Abstract
Tumor necrosis factor receptor 2 (TNFR2) is expressed on some tumor cells, such as myeloma, Hodgkin lymphoma, colon cancer and ovarian cancer, as well as immunosuppressive cells. There is increasingly evidence that TNFR2 expression in cancer microenvironment has significant implications in cancer progression, metastasis and immune evasion. Although nanomedicine has been extensively studied as a carrier of cancer immunotherapeutic agents, no study to date has investigated TNFR2-targeting nanomedicine in cancer treatment. From an epigenetic perspective, previous studies indicate that DNA demethylation might be responsible for high expressions of TNFR2 in cancer models. This perspective review discusses a novel therapeutic strategy based on nanomedicine that has the capacity to target TNFR2 along with inhibition of DNA demethylation. This approach may maximize the anti-cancer potential of nanomedicine-based immunotherapy and, consequently, markedly improve the outcomes of the management of patients with malignancy.
1. Introduction
Tumor necrosis factor (TNF) was first described in the early 1960s as its originally discovered role during tumor regression in rodents [1]. After its initial discovery, many research groups have studied the implications of TNF interactions with its receptors 1 and 2 (TNFR1 and TNFR2) in various inflammatory conditions [2,3]. As inflammation plays a key role in cancer progression and the microenvironment of cancer is controlled by inflammatory cells [4], previous studies have explored TNF-TNFRs interactions in cancer proliferation, metastasis and immune evasion [5]. These studies concluded that TNF works as a tumor-suppressive cytokine through interaction with TNFR1, which is expressed on both normal and tumor cells [6,7,8]. In contrast, TNFR2 expression was only reported on tumor cells and suppressive immune cells, suggesting that TNFR2 directly promotes cancer development [9]. Furthermore, the latest preclinical and clinical studies confirmed the implication of TNF-TNFR2 signaling in cancer proliferation and the suppression of immune response [10,11]. Consequently, blockade of TNF-TNFR2 axis could be a promising option for cancer treatment (Figure 1).
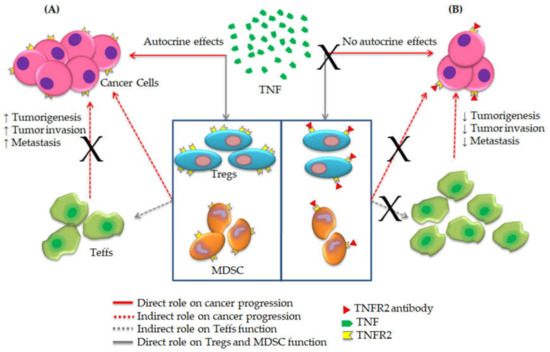
Figure 1.
The blockade mechanism of TNF-TNFR2 axis towards effective cancer immunotherapy. (A) TNFR2 is expressed on cancer cells and suppressive immune cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Binding of TNF to TNFR2 enhances cancer proliferation and immune evasion by expansion of Tregs and MDSCs along with the suppression of the immune response of effector T cells (Teffs). (B) Antibody blockade of TNFR2 inverts this mechanism and promotes effector functions against cancer (Amended from Al-Hatamleh et al., 2019 [16]).
On the other hand, nanotechnology provided several platforms in the field of immunotherapy and made significant advances towards safe and efficient targeting systems with positive clinical outcomes and minimal side effects; this is the so-called field of nanomedicine [12]. Despite the emerging trends of using nanoparticles in immunotherapy, no studies have investigated any type of nanocarriers to target TNFR2 on cancer cells. However, based on some previous novel reports about the effects of nanoparticles on immune homeostasis [13], especially using nanoparticles to promote TNFR2+Foxp3+ regulatory T cells (Tregs) in inflammatory models [14,15], we have suggested in our recent perspective review the possibility of blocking TNF-TNFR2 axis via nanoparticles [16].
