Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. IRB Approval
2.2. Sample Acquisition
2.3. FACS Analysis
2.4. Data Analysis
2.5. Statistical Analysis
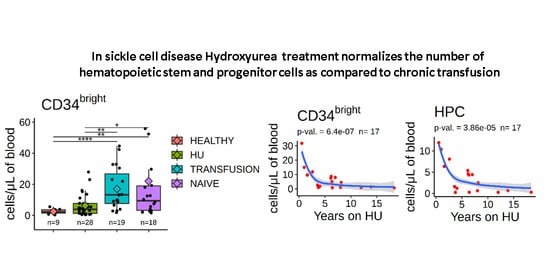
3. Results
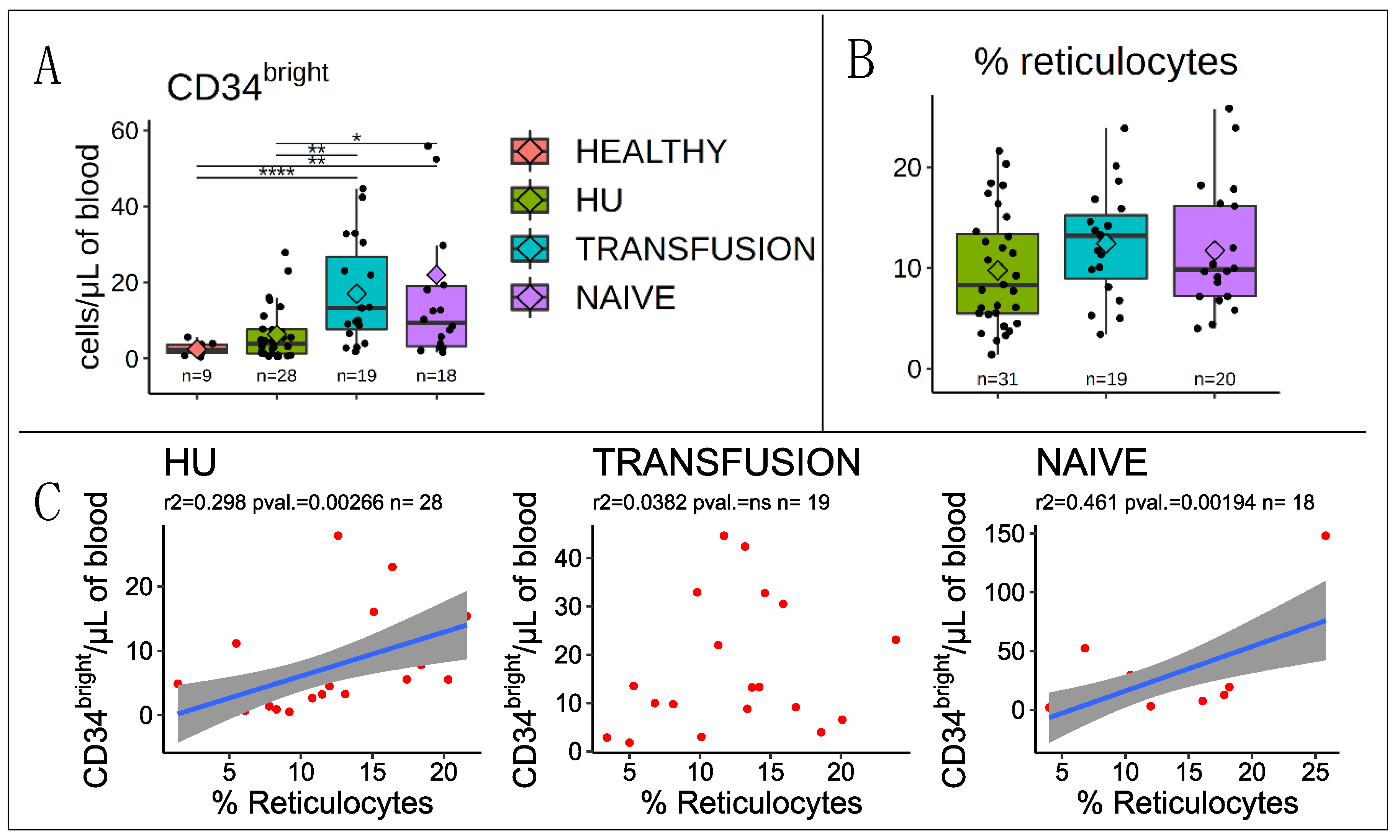
3.1. Correlation with Reticulocytes
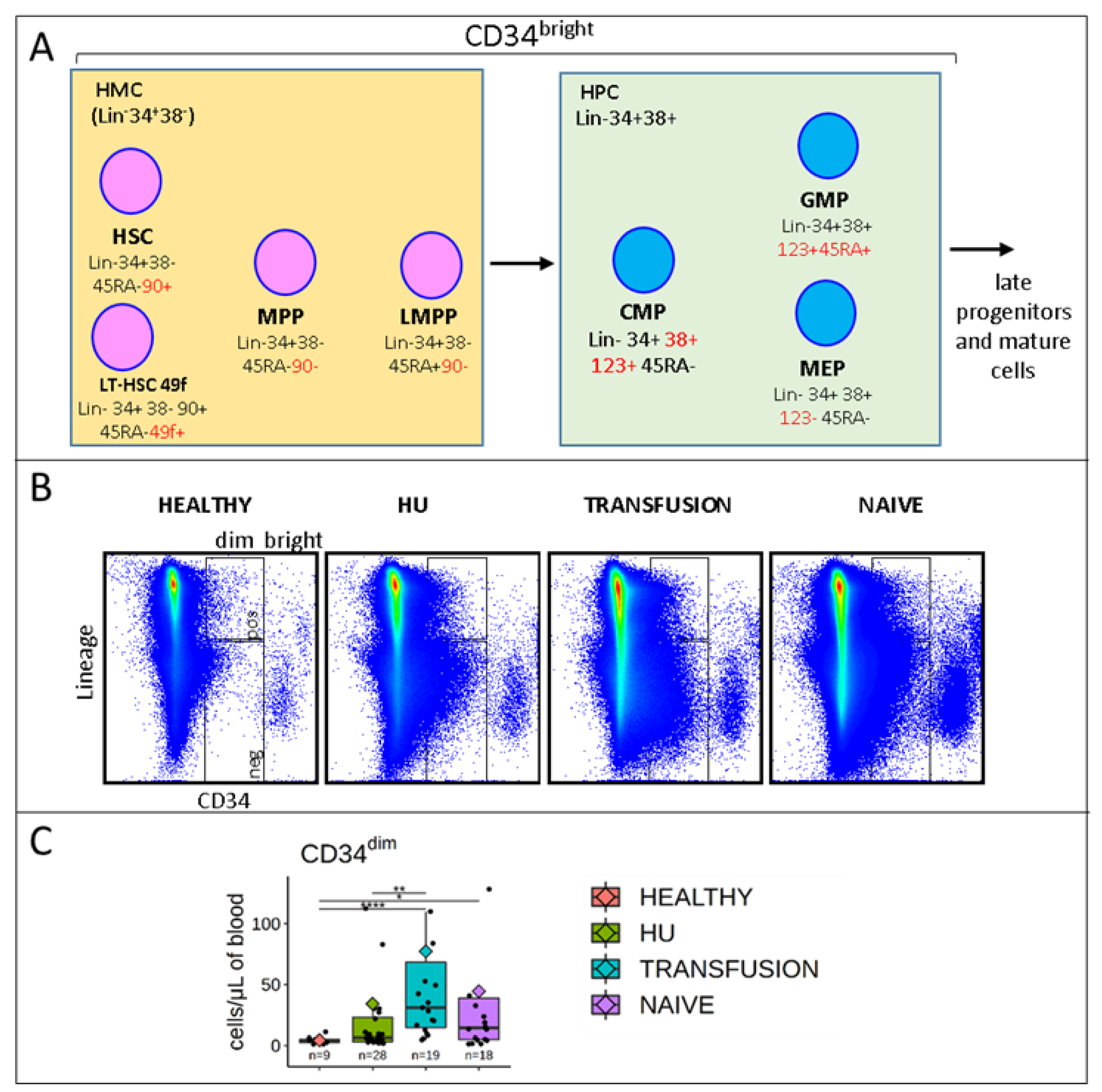
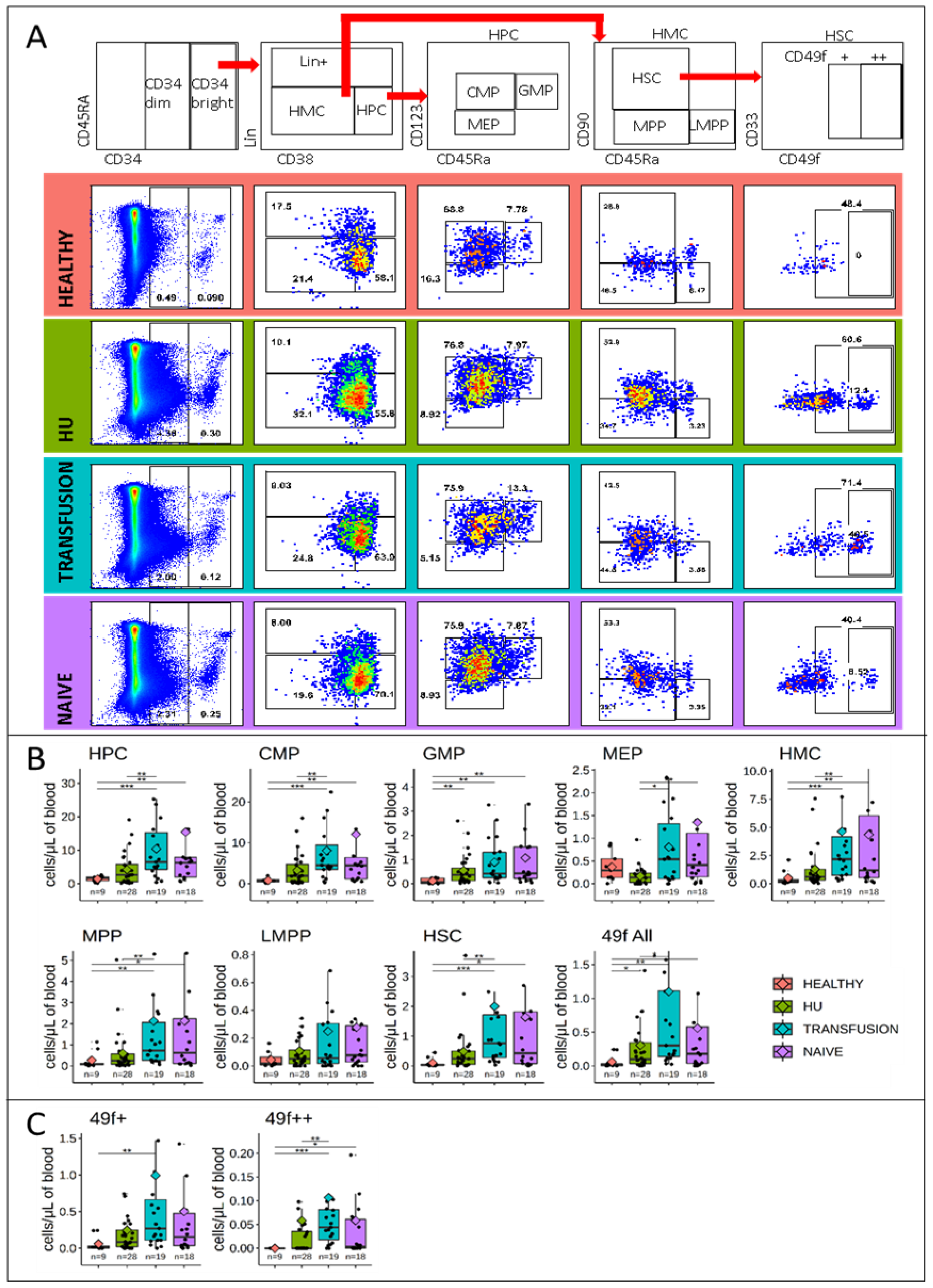
3.2. Stem and Progenitor Cells Quantification
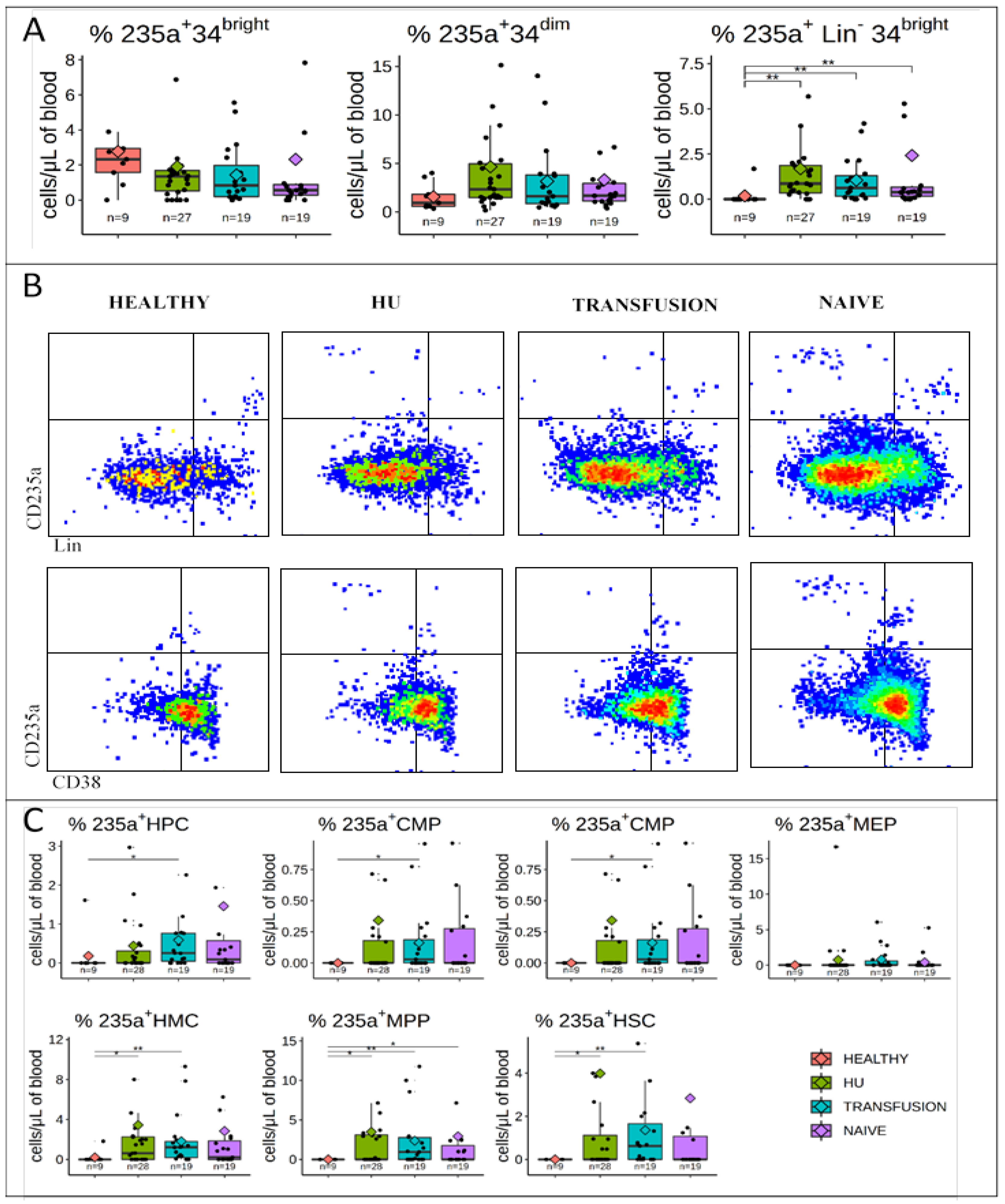
3.3. CD235a is Expressed in a Fraction of PB HMCs, MPPs, and HSCs of SCD Patients but not in Controls
3.4. CD49f is Expressed at Very High Levels in a Subset of HSCs from SCD Patients
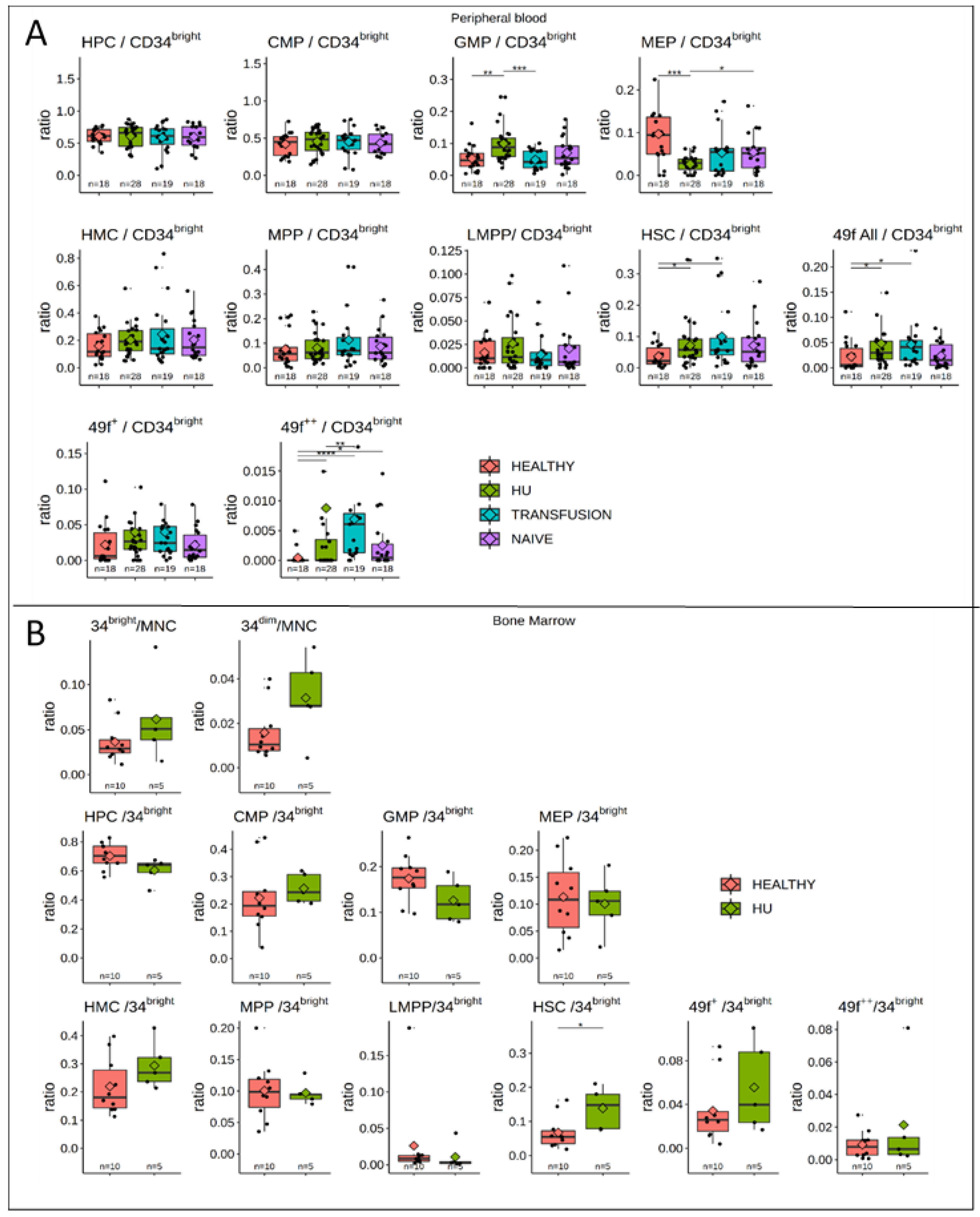
3.5. MPPs and HSCs and 49f Cells are Particularly Abundant in SCD
3.6. Bone Marrow Analysis
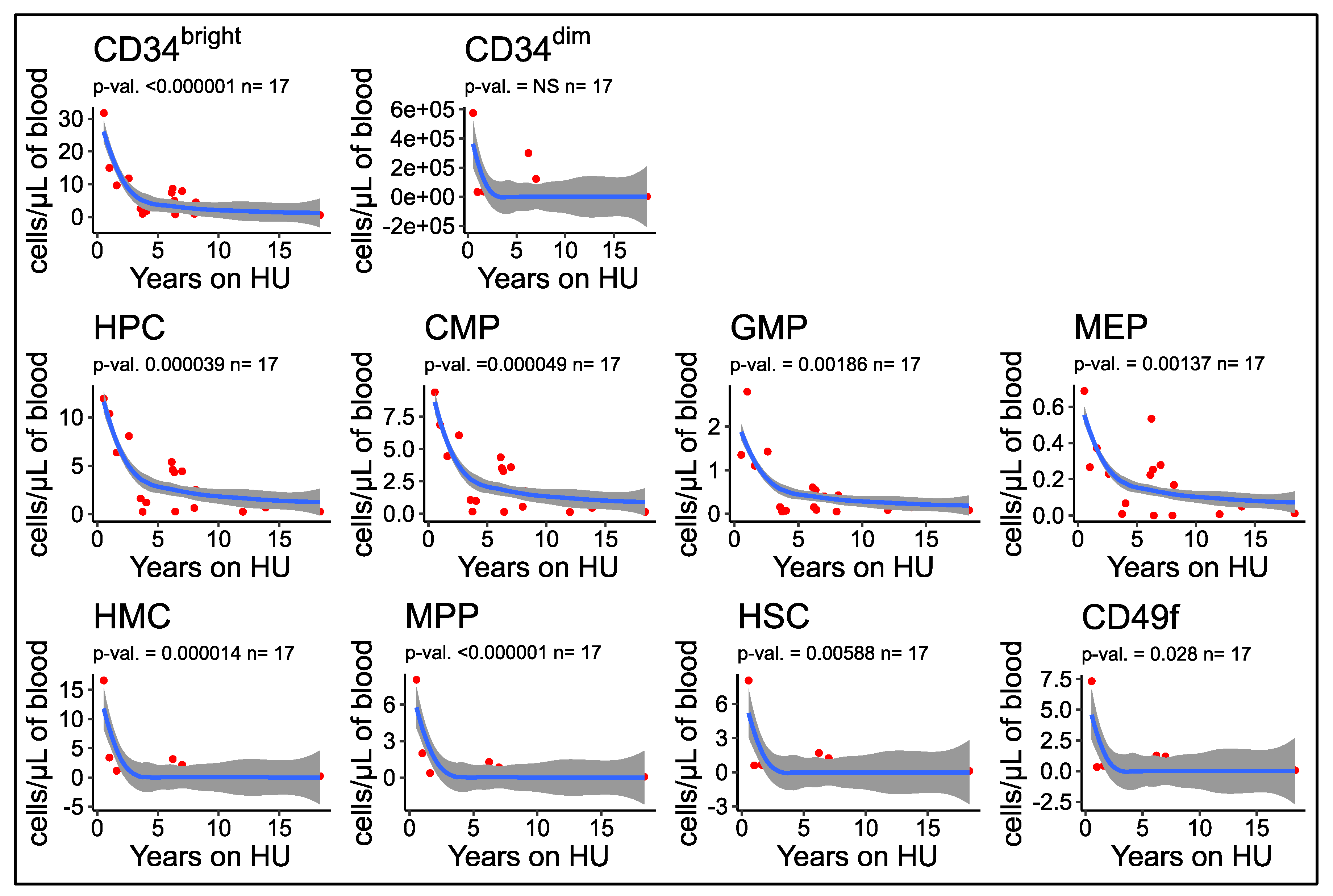
3.7. Number of Circulating HSPCs Decreases as a Function of the Duration of HU Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leonard, A.; Bonifacino, A.; Dominical, V.M.; Luo, M.; Haro-Mora, J.J.; Demirci, S.; Uchida, N.; Pierciey, F.J.; Tisdale, J.F.; Haro-Mora, J.J.; et al. Bone marrow characterization in sickle cell disease: Inflammation and stress erythropoiesis lead to suboptimal CD34 recovery. Br. J. Haematol. 2019, 186, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Grasso, J.A.; Sullivan, A.L.; Sullivan, L.W. Ultrastructural studies of the bone marrow in sickle cell anaemia. I. The structure of sickled erythrocytes and reticulocytes and their phagocytic destruction. Br. J. Haematol. 1975, 31, 135–148. [Google Scholar] [CrossRef]
- Grasso, J.A.; Sullivan, A.L.; Sullivan, L.W. Ultrastructural studies of the bone marrow in sickle cell anaemia. II. The morphology of erythropoietic cells and their response to deoxygenation in vitro. Br. J. Haematol. 1975, 31, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Krishnamurti, L.; Kutok, J.L.; Biernacki, M.; Rogers, S.; Zhang, W.; Antin, J.H.; Ritz, J. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood 2005, 106, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ogawa, M. Erythropoietic precursors in mice under erythropoietic stimulation and suppression. Exp. Hematol. 1977, 5, 141–148. [Google Scholar] [PubMed]
