Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Decellularization Protocol for Healthy and Cirrhotic Human 3D Liver Scaffolds
2.2. Scanning Electron Microscopy (SEM)
2.3. Second Harmonic Generation (SHG) and Imaging
2.4. Atomic Force Microscopy (AFM)
2.5. Collagen Proportionate Area Analysis
2.6. Proteomic Analysis
2.7. Bioengineering
2.8. Gene Expression
2.9. Protein Synthesis and Secretion
2.10. Statistical Analysis
3. Results
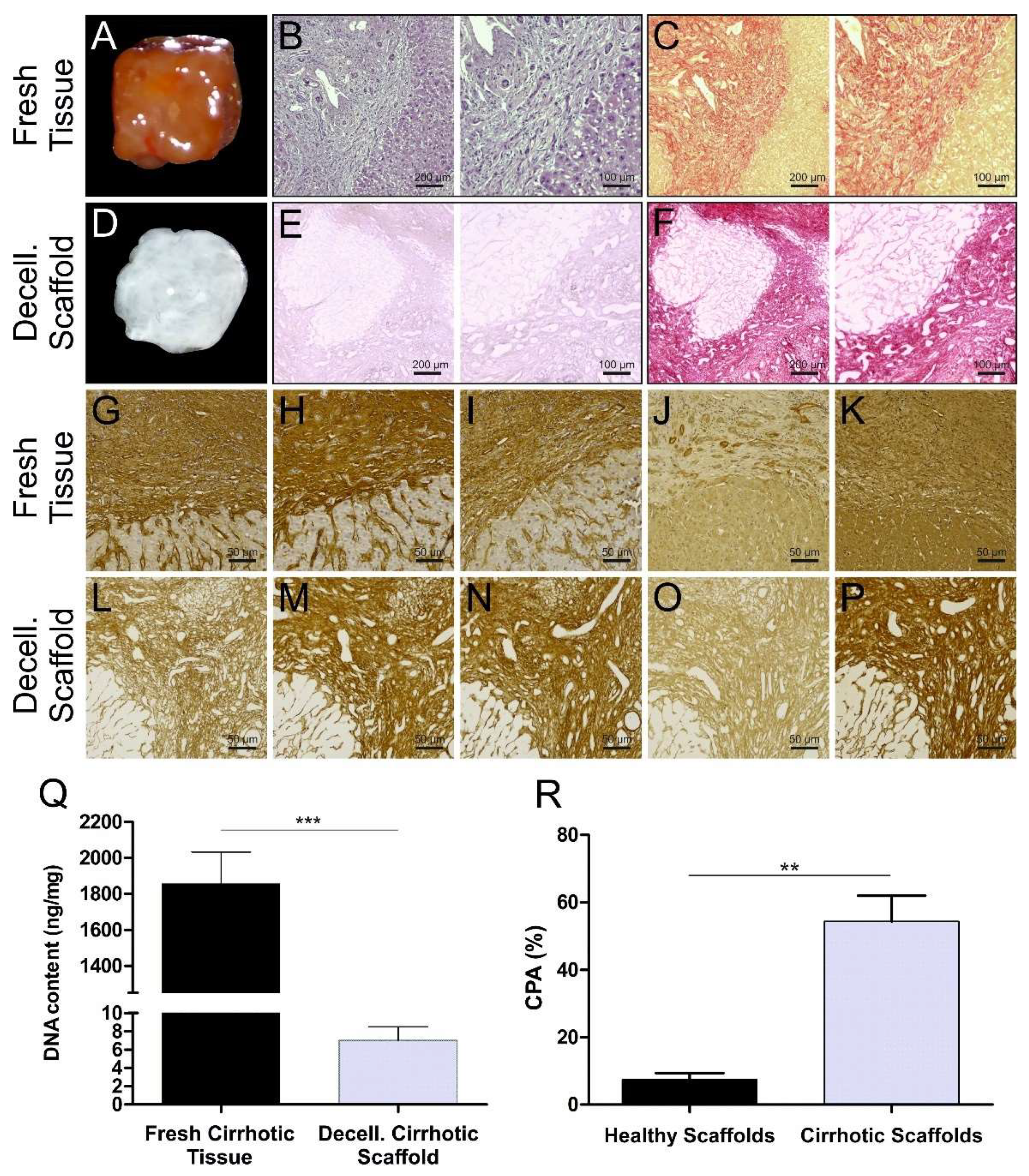
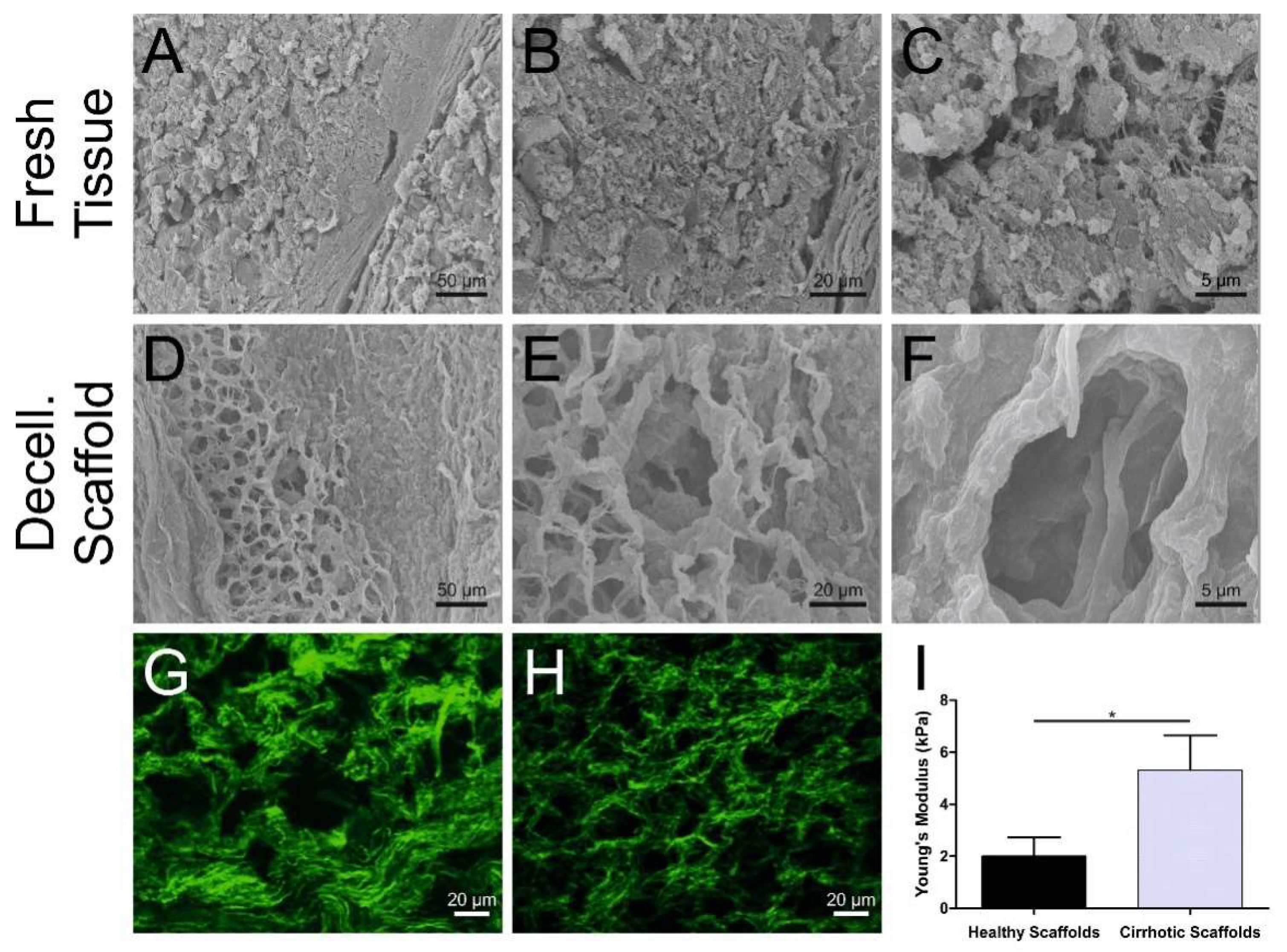
3.1. Cirrhotic Liver Tissue Scaffold Characterization
3.2. Proteomic Analysis of Healthy and Cirrhotic 3D Liver Scaffolds
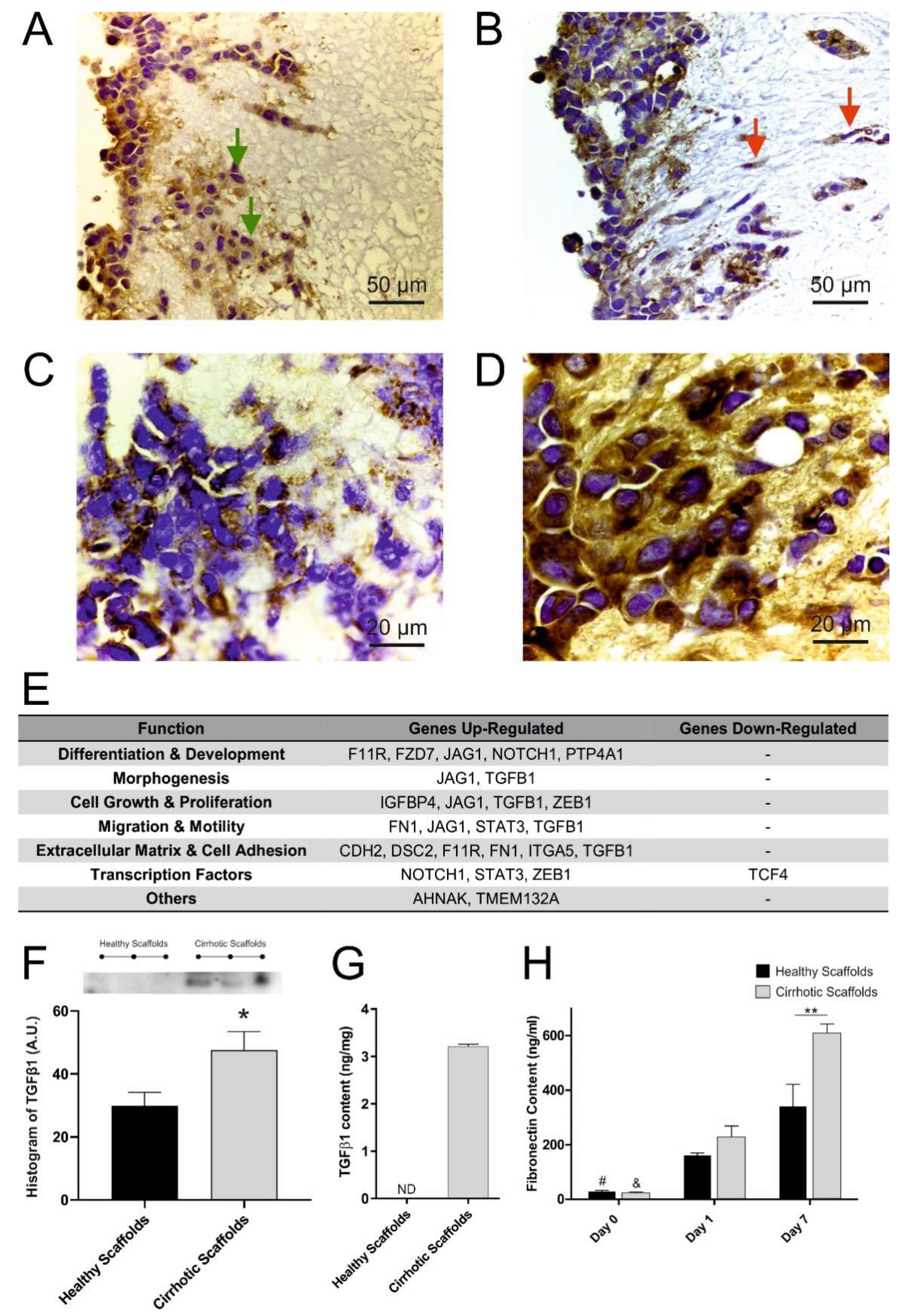
3.3. Cirrhotic ECM Drives EMT Induction
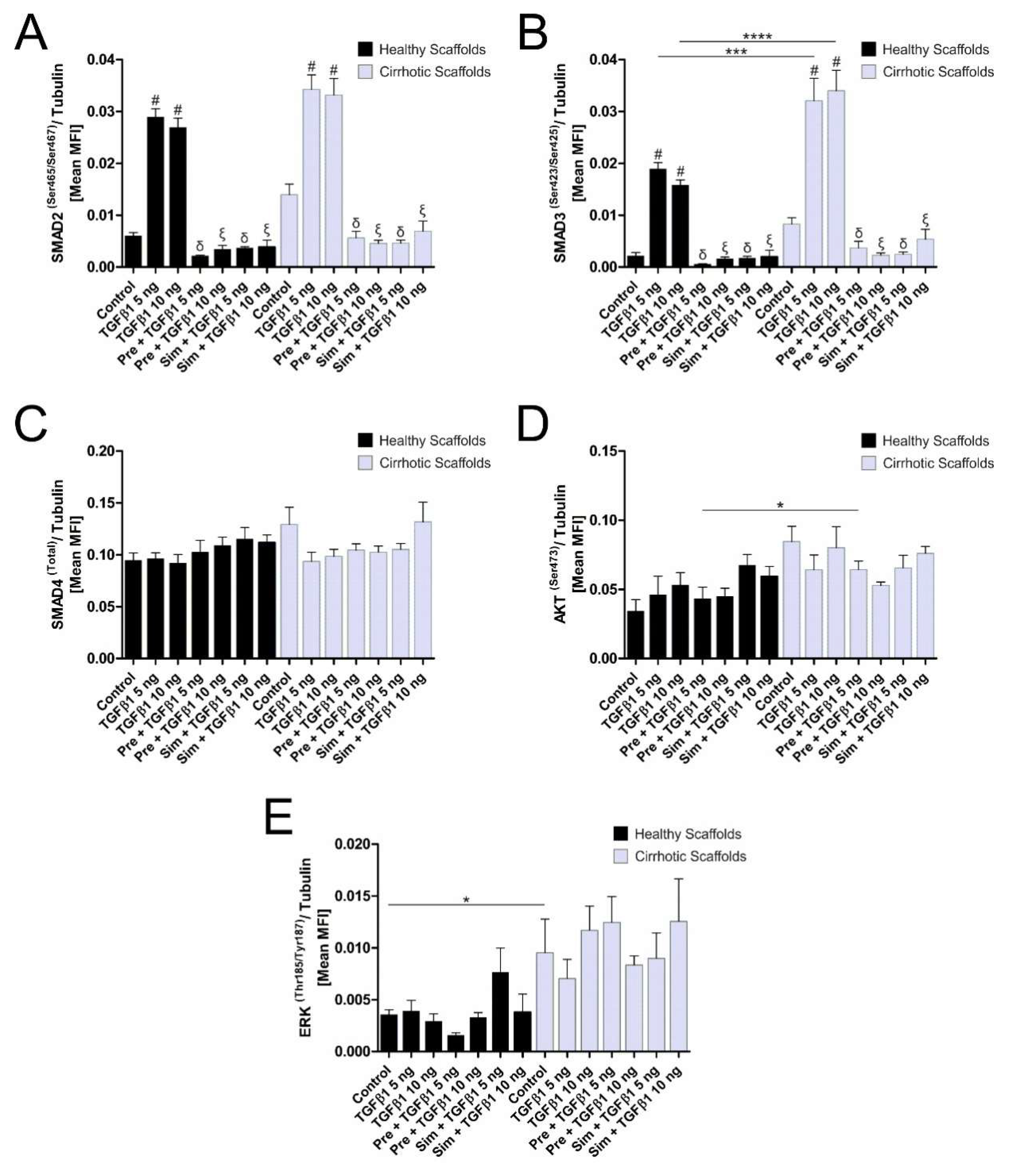
3.4. ECM Composition Affects TGFβ1-Induced Phosphorylation of Smad2/Smad3 Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.R.; Brugge, J.S. A stiff blow from the stroma: Collagen crosslinking drives tumor progression. Cancer Cell 2009, 16, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chen, C.S. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef]
- Freires, I.A.; Sardi, J.C.; de Castro, R.D.; Rosalen, P.L. Alternative Animal and Non-Animal Models for Drug Discovery and Development: Bonus or Burden? Pharm. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shanks, N.; Greek, R. Experimental use of nonhuman primates is not a simple problem. Nat. Med. 2008, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2013. [Google Scholar] [CrossRef]
