HbF Levels in Sickle Cell Disease Are Associated with Proportion of Circulating Hematopoietic Stem and Progenitor Cells and CC-Chemokines
Abstract
1. Introduction
2. Materials and Methods
2.1. IRB Approval
2.2. Sample Acquisition
2.3. Flow Cytometry Analysis
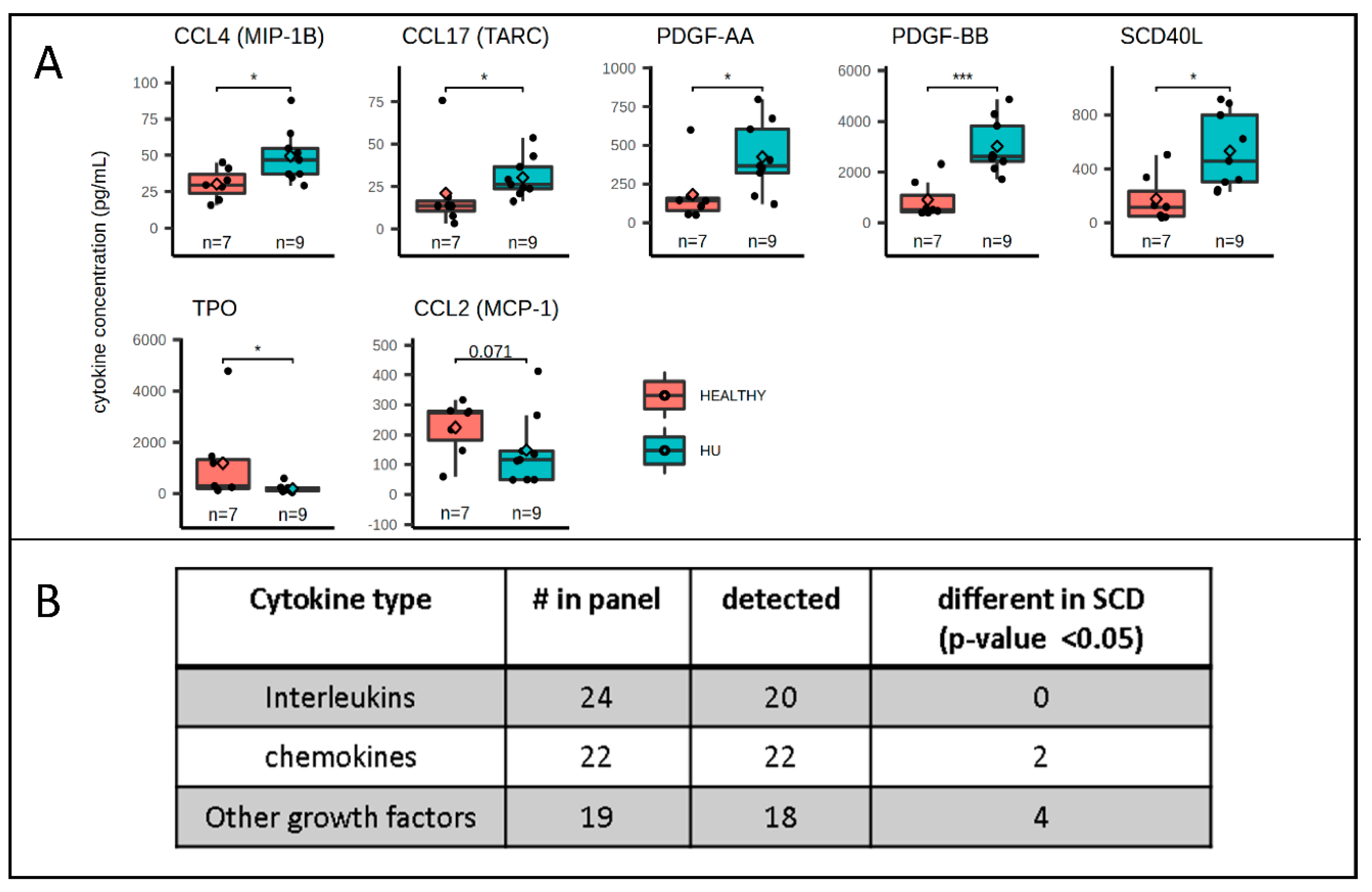
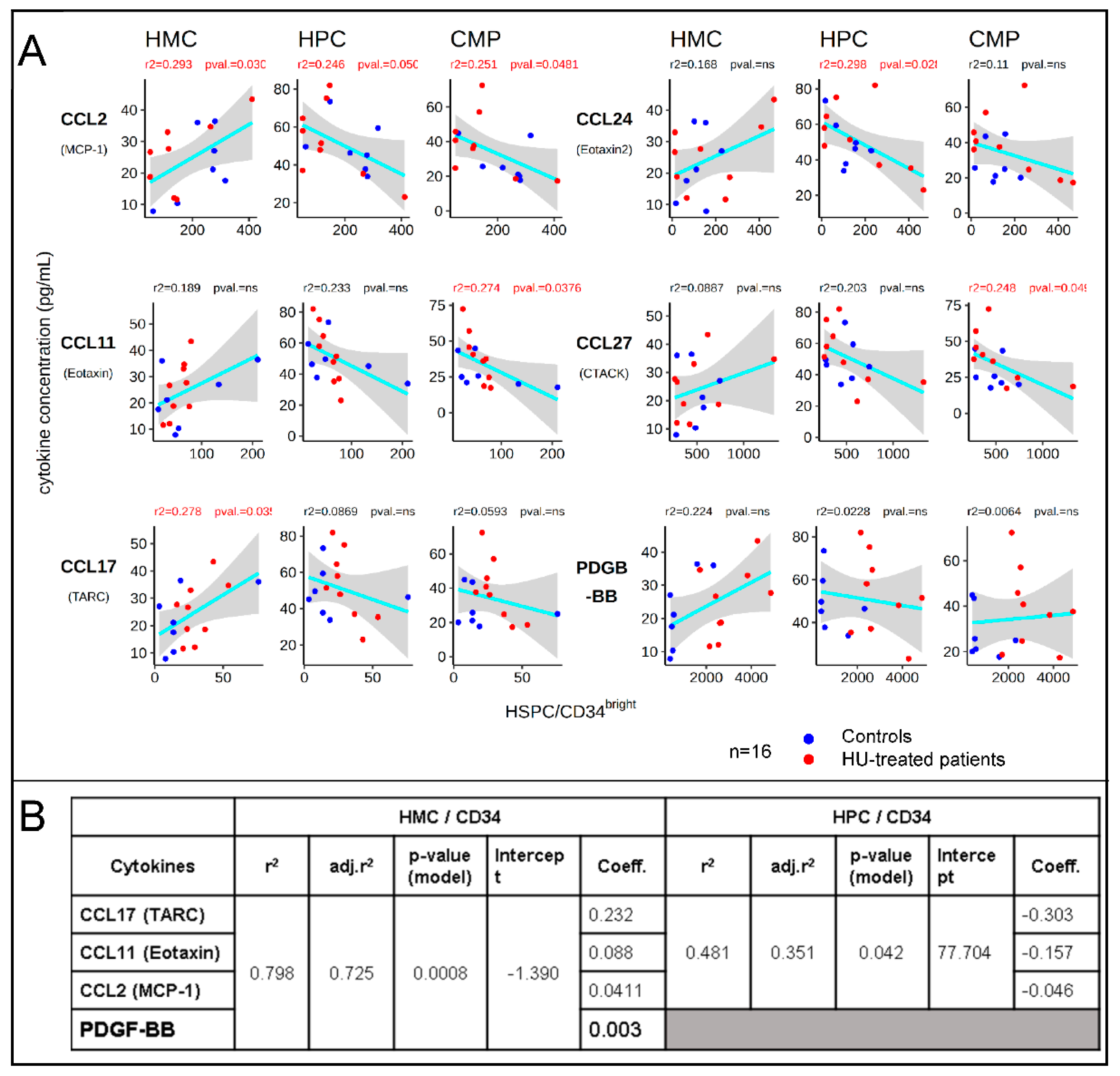
2.4. Cytokine Concentration Analysis
2.5. Statistical Analysis
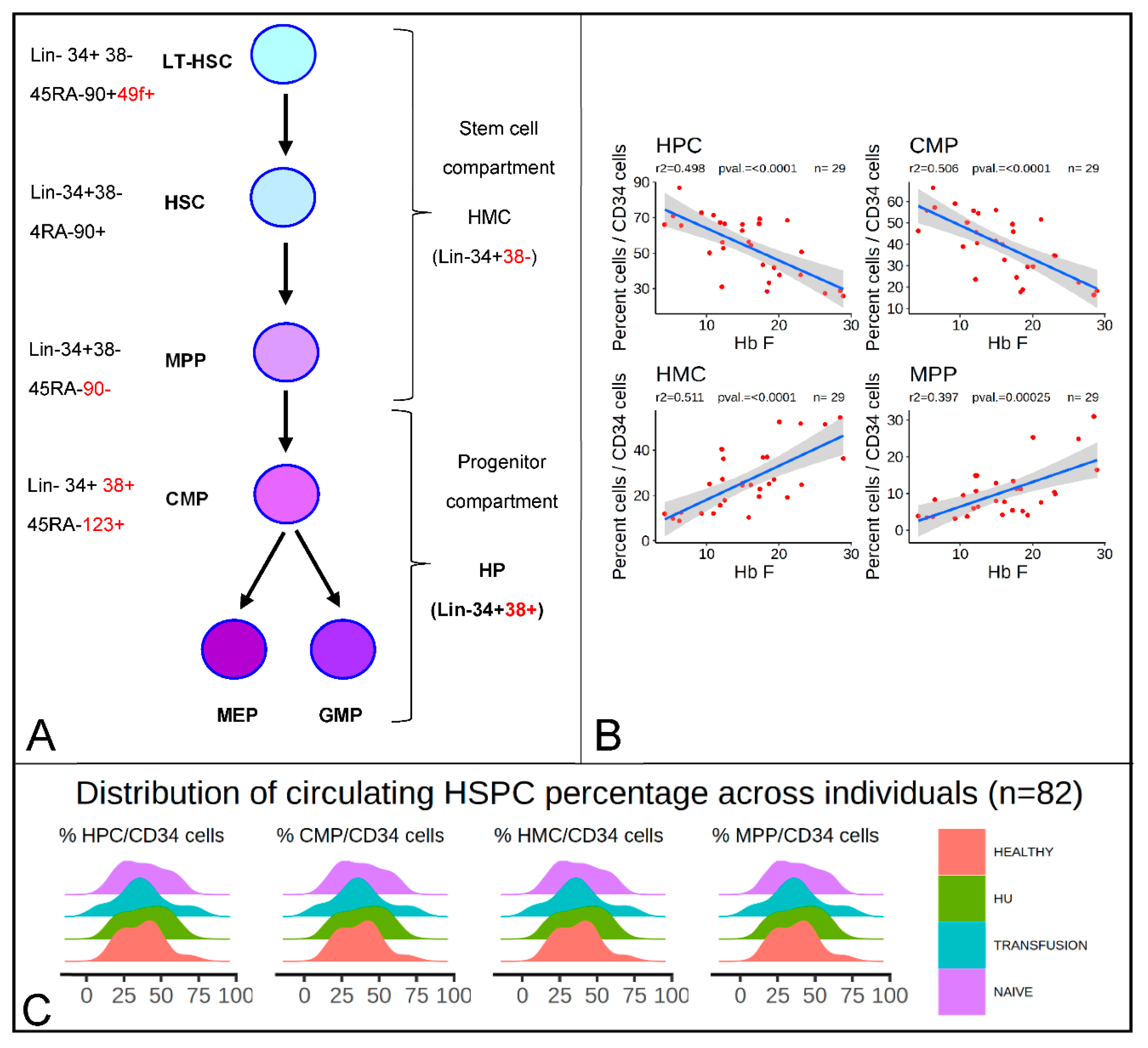
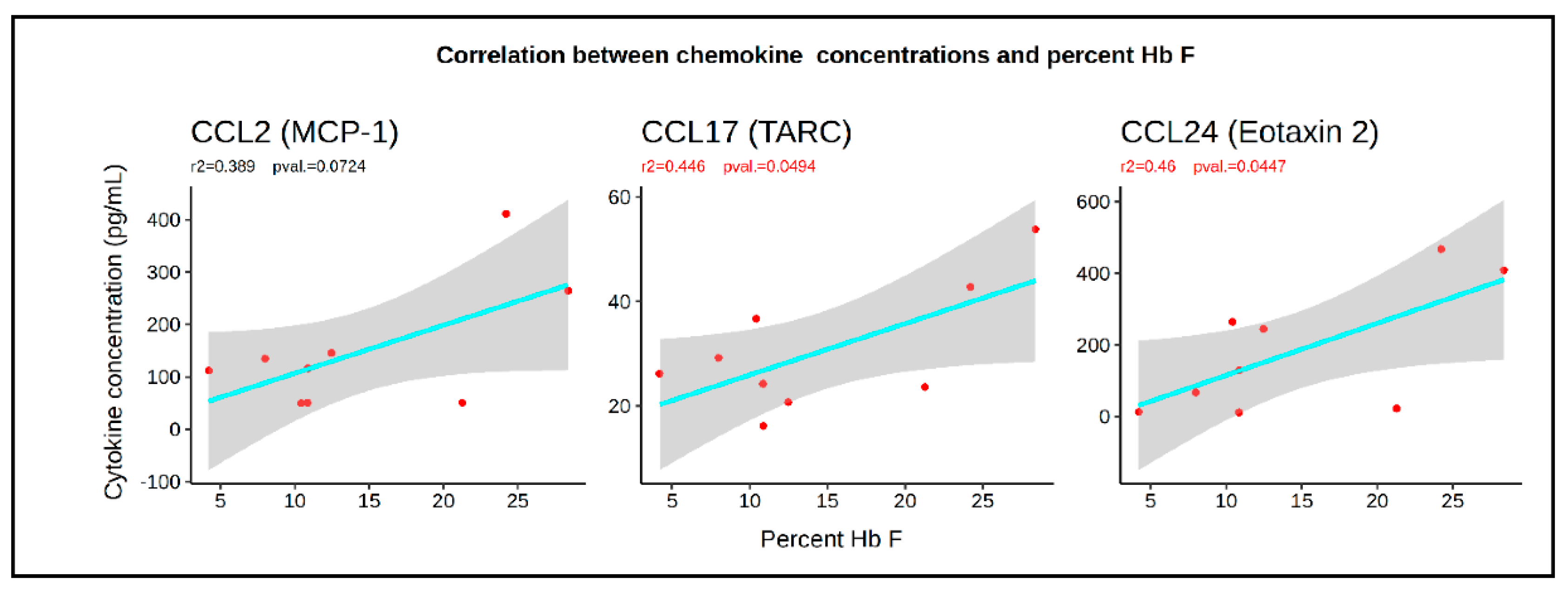
3. Results
3.1. HbF Levels Are Correlated with the Percentages of HMCs and HPCs
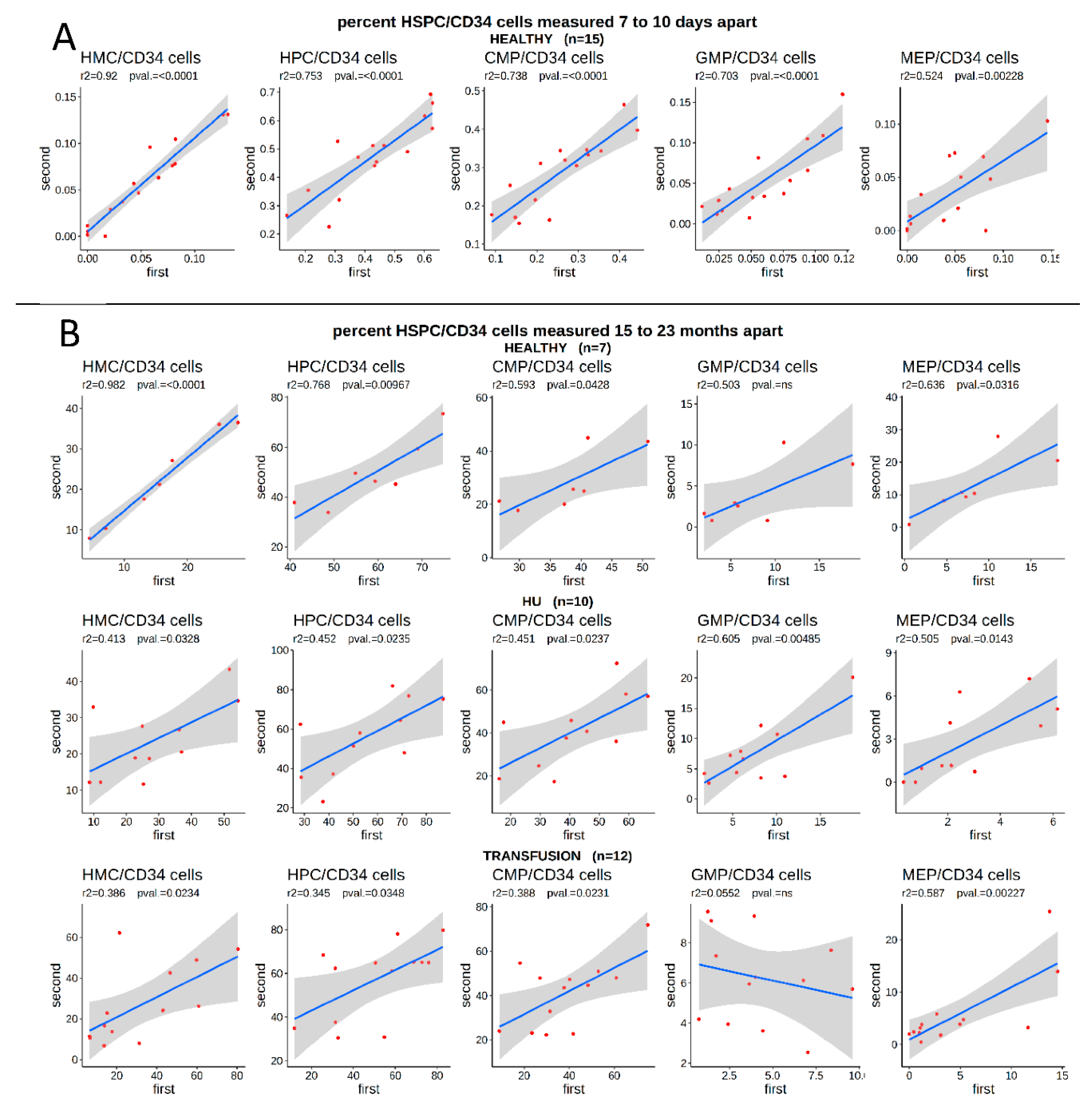
3.2. The Proportion of HSPCs Is Stable over Long Periods of Time in Both Controls and SCD Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Stuart, M.J.; Nagel, R.L. Sickle-cell disease. Lancet 2004, 364, 1343–1360. [Google Scholar] [CrossRef]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef]
- Piel, F.B.; Hay, S.I.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLoS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Achebe, M.M.; Alkindi, S.; Brown, R.C.; et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Telen, M.J. Beyond hydroxyurea: New and old drugs in the pipeline for sickle cell disease. Blood 2016, 127, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Green, N.S.; Ender, K.L.; Pashankar, F.; Driscoll, C.; Giardina, P.J.; Mullen, C.A.; Clark, L.N.; Manwani, D.; Crotty, J.; Kisselev, S.; et al. Candidate Sequence Variants and Fetal Hemoglobin in Children with Sickle Cell Disease Treated with Hydroxyurea. PLoS ONE 2013, 8, e55709. