Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Hematopoietic Cells
2.3. Enumeration of Hematopoietic Cells
2.4. Mobilization
2.5. Albumin Quantification
2.6. rhG-CSF Quantification
2.7. Statistics
3. Results
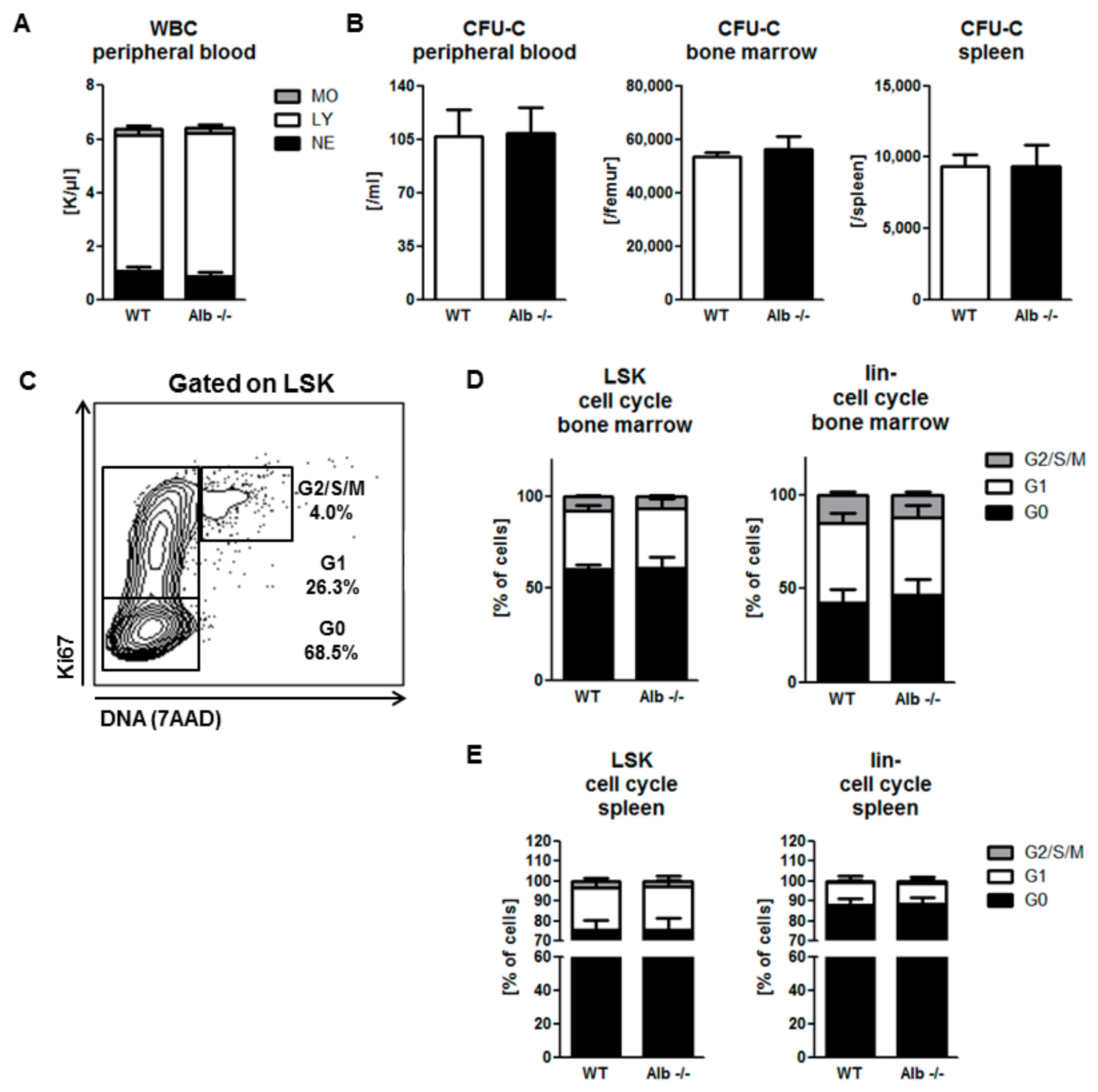
3.1. Homeostatic Hematopoiesis is Unaffected by Albumin Deficiency
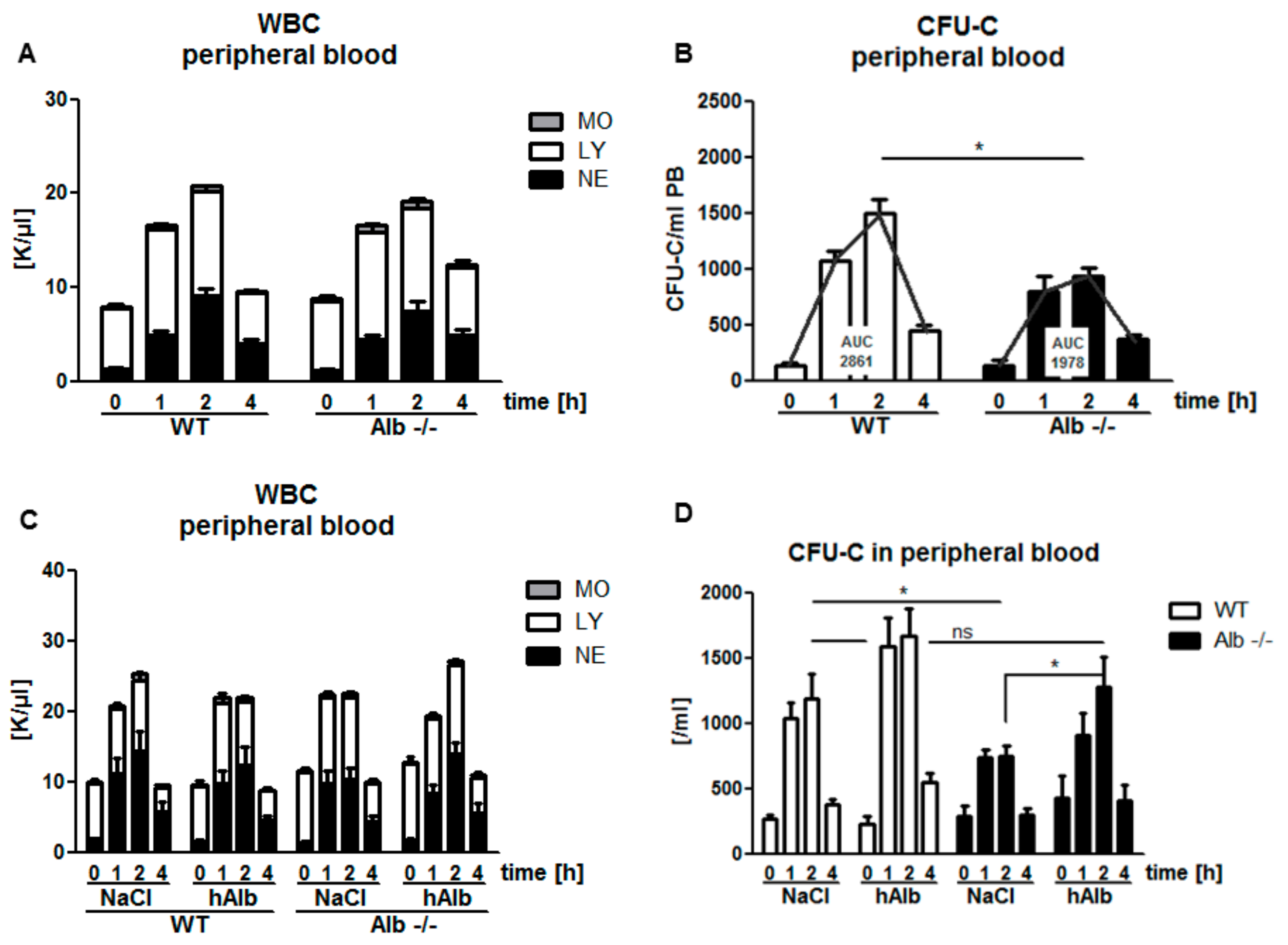
3.2. Role of Albumin in the Pharmacodynamics of the Small-Molecule CXCR4 Antagonist AMD3100
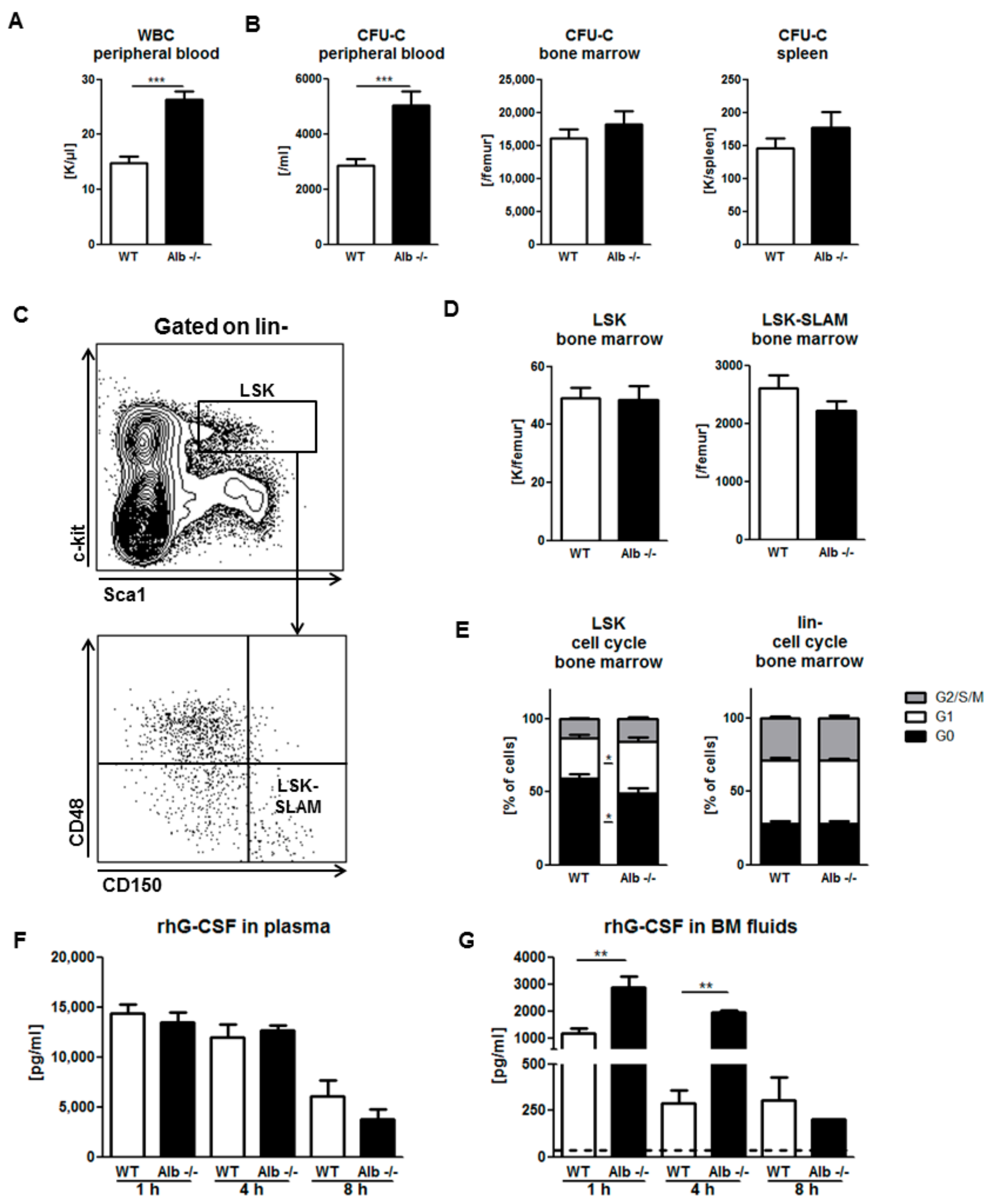
3.3. Role of Albumin in G-CSF-Induced Mobilization
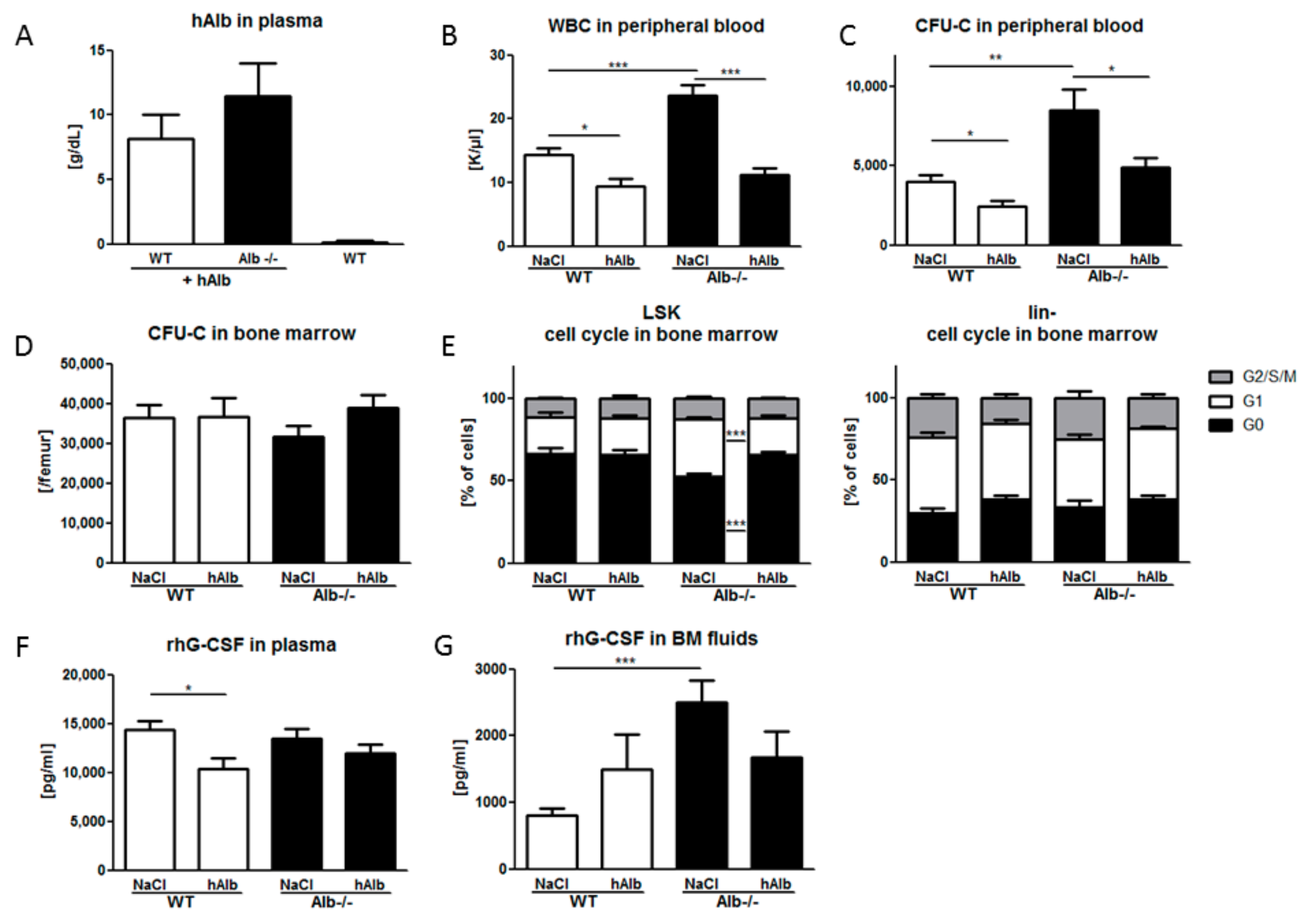
3.4. Human Albumin Substitution in the G-CSF Mobilization Setting
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.T. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic press: San Diego, CA, USA, 1995. [Google Scholar]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Meloun, B.; Moravek, L.; Kostka, V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975, 58, 134–137. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Bern, M.; Sand, K.M.; Nilsen, J.; Sandlie, I.; Andersen, J.T. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J. Control. Release. 2015, 211, 144–162. [Google Scholar] [CrossRef]
- Zhivkova, Z.D. Studies on drug-human serum albumin binding: The current state of the matter. Curr Pharm. Des. 2015, 21, 1817–1830. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Low, B.E.; Christianson, G.J.; Proetzel, G.; Sproule, T.J.; Wiles, M.V. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs 2015, 7, 344–351. [Google Scholar] [CrossRef]
- Koot, B.G.; Houwen, R.; Pot, D.J.; Nauta, J. Congenital analbuminaemia: Biochemical and clinical implications. A case report and literature review. Eur. J. Pediatr. 2004, 163, 664–670. [Google Scholar] [CrossRef]
- Bonig, H.; Papayannopoulou, T. Mobilization of hematopoietic stem/progenitor cells: General principles and molecular mechanisms. Methods Mol. Biol. 2012, 904, 1–14. [Google Scholar]
- Bonig, H.; Watts, K.L.; Chang, K.H.; Kiem, H.P.; Papayannopoulou, T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells 2009, 27, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Karpova, D.; Bonig, H. Concise Review: CXCR4/CXCL12 Signaling in Immature Hematopoiesis—Lessons From Pharmacological and Genetic Models. Stem Cells 2015, 33, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Holig, K. G-CSF in Healthy Allogeneic Stem Cell Donors. Transfus. Med. Hemother. 2013, 40, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.S.; Cameron, D.A.; Pettengell, R.; Bohlius, J.; Crawford, J.; Ellis, M.; Kearney, N.; Lyman, G.H.; Tjan-Heijnen, V.C.; Walewski, J.; et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur. J. Cancer 2006, 42, 2433–2453. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Nicola, N.A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J. Cell Physiol. 1983, 116, 198–206. [Google Scholar] [CrossRef]
- Morrison, S.J.; Wright, D.E.; Weissman, I.L. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl. Acad. Sci. USA 1997, 94, 1908–1913. [Google Scholar] [CrossRef]
- Wright, D.E.; Cheshier, S.H.; Wagers, A.J.; Randall, T.D.; Christensen, J.L.; Weissman, I.L. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood 2001, 97, 2278–2285. [Google Scholar] [CrossRef]
- Lapid, K.; Glait-Santar, C.; Gur-Cohen, S.; Canaani, J.; Kollet, O.; Lapidot, T. Egress and Mobilization of Hematopoietic Stem and Progenitor Cells: A Dynamic Multi-facet Process. Available online: https://www.stembook.org (accessed on 16 December 2019).