- Croizat, H.; Billett, H.H.; Nagel, R.L. Heterogeneity in the properties of burst-forming units of erythroid lineage in sickle cell anemia: DNA synthesis and burst-promoting activity production is related to peripheral hemoglobin F levels. Blood 1990, 75, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croizat, H.; Ponchio, L.; Nicolini, F.E.; Nagel, R.L.; Eaves, C.J.; Ponchio, L.; Nicolini, F.E.; Eaves, C.J. Primitive haematopoietic progenitors in the blood of patients with sickle cell disease appear to be endogenously mobilized. Br. J. Haematol. 2000, 111, 491–497. [Google Scholar] [CrossRef]
- Lamming, C.E.D.; Augustin, L.; Blackstad, M.; Lund, T.C.; Hebbel, R.P.; Verfaillie, C.M. Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis. J. Clin. Investig. 2003, 111, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Uchida, N.; Fujita, A.; Hsieh, M.M.; Bonifacino, A.C.; Krouse, A.E.; Metzger, M.E.; Donahue, R.E.; Tisdale, J.F. Bone Marrow as a Hematopoietic Stem Cell Source for Gene Therapy in Sickle Cell Disease: Evidence from Rhesus and SCD Patients. Hum. Gene Ther. Clin. Dev. 2017, 28, 136–144. [Google Scholar] [CrossRef]
- Ware, R.E. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010, 115, 5300–5311. [Google Scholar] [CrossRef]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumano, A.; Godin, I. Ontogeny of the hematopoietic system. Annu. Rev. Immunol. 2007, 25, 745–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeti, R.; Park, C.Y.; Weissman, I.L. Identification of a Hierarchy of Multipotent Hematopoietic Progenitors in Human Cord Blood. Cell Stem Cell 2007, 1, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notta, F.; Doulatov, S.; Laurenti, E.; Poeppl, A.; Jurisica, I.; Dick, J.E. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 2011, 333, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guzman, A.K.; Yan, Z.; Zhang, S.; Hu, M.Y.; Hamaneh, M.B.; Yu, Y.K.; Tolu, S.; Zhang, J.; Kanavy, H.E.; et al. Ultra-High-Frequency Reprogramming of Individual Long-Term Hematopoietic Stem Cells Yields Low Somatic Variant Induced Pluripotent Stem Cells. Cell Rep. 2019, 26, 2580–2592.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntsman, H.D.; Bat, T.; Cheng, H.; Cash, A.; Cheruku, P.S.; Fu, J.-F.; Keyvanfar, K.; Childs, R.W.; Dunbar, C.E.; Larochelle, A. Human hematopoietic stem cells from mobilized peripheral blood can be purified based on CD49f integrin expression. Blood 2015, 126, 1631–1633. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yan, Z.; Zhang, S.; Bartholdy, B.; Eaves, C.J.; Bouhassira, E.E. Clonal Origin in Normal Adults of All Blood Lineages and Circulating Hematopoietic Stem Cells. SSRN Electron. J. 2019. Online ahe. [Google Scholar] [CrossRef]
- Logan, A.C.; Weissman, I.L.; Shizuru, J.A. The road to purified hematopoietic stem cell transplants is paved with antibodies. Curr. Opin. Immunol. 2012, 24, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Paulson, R.F.; Shi, L.; Wu, D.-C. Stress erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Harandi, O.F.; Hedge, S.; Wu, D.-C.; McKeone, D.; Paulson, R.F. Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. J. Clin. Investig. 2010, 120, 4507–4519. [Google Scholar] [CrossRef] [Green Version]
- Woolthuis, C.M.; de Haan, G.; Huls, G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr. Opin. Immunol. 2011, 23, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Notta, F.; Zandi, S.; Takayama, N.; Dobson, S.; Gan, O.I.; Wilson, G.; Kaufmann, K.B.; McLeod, J.; Laurenti, E.; Dunant, C.F.; et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 2015, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, M.G.; Miyamoto, T.; Akashi, K.; Weissman, I.L. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. USA 2002, 99, 11872–11877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edvardsson, L.; Dykes, J.; Olofsson, T. Isolation and characterization of human myeloid progenitor populations--TpoR as discriminator between common myeloid and megakaryocyte/erythroid progenitors. Exp. Hematol. 2006, 34, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Goardon, N.; Marchi, E.; Atzberger, A.; Quek, L.; Schuh, A.; Soneji, S.; Woll, P.; Mead, A.; Alford, K.A.; Rout, R.; et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 2011, 19, 138–152. [Google Scholar] [CrossRef] [Green Version]
- Drissen, R.; Thongjuea, S.; Theilgaard-Mönch, K.; Nerlov, C. Identification of two distinct pathways of human myelopoiesis. Sci. Immunol. 2019, 4, eaau7148. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Luck, L.; Zeng, L.; Hiti, A.L.; Weinberg, K.I.; Malik, P. Human CD34+ and CD34+CD38− hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Exp. Hematol. 2004, 32, 483–493. [Google Scholar] [CrossRef]
- Will, B.; Zhou, L.; Vogler, T.O.; Ben-Neriah, S.; Schinke, C.; Tamari, R.; Yu, Y.; Bhagat, T.D.; Bhattacharyya, S.; Barreyro, L.; et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood 2012, 120, 2076–2086. [Google Scholar] [CrossRef]
- Wang, J.; Kimura, T.; Asada, R.; Harada, S.; Yokota, S.; Kawamoto, Y.; Fujimura, Y.; Tsuji, T.; Ikehara, S.; Sonoda, Y. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood 2003, 101, 2924–2931. [Google Scholar] [CrossRef] [Green Version]
- Sumide, K.; Matsuoka, Y.; Kawamura, H.; Nakatsuka, R.; Fujioka, T.; Asano, H.; Takihara, Y.; Sonoda, Y. A revised road map for the commitment of human cord blood CD34-negative hematopoietic stem cells. Nat. Commun. 2018, 9, 2202. [Google Scholar] [CrossRef] [PubMed]
- Carden, M.A.; Fasano, R.M.; Meier, E.R. Not all red cells sickle the same: Contributions of the reticulocyte to disease pathology in sickle cell anemia. Blood Rev. 2019, 100637. [Google Scholar] [CrossRef] [PubMed]