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.L.; Steward, S.; Howard, T.A.; Mortier, N.; Smeltzer, M.; Wang, Y.-D.; Ware, R.E. Epigenetic and molecular profiles of erythroid cells after hydroxyurea treatment in sickle cell anemia. Blood 2011, 118, 5664–5670. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Lu, Z.H.; Barton, F.B.; Terrin, M.L.; Charache, S.; Dover, G.J. Fetal hemoglobin in sickle cell anemia: Determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood 1997, 89, 1078–1088. [Google Scholar] [CrossRef]
- Ware, R.E.; Despotovic, J.M.; Mortier, N.A.; Flanagan, J.M.; He, J.; Smeltzer, M.P.; Kimble, A.C.; Aygun, B.; Wu, S.; Howard, T.; et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 2011, 118, 4985–4991. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Sankaran, V.G. Regulation of the fetal hemoglobin silencing factor BCL11A. Ann. N. Y. Acad. Sci. 2016, 1368, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Menzel, S.; Thein, S.L. Genetic Modifiers of Fetal Haemoglobin in Sickle Cell Disease. Mol. Diagn. Ther. 2019, 23, 235–244. [Google Scholar] [CrossRef]
- Raffield, L.M.; Ulirsch, J.C.; Naik, R.P.; Lessard, S.; Handsaker, R.E.; Jain, D.; Kang, H.M.; Pankratz, N.; Auer, P.L.; Bao, E.L.; et al. Common α-globin variants modify hematologic and other clinical phenotypes in sickle cell trait and disease. PLoS Genet. 2018, 14, e1007293. [Google Scholar] [CrossRef] [PubMed]
- Forget, B.G. Molecular basis of hereditary persistence of fetal hemoglobin. Ann. N. Y. Acad. Sci. 1998, 850, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ghatpande, S.S.; Choudhary, P.K.; Quinn, C.T.; Goodman, S.R. In vivo pharmaco-proteomic analysis of hydroxyurea induced changes in the sickle red blood cell membrane proteome. J. Proteom. 2010, 73, 619–626. [Google Scholar] [CrossRef]
- Chagas Costa, F.; Ferreira da Cunha, A.; Fattori, A.; de Sousa Peres, T.; Gilson Lacerda Costa, G.; Ferraz Machado, T.; Martins de Albuquerque, D.; Gambero, S.; Lanaro, C.; Olala Saad, S.T.; et al. Gene expression profiles of erythroid precursors characterise several mechanisms of the action of hydroxycarbamide in sickle cell anaemia. Br. J. Haematol. 2007, 136, 333–342. [Google Scholar] [CrossRef]
- Flanagan, J.M.; Steward, S.; Howard, T.A.; Mortier, N.A.; Kimble, A.C.; Aygun, B.; Hankins, J.S.; Neale, G.A.; Ware, R.E. Hydroxycarbamide alters erythroid gene expression in children with sickle cell anaemia. Br. J. Haematol. 2012, 157, 240–248. [Google Scholar] [CrossRef]