- Carloni, V.; Luong, T.V.; Rombouts, K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: More complicated than ever. Liver Int. 2014, 34, 834–843. [Google Scholar] [CrossRef]
- Giannelli, G.; Rani, B.; Dituri, F.; Cao, Y.; Palasciano, G. Moving towards personalised therapy in patients with hepatocellular carcinoma: The role of the microenvironment. Gut 2014, 63, 1668–1676. [Google Scholar] [CrossRef]
- Nault, J.C.; Galle, P.R.; Marquardt, J.U. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J. Hepatol. 2018. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.E.; Henderson, J.M.; Gorrell, M.D. Animal models for hepatocellular carcinoma. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 993–1002. [Google Scholar] [CrossRef]
- Budi, E.H.; Duan, D.; Derynck, R. Transforming Growth Factor-beta Receptors and Smads: Regulatory Complexity and Functional Versatility. Trends Cell Biol. 2017, 27, 658–672. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Telese, A.; Longato, L.; Urbani, L.; Robinson, B.; Hall, A.; Kong, K.; Frenguelli, L.; Marrone, G.; et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci. Rep. 2017, 7, 5534. [Google Scholar] [CrossRef]
- Calvaruso, V.; Burroughs, A.K.; Standish, R.; Manousou, P.; Grillo, F.; Leandro, G.; Maimone, S.; Pleguezuelo, M.; Xirouchakis, I.; Guerrini, G.P.; et al. Computer-assisted image analysis of liver collagen: Relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology 2009, 49, 1236–1244. [Google Scholar] [CrossRef]