Besides immunotherapy, the use of epigenetic modifications to influence the differentiation of programmed lineage pathways within the immune system has also emerged a few years ago [17]. For example, the inability to differentiate between Tregs and activated T cells neither through FOXP3 mRNA nor protein detection has made it difficult to study these cell subsets in the context of various diseases. To bypass this problem, previous studies have used DNA demethylation within the FOXP3 gene, to specifically identify Tregs in human peripheral tissue. Since FOXP3 is an essential marker for CD4+ Tregs and its expression plays a vital role in controlling the transcriptional program of Tregs, this approach could provide us valuable insight in understanding the molecular features of Tregs in healthy and disease states [18,19]. In other diseases like inflammatory bowel disease, gene expression profile was reported to be related to downregulation of TNFR2, thus promoting CD8+ T cell role in these types of inflammatory responses [20].
Furthermore there are large-scale of studies investigating the transcriptomic expressions of several immune checkpoints involved in cancer immune evasion as reviewed by Jamieson and Maker [21] but no studies to date have investigated DNA methylation within the TNFR2 gene or the transcriptomic expressions of TNFR2 in cancer models. Therefore, to improve the efficiency of cancer immunotherapy, we hypothesized that using DNA demethylation inhibitor along with nanomedicine targeting TNFR2 could be a novel effective approach towards cancer therapy.
2. Implication of TNFR2 in Cancer Development
Previous studies have shown that TNF preferentially binds to TNFR2 based on its higher affinity compared to TNFR1 [22,23]. TNFR1 is the primary mediator of TNF-induced apoptosis through its death domain (DD) which activates the nuclear factor kappa B (NF-κB) pathway [24]. TNFR2 also participates in enhancing apoptosis, by activating B cells and plays a crucial role in other pro-inflammatory responses, including proliferation of T cells [2]. Since Chen and his research group discovered TNFR2 for the first time in 2008, many reports have followed up on the potential impacts of TNFR2 expression on cancer cells [25,26,27,28]. As we mentioned before, these studies confirmed that TNFR2 was implicated in proliferation, metastasis and immune evasion of cancer cells by activating immunosuppressive cells.
When TNF-TNFR2 axis is activated, the intracellular domains activate the complexes consisting of TNF receptor-associated factor-2 (TRAF-2), cellular inhibitor of apoptosis protein-1 (cIAP-1) and cIAP2 resulting in the initiation of canonical and non-canonical activation of three main pathways, including NF-kB, activator protein 1 (AP1) and mitogen-activated protein kinases (MAPK) pathways [29,30]. The activity of these pathways both in cancer cells and immunosuppressive cells has led to the conclusion that TNFR2 contributes to cancer progression, expansion as well as stability of Tregs [16]. These pathways activate the phosphoinositide 3-kinases/protein Kinase B pathway (PI3K/Akt) signal transduction pathway that promotes survival and growth [31,32]. Further, NF-kB pathway promotes the transcription of genes responsible to cell proliferation and survival [33]. Activation of PI3K/Akt pathway reduces the differentiation of T helper 17 cells (Th17), which is associated with increased phosphorylation of signal transducer and activator of transcription 5 (STAT5) [33]. The STAT5 got a critical role in the function and activation of Tregs and it is associated with suppression of the anti-tumor activity of Teffs and an increase in proliferation, survival and immune invasion of cancer cells [34]. The suppressor mechanism of STAT5 is based on enhancing the secretion of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [35]. NF-kB also leads to increased IL-2 expressions via activating its promoter, which enhances expansion and stability of Tregs that expressing abundant amounts of the IL-2 receptor (IL-2R) associated with improving their suppressor function [36]. The overall description of the implication of TNFR2 activation in cancer cells progression and promoting immunosuppressive cells is summarized in Figure 2 [29,33,37,38].
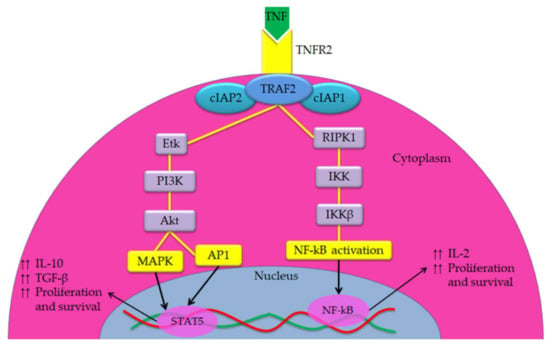
Figure 2.