- Pourfarzad, F.; von Lindern, M.; Azarkeivan, A.; Hou, J.; Kia, S.K.; Esteghamat, F.; van IJcken, W.; Philipsen, S.; Najmabadi, H.; Grosveld, F. Hydroxyurea responsiveness in β-thalassemic patients is determined by the stress response adaptation of erythroid progenitors and their differentiation propensity. Haematologica 2013, 98, 696–704. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Kwon, J.; Kim, S.N.; Kim, J.E.; Koh, W.S.; Kim, E.-J.; Chung, M.-K.; Han, S.-S.; Song, C.W. cDNA microarray gene expression profiling of hydroxyurea, paclitaxel, and p-anisidine, genotoxic compounds with differing tumorigenicity results. Environ. Mol. Mutagen. 2003, 42, 91–97. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, K.; Sun, C.-W.; Pawlik, K.M.; Townes, T.M. KLF1 regulates BCL11A expression and γ- to β-globin gene switching. Nat. Genet. 2010, 42, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.; Papadopoulos, P.; Georgitsi, M.; Gutiérrez, L.; Grech, G.; Fanis, P.; Phylactides, M.; Verkerk, A.J.M.H.; van der Spek, P.J.; Scerri, C.A.; et al. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat. Genet. 2010, 42, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Grieco, A.J.; Billett, H.H.; Green, N.S.; Driscoll, M.C.; Bouhassira, E.E. Variation in Gamma-Globin Expression before and after Induction with Hydroxyurea Associated with BCL11A, KLF1 and TAL1. PLoS ONE 2015, 10, e0129431. [Google Scholar] [CrossRef] [PubMed]