- Li, Q.; Uygun, B.E.; Geerts, S.; Ozer, S.; Scalf, M.; Gilpin, S.E.; Ott, H.C.; Yarmush, M.L.; Smith, L.M.; Welham, N.V.; et al. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials 2016, 75, 37–46. [Google Scholar] [CrossRef]
- Longato, L.; Andreola, F.; Davies, S.S.; Roberts, J.L.; Fusai, G.; Pinzani, M.; Moore, K.; Rombouts, K. Reactive gamma-ketoaldehydes as novel activators of hepatic stellate cells in vitro. Free Radic. Biol. Med. 2017, 102, 162–173. [Google Scholar] [CrossRef]
- Marrone, G.; De Chiara, F.; Bottcher, K.; Levi, A.; Dhar, D.; Longato, L.; Mazza, G.; Zhang, Z.; Marrali, M.; Iglesias, A.F.; et al. The AMPK-v-ATPase-pH axis: A key regulator of the pro-fibrogenic phenotype of human hepatic stellate cells. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Bager, C.L.; Gudmann, N.; Willumsen, N.; Leeming, D.J.; Karsdal, M.A.; Bay-Jensen, A.C.; Hogdall, E.; Balslev, I.; He, Y. Quantification of fibronectin as a method to assess ex vivo extracellular matrix remodeling. Biochem. Biophys. Res. Commun. 2016, 478, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Griggs, L.A.; Hassan, N.T.; Malik, R.S.; Griffin, B.P.; Martinez, B.A.; Elmore, L.W.; Lemmon, C.A. Fibronectin fibrils regulate TGF-beta1-induced Epithelial-Mesenchymal Transition. Matrix Biol. 2017, 60–61, 157–175. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Kim, Y.; Sohn, A.; Yeo, I.; Yu, S.J.; Yoon, J.H.; Park, T.; Kim, Y. Serum fibronectin distinguishes the early stages of hepatocellular carcinoma. Sci. Rep. 2017, 7, 9449. [Google Scholar] [CrossRef]