- Detterich, J.; Alexy, T.; Rabai, M.; Wenby, R.; Dongelyan, A.; Coates, T.; Wood, J.; Meiselman, H. Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion 2013, 53, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detterich, J.A.; Sangkatumvong, S.; Kato, R.; Dongelyan, A.; Bush, A.; Khoo, M.; Meiselman, H.J.; Coates, T.D.; Wood, J.C. Patients with sickle cell anemia on simple chronic transfusion protocol show sex differences for hemodynamic and hematologic responses to transfusion. Transfusion 2013, 53, 1059–1068. [Google Scholar] [CrossRef] [Green Version]
- Vodyanik, M.A.; Thomson, J.A.; Slukvin, I.I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood 2006, 108, 2095–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, E.N.; Zhang, S.; Yan, Z.; Suzuka, S.; Roberts, K.; Wang, K.; Bouhassira, E.E. PSC-RED and MNC-RED: Albumin-free and low-transferrin robust erythroid differentiation protocols to produce human enucleated red blood cells. Exp. Hematol. 2019. [Google Scholar] [CrossRef]
- Krebsbach, P.H.; Villa-Diaz, L.G. The Role of Integrin α6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017, 26, 1090–1099. [Google Scholar] [CrossRef]
- Fares, I.; Chagraoui, J.; Gareau, Y.; Gingras, S.; Ruel, R.; Mayotte, N.; Csaszar, E.; Knapp, D.J.H.F.; Miller, P.; Ngom, M.; et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science (80-.) 2014, 345, 1509–1512. [Google Scholar] [CrossRef] [Green Version]
- Hua, P.; Roy, N.; de la Fuente, J.; Wang, G.; Thongjuea, S.; Clark, K.; Roy, A.; Psaila, B.; Ashley, N.; Harrington, Y.; et al. Single-cell analysis of bone marrow-derived CD34+ cells from children with sickle cell disease and thalassemia. Blood 2019, 134, 2111–2115. [Google Scholar] [CrossRef]
- Stricker, R.B.; Linker, C.A.; Crowley, T.J.; Embury, S.H. Hematologic malignancy in sickle cell disease: Report of four cases and review of the literature. Am. J. Hematol. 1986, 21, 223–230. [Google Scholar] [CrossRef]
- Brunson, A.; Keegan, T.H.M.; Bang, H.; Mahajan, A.; Paulukonis, S.; Wun, T. Increased risk of leukemia among sickle cell disease patients in California. Blood 2017, 130, 1597–1599. [Google Scholar] [CrossRef] [Green Version]