- Conley, C.L.; Weatherall, D.J.; Richardson, S.N.; Shepard, M.K.; Charache, S. Hereditary persistence of fetal hemoglobin: A study of 79 affected persons in 15 Negro families in Baltimore. Blood 1963, 21, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Tolu, S.S.; Wang, K.; Yan, Z.; Zhang, S.; Roberts, K.; Crouch, A.S.; Sebastian, G.; Chaitowitz, M.; Fornari, E.D.; Schwechter, E.M.; et al. Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells. Cells 2020, 9, 2159. [Google Scholar] [CrossRef]
- Croizat, H. Circulating cytokines in sickle cell patients during steady state. Br. J. Haematol. 1994, 87, 592–597. [Google Scholar] [CrossRef]
- Lamming, C.E.D.; Augustin, L.; Blackstad, M.; Lund, T.C.; Hebbel, R.P.; Verfaillie, C.M. Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis. J. Clin. Investig. 2003, 111, 811–819. [Google Scholar] [CrossRef]
- Lucas, D. Leukocyte Trafficking and Regulation of Murine Hematopoietic Stem Cells and Their Niches. Front. Immunol. 2019, 10, 387. [Google Scholar] [CrossRef]
- Granick, J.L.; Simon, S.I.; Borjesson, D.L. Hematopoietic Stem and Progenitor Cells as Effectors in Innate Immunity. Bone Marrow Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Robbins, C.S.; Chudnovskiy, A.; Rauch, P.J.; Figueiredo, J.-L.; Iwamoto, Y.; Gorbatov, R.; Etzrodt, M.; Weber, G.F.; Ueno, T.; van Rooijen, N.; et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012, 125, 364–374. [Google Scholar] [CrossRef]