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef]
- Kelley, R.K.; Gane, E.; Assenat, E.; Siebler, J.; Galle, P.R.; Merle, P.; Hourmand, I.O.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; et al. A Phase 2 Study of Galunisertib (TGF-beta1 Receptor Type I Inhibitor) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00056. [Google Scholar] [CrossRef]
- Howlett, A.R.; Bissell, M.J. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993, 2, 79–89. [Google Scholar] [PubMed]
- Bhat, R.; Bissell, M.J. Of plasticity and specificity: Dialectics of the micro- and macro-environment and the organ phenotype. Wiley Interdiscip. Rev. Membr. Transp. Signal 2014, 3, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Manon-Jensen, T.; Genovese, F.; Kristensen, J.H.; Nielsen, M.J.; Sand, J.M.; Hansen, N.U.; Bay-Jensen, A.C.; Bager, C.L.; Krag, A.; et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. 2015, 308, G807–G830. [Google Scholar] [CrossRef] [PubMed]
- Gissen, P.; Arias, I.M. Structural and functional hepatocyte polarity and liver disease. J. Hepatol. 2015, 63, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Dituri, F.; Mancarella, S.; Cigliano, A.; Chieti, A.; Giannelli, G. TGF-β as Multifaceted Orchestrator in HCC Progression: Signaling, EMT, Immune Microenvironment, and