Overview of the TNF-TNFR2 signaling pathway. In both cancer cell and immunosuppressive cells, TNFR2 is activated by both soluble TNF (sTNF) and membrane-bound TNF (mTNF) but it is fully activated by mTNF. TNFR2 does not interact with an intracellular DD, while it interacts with complex I that consists of TRAF2 with cIAP1 and cIAP2 and induction of homeostatic signals. The signals travel from complex I either via receptor-interacting serine/threonine-protein kinase 1 (RIPK1) or Etk (a member of the Btk tyrosine kinase family). RIPK1 trigger NF-κB via the IkB kinase (IKK) complex, which results in increasing the transcription of several genes associated positively with cell survival and proliferation. However, the Etk, through the PI3K/Akt pathway, is able to activate both AP1 and MAPK signaling pathways, which activate the promoter of proliferation, survival and other transcription factors. Further, it is associated with enhancing the phosphorylation of STAT5 that play a crucial role in immunosuppression.
Tregs express higher levels of TNFR2 than any other immunosuppressive cell, indicating a crucial role of Tregs in cancer microenvironment [39]. Therefore, TNFR2 has emerged in recent years as a potential target of cancer immunotherapy, although the intracellular mechanisms of TNFR2 in cancers are still unclear to some extent [9]. Table 1 shows several studies on the implication of TNFR2 in cancer development and suppressive immune responses. It is worth mentioning that, although several anti-cancer immunotherapeutics have been approved by the Food and Drug Administration (FDA) [12], to date there is no drug approved by the FDA as an TNFR2-antagonist.

Table 1.
List of studies that directly investigated the significant role of TNFR2 in cancer development and immune evasion.
3. Nanomedicine Applications for Cancer Immunotherapy
The introduction of advanced nanotechnology in the medical field aimed to better prevention, diagnostics and therapy of diseases, is called nanomedicine [41]. Incorporating therapeutic drugs with nano-objects (NOs) showed promising results in avoiding systemic side effects of drugs by improving its precision in targeting at the cellular level [42,43]. Most of the NOs are objects with a spherical shape but all the NOs are with nanometric size (most often below 200 nm) [44]. Thanks to their small size, relatively close to that of proteins, these NOs easily cross the different barriers immunity. Moreover, according to their chemical composition, these NOs are able to encapsulate and protect hydrophilic or/and hydrophobic drugs against any enzymatic, chemical or physical degradation, all along their transport to the target cells [44]. The NOs currently used in the biomedical field are complex systems that can be made of various components according to the desired application. Researchers utilized several types of drug-loaded NOs (nanocarriers) to enhance cancer therapy, including organic, inorganic and hybrid nanoparticles (NPs) (Table 2) [45]. Nanomedicine provided many effective approaches in cancer immunotherapy, while nano-based drug delivery has received considerable interest as well (Figure 3).

Table 2.
Drug delivery systems for anti-tumor drugs.
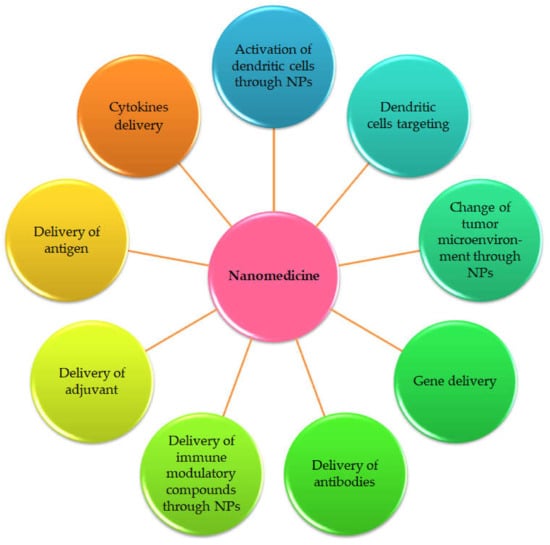
Figure 3.
Nanomedicine strategies to improve cancer immunotherapy as suggested by Qiu et al., 2017 [12].