- Bonig, H.; Papayannopoulou, T. Hematopoietic stem cell mobilization: Updated conceptual renditions. Leukemia 2013, 27, 24–31. [Google Scholar] [CrossRef]
- Klein, G.; Schmal, O.; Aicher, W.K. Matrix metalloproteinases in stem cell mobilization. Matrix Biol. 2015, 44–46, 175–183. [Google Scholar] [CrossRef]
- Christopher, M.J.; Liu, F.; Hilton, M.J.; Long, F.; Link, D.C. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood 2009, 114, 1331–1339. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; David, T.S.; Avi, M.; Grigori, N.; Grigori, N.E.; et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Levesque, J.P. Mechanisms of hematopoietic stem cell mobilization: When innate immunity assails the cells that make blood and bone. Exp. Hematol. 2006, 34, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Poursine-Laurent, J.; Link, D.C. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not flt-3 ligand. Blood 1997, 90, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Orschell, C.M.; Clapp, D.W.; Hangoc, G.; Cooper, S.; Plett, P.A.; Liles, W.C.; Li, X.; Graham-Evans, B.; Timothy, B.C.; et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005, 201, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, C.W.; Flexner, C.; MacFarland, R.T.; Giandomenico, C.; Fuchs, E.J.; Redpath, E.; Bridger, G.; Henson, G.W. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Chemother. 2000, 44, 1667–1673. [Google Scholar] [CrossRef]
- Stute, N.; Santana, V.M.; Rodman, J.H.; Schell, M.J.; Ihle, J.N.; Evans, W.E. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood 1992, 79, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Sturgill, M.G.; Huhn, R.D.; Drachtman, R.A.; Ettinger, A.G.; Ettinger, L.J. Pharmacokinetics of intravenous recombinant human granulocyte colony-stimulating factor (rhG-CSF) in children receiving myelosuppressive cancer chemotherapy: Clearance increases in relation to absolute neutrophil count with repeated dosing. Am. J. Hematol. 1997, 54, 124–130. [Google Scholar] [CrossRef]
- EMEA. CHMP Assessment Report for Mozobil 2009. Available online: https://www.ema.europa.eu/en/documents/assessment-report/mozobil-epar-public-assessment-report_en.pdf (accessed on 16 December 2019).