Novel Therapeutic Perspectives. Semin. Liver Dis. 2019, 39, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Kramer, K.; Sindhi, N.; Sarkar, P.; Upton, M.; Kalluri, R. De-differentiation of primary human hepatocytes depends on the composition of specialized liver basement membrane. Mol. Cell. Biochem. 2006, 283, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef]

| Gene Name | p-Value a = 10−5, b = 10−4, c = 10−3, d = 10−2 | Gene Name | p-Value a = 10−5, b = 10−4, c = 10−3, d = 10−2 | Gene Name | p-Value a = 10−5, b = 10−4, c = 10−3, d = 10−2 |
|---|---|---|---|---|---|
| FGG | 4.9 × 10 a | NUDC | 1.4 × 10 d | THNSL1 | 3.0 × 10 d |
| FKBP4 | 6.1 × 10 a | HSP90AA1 | 1.6 × 10 d | SEPHS2 | 3.0 × 10 d |
| COL6A6 | 1.4 × 10 b | CSNK1A1 | 1.7 × 10 d | BDH1 | 3.0 × 10 d |
| NECAP2 | 5.9 × 10 b | RBM39 | 1.7 × 10 d | COL12A1 | 3.1 × 10 d |
| ECM1 | 7.2 × 10 b | SEC24C | 1.7 × 10 d | PSMD2 | 3.1 × 10 d |
| SCYL2 | 9.1 × 10 b | MMAB | 1.7 × 10 d | PAPSS2 | 3.1 × 10 d |
| FGB | 9.1 × 10 b | FKBP5 | 1.7 × 10 d | MARS | 3.2 × 10 d |
| CCT4 | 9.9 × 10 b | ARAF | 1.7 × 10 d | SEC31A | 3.2 × 10 d |
| GSPT1 | 1.1 × 10 c | GNE | 1.8 × 10 d | LGMN | 3.3 × 10 d |
| HUWE1 | 1.1 × 10 c | CCT3 | 1.8 × 10 d | HSPA4L | 3.3 × 10 d |
| HSPA4 | 1.2 × 10 c | SAR1A | 1.8 × 10 d | EPRS | 3.3 × 10 d |
| SEC24B | 1.6 × 10 c | SDSL | 1.9 × 10 d | CHORDC1 | 3.4 × 10 d |
| HSPD1 | 1.8 × 10 c | LUM | 1.9 × 10 d | FGL1 | 3.6 × 10 d |
| LONP1 | 1.9 × 10 c | NADK2 | 2.0 × 10 d | COL6A1 | 3.6 × 10 d |