- Rundberg Nilsson, A.; Soneji, S.; Adolfsson, S.; Bryder, D.; Pronk, C.J. Human and Murine Hematopoietic Stem Cell Aging Is Associated with Functional Impairments and Intrinsic Megakaryocytic/Erythroid Bias. PLoS ONE 2016, 11, e0158369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, W.W.; Schrier, S.L.; Weissman, I.L. Age-associated changes in human hematopoietic stem cells. Semin. Hematol. 2017, 54, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Parmar, K.; Mouly, E.; Delord, M.; Kim, J.M.; Regairaz, M.; Pla, M.; Vasquez, N.; Zhang, Q.-S.; Pondarre, C.; et al. Bone Marrow Failure in Fanconi Anemia Is Triggered by an Exacerbated p53/p21 DNA Damage Response that Impairs Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 2012, 11, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Lataillade, J.-J.; Pierre-Louis, O.; Hasselbalch, H.C.; Uzan, G.; Jasmin, C.; Martyré, M.-C.; Le Bousse-Kerdilès, M.-C. French INSERM and the European EUMNET Networks on Myelofibrosis Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood 2008, 112, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Mortera-Blanco, T.; Dimitriou, M.; Woll, P.S.; Karimi, M.; Elvarsdottir, E.; Conte, S.; Tobiasson, M.; Jansson, M.; Douagi, I.; Moarii, M.; et al. SF3B1-initiating mutations in MDS-RSs target lymphomyeloid hematopoietic stem cells. Blood 2017, 130, 881–890. [Google Scholar] [CrossRef] [Green Version]
| All Patients | Hydroxyurea ∞ | Transfusion α | Naïve ¥ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Adult | Pediatric | Total | Adult | Pediatric | Total | Adult | Pediatric | Total | Adult | Pediatric | |
| count | 71 | 44 | 27 | 32 | 21 | 11 | 19 | 10 | 9 | 20 | 13 | 7 |
| Age | 26.11 ± 12.24 | 33.09 ± 10.21 | 14.31 ± 2.25 | 28.06 ± 13.67 | 35.05 ± 11.06 | 13.4 ± 7.3 | 21.11 ± 7.3 | 26.7 ± 5.39 | 14.89 ± 2.64 | 27.85 ± 12.3 | 34.85 ± 9.6 | 14.86 ± 1.12 |
