immunol. Cell Biol. 2020. [Google Scholar] [CrossRef]



| Nonatopic | Atopic | p | |
|---|---|---|---|
| Number | 17 | 18 | |
| Age, years (mean ± SE) | 30.2 ± 4.11 | 32.9 ± 3.26 | 0.87 |
| Sex (male/female) | 7/10 | 9/9 | 0.82 |
| IgE-specific reactivity (n/%) | |||
| Dermatophagoides pteronyssinus | 0/0 | 18/100 | |
| Dermatophagoides farinae | 0/0 | 18/100 | |
| Aspergillus fumigatus | 0/0 | 9/50 | |
| Penicillium notatum | 0/0 | 4/22 | |
| Alternaria alternata | 0/0 | 3/16 | |
| Canis familiaris | 0/0 | 2/11 | |
| Felis domesticus | 0/0 | 2/11 | |
| Cladosporium herbarum | 0/0 | 0/0 | |
| SPT reactivity (n/%) | |||
| Dermatophagoides pteronyssinus | 0/0 | 17/93 | |
| Dermatophagoides farinae | 0/0 | 16/88 | |
| Aspergillus fumigatus | 0/0 | 6/33 | |
| Penicillium notatum | 0/0 | 3/16 | |
| Alternaria alternata | 0/0 | 2/11 | |
| Canis familiaris | 0/0 | 2/11 | |
| Felis domesticus | 0/0 | 1/5 | |
| Cladosporium herbarum | 0/0 | 1/5 | |
| SPT reactivity (n/%) | |||
| Dual | 0/0 | 0/0 | |
| Triple | 0/0 | 9/50 | |
| Quadruple | 0/0 | 6/33 | |
| Quintuple | 0/0 | 3/16 | |
| Others | |||
| Clinically allergic | 0/0 | 18/100 | |
| Regular use of antihistamines | 0/0 | 18/100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, A.H.S.; Lira, A.A.d.L.; de-Oliveira, M.G.; de Sousa, T.R.; Sgnotto, F.d.R.; Duarte, A.J.d.S.; Victor, J.R. The Potential of IgG to Induce Murine and Human Thymic Maturation of IL-10+ B Cells (B10) Revealed in a Pilot Study. Cells 2020, 9, 2239. https://doi.org/10.3390/cells9102239
Inoue AHS, Lira AAdL, de-Oliveira MG, de Sousa TR, Sgnotto FdR, Duarte AJdS, Victor JR. The Potential of IgG to Induce Murine and Human Thymic Maturation of IL-10+ B Cells (B10) Revealed in a Pilot Study. Cells. 2020; 9(10):2239. https://doi.org/10.3390/cells9102239
Chicago/Turabian StyleInoue, Amanda Harumi Sabô, Aline Aparecida de Lima Lira, Marília Garcia de-Oliveira, Thamires Rodrigues de Sousa, Fábio da Ressureição Sgnotto, Alberto José da Silva Duarte, and Jefferson Russo Victor. 2020. "The Potential of IgG to Induce Murine and Human Thymic Maturation of IL-10+ B Cells (B10) Revealed in a Pilot Study" Cells 9, no. 10: 2239. https://doi.org/10.3390/cells9102239
APA StyleInoue, A. H. S., Lira, A. A. d. L., de-Oliveira, M. G., de Sousa, T. R., Sgnotto, F. d. R., Duarte, A. J. d. S., & Victor, J. R. (2020). The Potential of IgG to Induce Murine and Human Thymic Maturation of IL-10+ B Cells (B10) Revealed in a Pilot Study. Cells, 9(10), 2239. https://doi.org/10.3390/cells9102239