- Filshie, R. Cytokines in Haemopoietic Progenitor Mobilisation for Peripheral Blood Stem Cell Transplantation. Curr. Pharm. Des. 2002, 8, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; He, S.J.; Yang, J. MCP-1/CCL2 Mediated by Autocrine Loop of PDGF-BB Promotes Invasion of Lung Cancer Cell by Recruitment of Macrophages Via CCL2-CCR2 Axis. J. Interferon Cytokine Res. 2019, 39, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Van Coillie, E.; Van Damme, J.; Opdenakker, G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999, 10, 61–86. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Kim, C.H.; Cooper, S.H.; Hangoc, G.; Hromas, R.; Pelus, L.M. Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors. Ann. N. Y. Acad. Sci. 1999, 872, 142–162, discussion 163. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.J.; Wright, E.G.; Hewick, R.; Wolpe, S.D.; Wilkie, N.M.; Donaldson, D.; Lorimore, S.; Pragnell, I.B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature 1990, 344, 442–444. [Google Scholar] [CrossRef]
- Pragnell, I.B.; Wright, E.G.; Lorimore, S.A.; Adam, J.; Rosendaal, M.; DeLamarter, J.F.; Freshney, M.; Eckmann, L.; Sproul, A.; Wilkie, N. The effect of stem cell proliferation regulators demonstrated with an in vitro assay. Blood 1988, 72, 196–201. [Google Scholar] [CrossRef]