| PSMD11 | 2.8 × 10 c | COASY | 2.0 × 10 d | TRAP1 | 3.6 × 10 d |
| BGN | 3.5 × 10 c | EIF4G2 | 2.0 × 10 d | HNRNPK | 3.7 × 10 d |
| TCP1 | 3.5 × 10 c | FN1 | 2.0 × 10 d | CBS | 3.8 × 10 d |
| UBR4 | 3.7 × 10 c | ACAD11 | 2.1 × 10 d | COPG1 | 3.8 × 10 d |
| CCT2 | 4.3 × 10 c | APPL1 | 2.1 × 10 d | RECQL | 3.9 × 10 d |
| AASS | 4.4 × 10 c | EIF2B5 | 2.2 × 10 d | MON2 | 3.9 × 10 d |
| RAB2A | 4.6 × 10 c | GALT | 2.3 × 10 d | COL6A2 | 3.9 × 10 d |
| CCT7 | 5.0 × 10 c | EIF4G1 | 2.3 × 10 d | TOLLIP | 3.9 × 10 d |
| POLDIP2 | 5.3 × 10 c | TPD52L1 | 2.4 × 10 d | NAGS | 4.0 × 10 d |
| EEF1G | 5.9 × 10 c | SERPINF2 | 2.4 × 10 d | CHST13 | 4.3 × 10 d |
| EIF3D | 7.9 × 10 c | RRAGC | 2.4 × 10 d | TAGLN2 | 4.5 × 10 d |
| FGA | 1.0 × 10 d | PCYT2 | 2.5 × 10 d | SEC23B | 4.6 × 10 d |
| CYB5R1 | 1.0 × 10 d | GYS2 | 2.5 × 10 d | SRP72 | 4.6 × 10 d |
| PRB1 | 1.1 × 10 d | AHSA1 | 2.5 × 10 d | IDH3A | 4.7 × 10 d |
| EIF2S3 | 1.1 × 10 d | CTSZ | 2.6 × 10 d | HSP90AB1 | 4.7 × 10 d |
| MLYCD | 1.2 × 10 d | DARS | 2.6 × 10 d | PRMT1 | 4.8 × 10 d |
| USP9X | 1.2 × 10 d | DDX21 | 2.7 × 10 d | SPRYD4 | 4.8 × 10 d |
| TFG | 1.3 × 10 d | NAMPT | 2.9 × 10 d | ACAD9 | 4.8 × 10 d |
| TUBB2B | 1.4 × 10 d | NEDD4 | 2.9 × 10 d | DCXR | 4.8 × 10 d |
| BZW1 | 1.4 × 10 d | FTL | 3.0 × 10 d |
| Gene Name | p-Valuea a = 10−5, b = 10−4, c = 10−3, d = 10−2 | Gene Name | p-Valuea a = 10−5, b = 10−4, c = 10−3, d = 10−2 | Gene Name | p-Valuea a = 10−5, b = 10−4, c = 10−3, d = 10−2 |