| Sex | M = 36 F = 34 | M = 22 F = 22 | M = 14 F = 13 | M = 16 F = 15 | M = 11 F = 10 | M = 5 F = 5 | M = 12 F = 7 | M = 7 F = 3 | M = 5 F = 4 | M = 8 F = 12 | M = 4 F = 9 | M = 4 F = 3 |
| Hb g/dL | 9.13 ± 1.37 | 9.27 ± 1.43 | 8.9 ± 1.22 | 9.5 ± 1.24 | 9.53 ± 1.37 | 9.43 ± 0.99 | 9.39 ± 0.99 | 9.66 ± 0.9 | 9.09 ± 0.99 | 8.33 ± 1.51 | 8.55 ± 1.58 | 7.9 ± 1.28 |
| Hb S % | 64.77 ± 23.59 | 66.84 ± 22.37 | 61.27 ± 25.12 | 74.3 ± 11.46 | 75.84 ± 9.28 | 71.06 ± 12.14 | 33.2 ± 12.14 | 34.73 ± 12.67 | 31.5 ± 11.29 | 80 ± 16.86 | 77.01 ± 20.11 | 85.54 ± 3.68 |
| Hb F% | 10.91 ± 7.6 | 10.77 ± 8.58 | 11.16 ± 5.57 | 16.24 ± 6.94 | 16.67 ± 7.43 | 15.34 ± 4.26 | 5.87 ± 4.26 | 3.07 ± 2.03 | 8.98 ± 3.91 | 7.44 ± 5.48 | 7.14 ± 6.45 | 7.98 ± 2.87 |
| Hematocrit % | 26.63 ± 3.78 | 26.92 ± 3.98 | 26.15 ± 3.37 | 27.03 ± 3.55 | 27.06 ± 3.75 | 26.97 ± 2.62 | 28.08 ± 2.62 | 28.71 ± 2.53 | 27.39 ± 2.54 | 24.64 ± 4.22 | 25.32 ± 4.57 | 23.37 ± 3.11 |
| MCV fL | 92.49 ± 11.22 | 93.7 ± 11.77 | 90.43 ± 9.87 | 101.38 ± 8.76 | 102.79 ± 9.31 | 98.44 ± 3.75 | 88.25 ± 3.75 | 86.47 ± 3.18 | 90.22 ± 3.32 | 82.73 ± 8.54 | 84.59 ± 8.06 | 79.26 ± 8.32 |
| Platelet k/μL | 341.69 ± 142.15 | 310.16 ± 123.8 | 395.04 ± 154.71 | 387.39 ± 141.79 | 368.86 ± 118.99 | 426.3 ± 128.23 | 273.37 ± 128.23 | 194.2 ± 43.11 | 361.33 ± 133.97 | 335.75 ± 127.39 | 304.54 ± 107.85 | 393.71 ± 139.99 |
| WBC k/μL | 10,042 ± 3741.03 | 9461 ± 3596 | 11,026 ± 3774 | 8922 ± 3228 | 8766 ± 3134 | 9250 ± 3049 | 10,205 ± 3049 | 9600 ± 2982 | 10,877 ± 2980 | 11,625 ± 4417 | 10,476 ± 4386 | 13,757 ± 3609 |
| Reticulo-cyte % | 11.04 ± 5.73 | 10.73 ± 5.61 | 11.56 ± 5.9 | 9.75 ± 5.6 | 10.15 ± 5.24 | 8.92 ± 5.24 | 12.41 ± 5.24 | 13.01 ± 6.25 | 11.76 ± 3.71 | 11.73 ± 5.96 | 9.92 ± 5.17 | 15.07 ± 5.87 |
| Total Bilirubin mg/dL | 3.42 ± 2.4 | 3.45 ± 2.73 | 3.38 ± 1.69 | 2.56 ± 1.52 | 2.51 ± 1.34 | 2.68 ± 3.19 | 4.61 ± 3.19 | 5.23 ± 4.03 | 3.91 ± 1.61 | 3.64 ± 2.07 | 3.61 ± 2.44 | 3.69 ± 1.12 |
| Ferritin ng/mL | 1251 ± 1744 | 1262 ± 1931 | 1230 ± 1249 | 526 ± 589 | 563 ± 653 | 401 ± 250 | 2752 ± 2258 | 3093 ± 2920 | 2374 ± 1013 | 717 ± 1156 | 929 ± 1300 | 167 ± 80 |