- Su, S.; Mukaida, N.; Wang, J.; Zhang, Y.; Takami, A.; Nakao, S.; Matsushima, K. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1alpha by interacting mainly with a C-C chemokine receptor, CCR1. Blood 1997, 90, 605–611. [Google Scholar] [CrossRef]
- Patel, V.P.; Kreider, B.L.; Li, Y.; Li, H.; Leung, K.; Salcedo, T.; Nardelli, B.; Pippalla, V.; Gentz, S.; Thotakura, R.; et al. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J. Exp. Med. 1997, 185, 1163–1172. [Google Scholar] [CrossRef]
- Staversky, R.J.; Byun, D.K.; Georger, M.A.; Zaffuto, B.J.; Goodman, A.; Becker, M.W.; Calvi, L.M.; Frisch, B.J. The Chemokine CCL3 Regulates Myeloid Differentiation and Hematopoietic Stem Cell Numbers. Sci. Rep. 2018, 8, 14691. [Google Scholar] [CrossRef]
- Si, Y.; Tsou, C.-L.; Croft, K.; Charo, I.F. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J. Clin. Investig. 2010, 120, 1192–1203. [Google Scholar] [CrossRef]
- Dutta, P.; Sager, H.B.; Stengel, K.R.; Naxerova, K.; Courties, G.; Saez, B.; Silberstein, L.; Heidt, T.; Sebas, M.; Sun, Y.; et al. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 2015, 16, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Ramoni, M.F.; Nolan, V.; Baldwin, C.T.; Steinberg, M.H. Genetic dissection and prognostic modeling of overt stroke in sickle cell anemia. Nat. Genet. 2005, 37, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-H.; Garstka, M.A.; Li, Z.-F. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol. Immunol. 2020, 117, 201–215. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minniti, C.P.; Tolu, S.S.; Wang, K.; Yan, Z.; Robert, K.; Zhang, S.; Crouch, A.S.; Uehlinger, J.; Manwani, D.; Bouhassira, E.E. HbF Levels in Sickle Cell Disease Are Associated with Proportion of Circulating Hematopoietic Stem and Progenitor Cells and CC-Chemokines. Cells 2020, 9, 2199. https://doi.org/10.3390/cells9102199
Minniti CP, Tolu SS, Wang K, Yan Z, Robert K, Zhang S, Crouch AS, Uehlinger J, Manwani D, Bouhassira EE. HbF Levels in Sickle Cell Disease Are Associated with Proportion of Circulating Hematopoietic Stem and Progenitor Cells and CC-Chemokines. Cells. 2020; 9(10):2199. https://doi.org/10.3390/cells9102199
Chicago/Turabian StyleMinniti, Caterina P., Seda S. Tolu, Kai Wang, Zi Yan, Karl Robert, Shouping Zhang, Andrew S. Crouch, Joan Uehlinger, Deepa Manwani, and Eric E. Bouhassira. 2020. "HbF Levels in Sickle Cell Disease Are Associated with Proportion of Circulating Hematopoietic Stem and Progenitor Cells and CC-Chemokines" Cells 9, no. 10: 2199. https://doi.org/10.3390/cells9102199
APA StyleMinniti, C. P., Tolu, S. S., Wang, K., Yan, Z., Robert, K., Zhang, S., Crouch, A. S., Uehlinger, J., Manwani, D., & Bouhassira, E. E. (2020). HbF Levels in Sickle Cell Disease Are Associated with Proportion of Circulating Hematopoietic Stem and Progenitor Cells and CC-Chemokines. Cells, 9(10), 2199. https://doi.org/10.3390/cells9102199