|---|---|---|---|---|---|
| IGKV3-11 | 2.1 × 10 a | MYO1C | 1.2 × 10 d | SDHA | 3.2 × 10 d |
| BST1 | 1.3 × 10 b | GPX3 | 1.3 × 10 d | IGKV1D-33 | 3.3 × 10 d |
| IGKV2D-28 | 3.5 × 10 b | CTSS | 1.4 × 10 d | FLNA | 3.4 × 10 d |
| NDUFB3 | 6.2 × 10 b | SEC22B | 1.7 × 10 d | LAMA2 | 3.4 × 10 d |
| DNAJB9 | 8.2 × 10 b | MYOF | 1.7 × 10 d | CD47 | 3.4 × 10 d |
| FHL2 | 1.4 × 10 c | ADH1B | 1.8 × 10 d | EHD2 | 3.4 × 10 d |
| TMEM43 | 1.4 × 10 c | FBLN1 | 1.9 × 10 d | CHST4 | 3.5 × 10 d |
| EFEMP1 | 1.6 × 10 c | TBL2 | 1.9 × 10 d | EMILIN1 | 3.6 × 10 d |
| LXN | 1.6 × 10 c | H3F3B | 2.0 × 10 d | NCKAP1 | 3.7 × 10 d |
| IGKV1-5 | 1.7 × 10 c | RRAS | 2.0 × 10 d | NOP56 | 3.7 × 10 d |
| HSD17B12 | 2.6 × 10 c | VCAN | 2.0 × 10 d | COL5A1 | 3.7 × 10 d |
| CLTC | 4.3 × 10 c | HNRNPM | 2.3 × 10 d | ADH1A | 3.7 × 10 d |
| C1QB | 4.9 × 10 c | IGKV3-20 | 2.4 × 10 d | IGLC3 | 3.9 × 10 d |
| IGHG1 | 5.1 × 10 c | TGFB1I1 | 2.4 × 10 d | TNS3 | 4.0 × 10 d |
| CTSG | 5.5 × 10 c | SERPINA1 | 2.5 × 10 d | GNB1 | 4.0 × 10 d |
| MFAP4 | 6.7 × 10 c | GNAI2 | 2.6 × 10 d | PKD1L1 | 4.1 × 10 d |
| IGKV3-15 | 8.4 × 10 c | VDAC2 | 2.7 × 10 d | CAV1 | 4.4 × 10 d |
| FBLN5 | 8.7 × 10 c | FBN1 | 2.8 × 10 d | MEMO1 | 4.4 × 10 d |
| IGFBP7 | 9.2 × 10 c | LTBP1 | 2.8 × 10 d | HBB | 4.4 × 10 d |
| TRAM1 | 1.0 × 10 d | APOC3 | 2.8 × 10 d | LGALS3 | 4.5 × 10 d |
| HLA-DRA | 1.0 × 10 d | FLOT1 | 2.9 × 10 d | LTBP4 | 4.6 × 10 d |
| S100A9 | 1.0 × 10 d | THSD4 | 3.1 × 10 d | RPL10 | 4.7 × 10 d |