| LDH U/L | 451.65 ± 150.71 | 433.57 ± 161.02 | 482.27 ± 125.65 | 429.26 ± 139.62 | 416.5 ± 152.36 | 456.10 ± 103.03 | 442.68 ± 145.74 | 399.40 ± 141.56 | 490.8 ± 134.82 | 494.90 ± 162.43 | 487.46 ± 174.82 | 508.71 ± 135.40 |
| N = 5 | Average | Standard Deviation |
|---|---|---|
| Years on HU | 4.22 | 1.05 |
| WBC k/μL | 11.80 | 1.64 |
| Hemoglobin g/dL | 10.38 | 1.68 |
| Hematocrit % | 29.90 | 4.87 |
| MCV fL | 94.30 | 8.95 |
| Platelet k/μL | 407.50 | 90.93 |
| Reticulocyte % | 9.25 | 2.55 |
| Hemoglobin F% | 12.65 | 1.81 |
| Hemoglobin S% | 80.33 | 2.73 |
| Hemoglobin A2% | 4.17 | 0.79 |
| Total Bilirubin mg/dL | 2.55 | 0.50 |
| LDH U/L | 489.00 | 184.32 |
| Ferritin ng/mL | 313.00 | 136.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolu, S.S.; Wang, K.; Yan, Z.; Zhang, S.; Roberts, K.; Crouch, A.S.; Sebastian, G.; Chaitowitz, M.; Fornari, E.D.; Schwechter, E.M.; et al. Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells. Cells 2020, 9, 2159. https://doi.org/10.3390/cells9102159
Tolu SS, Wang K, Yan Z, Zhang S, Roberts K, Crouch AS, Sebastian G, Chaitowitz M, Fornari ED, Schwechter EM, et al. Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells. Cells. 2020; 9(10):2159. https://doi.org/10.3390/cells9102159
Chicago/Turabian StyleTolu, Seda S., Kai Wang, Zi Yan, Shouping Zhang, Karl Roberts, Andrew S. Crouch, Gracy Sebastian, Mark Chaitowitz, Eric D. Fornari, Evan M. Schwechter, and et al. 2020. "Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells" Cells 9, no. 10: 2159. https://doi.org/10.3390/cells9102159
APA StyleTolu, S. S., Wang, K., Yan, Z., Zhang, S., Roberts, K., Crouch, A. S., Sebastian, G., Chaitowitz, M., Fornari, E. D., Schwechter, E. M., Uehlinger, J., Manwani, D., Minniti, C. P., & Bouhassira, E. E. (2020). Characterization of Hematopoiesis in Sickle Cell Disease by Prospective Isolation of Stem and Progenitor Cells. Cells, 9(10), 2159. https://doi.org/10.3390/cells9102159