- Bonig, H.; Priestley, G.V.; Oehler, V.; Papayannopoulou, T. Hematopoietic progenitor cells (HPC) from mobilized peripheral blood display enhanced migration and marrow homing compared to steady-state bone marrow HPC. Exp. Hematol. 2007, 35, 326–334. [Google Scholar] [CrossRef]
- Winkler, I.G.; Wiercinska, E.; Barbier, V.; Nowlan, B.; Bonig, H.; Levesque, J.P. Mobilization of hematopoietic stem cells with highest self-renewal by G-CSF precedes clonogenic cell mobilization peak. Exp. Hematol. 2016, 44, 303–314. [Google Scholar] [CrossRef]
- Bonig, H.; Priestley, G.V.; Nilsson, L.M.; Jiang, Y.; Papayannopoulou, T. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood 2004, 104, 2299–2306. [Google Scholar] [CrossRef]
- Rosenkilde, M.M.; Gerlach, L.O.; Jakobsen, J.S.; Skerlj, R.T.; Bridger, G.J.; Schwartz, T.W. Molecular mechanism of AMD3100 antagonism in the CXCR4 receptor: Transfer of binding site to the CXCR3 receptor. J. Biol. Chem. 2004, 279, 3033–3041. [Google Scholar] [CrossRef]
- Merlin, E.; Piguet, C.; Auvrignon, A.; Rubie, H.; Demeocq, F.; Kanold, J. The pros and cons of split-dose granulocyte colony-stimulating factor alone rather than a single high dose for hematopoietic progenitor cell mobilization in small children (<15 kg) with solid tumors. Haematologica 2006, 91, 1004–1005. [Google Scholar]
- Carrion, R.; Serrano, D.; Gomez-Pineda, A.; Diez-Martin, J.L. A randomised study of 10 microg/kg/day (single dose) vs 2 x 5 microg/kg/day (split dose) G-CSF as stem cell mobilisation regimen in high-risk breast cancer patients. Bone Marrow Transplant. 2003, 32, 563–567. [Google Scholar] [CrossRef][Green Version]
- Van Der Auwera, P.; Platzer, E.; Xu, Z.X.; Schulz, R.; Feugeas, O.; Capdeville, R.; David, J.E. Pharmacodynamics and pharmacokinetics of single doses of subcutaneous pegylated human G-CSF mutant (Ro 25-8315) in healthy volunteers: Comparison with single and multiple daily doses of filgrastim. Am. J. Hematol. 2001, 66, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kaneko, T. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor in the rat. Single and multiple dosing studies. Drug Metab. Dispos. 1991, 19, 200–204. [Google Scholar] [PubMed]
- Kroger, N.; Renges, H.; Kruger, W.; Gutensohn, K.; Loliger, C.; Carrero, I.; Lourdes, C.; Axel, R.Z. A randomized comparison of once versus twice daily recombinant human granulocyte colony-stimulating factor (filgrastim) for stem cell mobilization in healthy donors for allogeneic transplantation. Br. J. Haematol. 2000, 111, 761–765. [Google Scholar] [PubMed]
- Tanaka, H.; Satake-Ishikawa, R.; Ishikawa, M.; Matsuki, S.; Asano, K. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor conjugated to polyethylene glycol in rats. Cancer Res. 1991, 51, 3710–3714. [Google Scholar]
- Kim, M.G.; Han, N.; Lee, E.K.; Kim, T. Pegfilgrastim vs filgrastim in PBSC mobilization for autologous hematopoietic SCT: A systematic review and meta-analysis. Bone Marrow Transplant. 2015, 50, 523–530. [Google Scholar] [CrossRef]
- Do, B.H.; Kang, H.J.; Song, J.A.; Nguyen, M.T.; Park, S.; Yoo, J.; Do, B.H.; Kang, H.J.; Song, J.A.; Nguyen, M.T.; et al. Granulocyte colony-stimulating factor (GCSF) fused with Fc Domain produced from E. coli is less effective than Polyethylene Glycol-conjugated GCSF. Sci Rep. 2017, 7, 6480. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Tian, H.; Chen, X.; Cai, D.; Yao, W.; Gao, X. Extending the serum half-life of G-CSF via fusion with the domain III of human serum albumin. Biomed. Res. Int. 2013, 2013, 107238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Wen, X.F.; Yang, Z.Y.; Wu, Y.L.; Lu, Y.; Zhou, L.F. Development and characterization of a novel fusion protein of a mutated granulocyte colony-stimulating factor and human serum albumin in Pichia pastoris. PLoS ONE 2014, 9, e115840. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.C.; Guttman, D.E. The binding of drugs by plasma proteins. J. Pharm. Sci. 1968, 57, 895–918. [Google Scholar] [CrossRef] [PubMed]
- Vallner, J.J. Binding of drugs by albumin and plasma protein. J. Pharm. Sci. 1977, 66, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Gonzalez, D.; Derendorf, H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2010, 99, 1107–1122. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danner, E.; Bonig, H.; Wiercinska, E. Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice. Cells 2020, 9, 4. https://doi.org/10.3390/cells9010004
Danner E, Bonig H, Wiercinska E. Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice. Cells. 2020; 9(1):4. https://doi.org/10.3390/cells9010004
Chicago/Turabian StyleDanner, Eva, Halvard Bonig, and Eliza Wiercinska. 2020. "Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice" Cells 9, no. 1: 4. https://doi.org/10.3390/cells9010004
APA StyleDanner, E., Bonig, H., & Wiercinska, E. (2020). Albumin Modifies Responses to Hematopoietic Stem Cell Mobilizing Agents in Mice. Cells, 9(1), 4. https://doi.org/10.3390/cells9010004