| FLOT2 | 1.0 × 10 d | PDLIM7 | 3.2 × 10 d | AKR7L | 4.8 × 10 d |
| LOXL1 | 1.1 × 10 d | FBLN2 | 3.2 × 10 d | COL10A1 | 4.9 × 10 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, G.; Telese, A.; Al-Akkad, W.; Frenguelli, L.; Levi, A.; Marrali, M.; Longato, L.; Thanapirom, K.; Vilia, M.G.; Lombardi, B.; et al. Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition. Cells 2020, 9, 83. https://doi.org/10.3390/cells9010083
Mazza G, Telese A, Al-Akkad W, Frenguelli L, Levi A, Marrali M, Longato L, Thanapirom K, Vilia MG, Lombardi B, et al. Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition. Cells. 2020; 9(1):83. https://doi.org/10.3390/cells9010083
Chicago/Turabian StyleMazza, Giuseppe, Andrea Telese, Walid Al-Akkad, Luca Frenguelli, Ana Levi, Martina Marrali, Lisa Longato, Kessarin Thanapirom, Maria Giovanna Vilia, Benedetta Lombardi, and et al. 2020. "Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition" Cells 9, no. 1: 83. https://doi.org/10.3390/cells9010083
APA StyleMazza, G., Telese, A., Al-Akkad, W., Frenguelli, L., Levi, A., Marrali, M., Longato, L., Thanapirom, K., Vilia, M. G., Lombardi, B., Crowley, C., Crawford, M., Karsdal, M. A., Leeming, D. J., Marrone, G., Bottcher, K., Robinson, B., Del Rio Hernandez, A., Tamburrino, D., ... Rombouts, K. (2020). Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-β1 Epithelial Mesenchymal Transition. Cells, 9(1), 83. https://doi.org/10.3390/cells9010083