Owing to their properties as effective antigen-presenting cells (APCs), dendritic cells (DCs) have been utilized in cell-mediated therapeutic vaccination via several strategies to present antigens against cancer cells [57]. Therefore, it was hypothesized that targeting DCs with antigens and adjuvants loaded with NPs might be an effective strategy to adopt the use of DCs in cancer immunotherapy [58,59]. So far, one study has successfully used a poly-lactic-co-glycolic acid (PLGA)-based NPs in targeting DCs via biotinylated antibodies and it was enhanced T cell immunity [60]. In this study, targeting more DCs subsets was significantly correlated with more T cell activation [60].
Utilizing NPs to change tumor microenvironment can produce an immunosuppressive environment based on the combination of NPs with several immunotherapy applications, including chemotherapy, cytokines and many biomolecules towards releasing soluble cytokine mediators and attracting immunosuppressive cells [12]. A study on advanced melanoma model showed that combining of polyethylene glycol (PEG) conjugated curcumin and vaccine, dramatically decreased the number of immunosuppressive cells and reduced the levels of IL-6 and chemokine ligand 2, while showing an increase in levels of proinflammatory cytokines and elevation in the CD8+ T-cells [61]. In gene delivery, several NPs have been effectively utilized to deliver small interfering RNA (siRNA) mainly to TGF-β, cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), programmed death-ligand 1 (PD-L1) and PD-L2. These approaches showed a significant inhibition in cancer development due to increase of CD8+ T cells and decreased of immunosuppressive cells [62,63,64].
Antibody-based treatments are a promising approaches in cancer immunotherapy and emerged due to their high degree of specificity to target precision sites implicated in cancer development [65]. However, there is a need for novel delivery strategies to reach the highest levels of specificity in antibody targeting, while trying to avoid off-target side effects as much as possible. Antibody delivery has been reported to be the most common nanomedicine application in terms of immunotherapy [66]. NPs have been utilized in co-delivery of several antibodies to blockade CTLA-4, PD-1 and PDL-1 in different cancer models [67,68]. It also successfully improved the immunotherapeutic antibodies inhibit cancer’s immunosuppressive checkpoint pathways, thus inhibiting Tregs, activation of Teffs and fight cancer cells [69,70]. Although the promising findings were reported by studies of antibody-based drugs which have been incorporated with NPs to target significant receptors on cancer cells and immune cells in cancer microenvironment, no studies to date have combined TNFR2 antibodies with NPs towards an effective immunotherapy approach. Therefore, we hypothesized that the utilization of NPs specific ligands to delivery TNFR2 antibodies would alter immunological status of cancer microenvironment; results in fight cancer cells and immunosuppressive cells specifically (Figure 4).
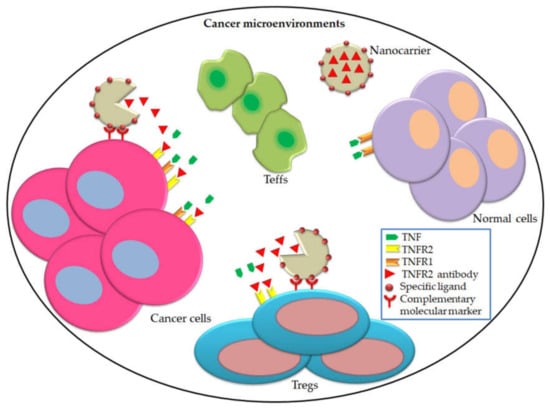
Figure 4.
Nanoparticles-targeting TNFR2 exhibit their effects on cancer cells and Tregs. We hypothesized that based on the ability of nanoparticles with specific ligands to targeting cancer cells, it is possible to incorporate TNFR2 antibodies with this kind of nanoparticles. Consequently, TNFR2 antibodies will be released at the cancer microenvironment, which is in turn, will blockade TNFR2 on cancer cells and Tregs. This modulation will result in suppressing Tregs and activate Teffs, thus less tumorigenesis, tumor invasion and metastasis.
4. DNA Demethylation and Immune Evasion
The 5-methylcytosines within CG dinucleotides (CpG islands) are establishing to keep the balance of normal development and this process called DNA methylation [71]. As cancer progression results in many modifications in the normal mechanism of gene expression, demethylation of DNA is emerged to be one of the critical mechanisms that leads to modify gene expression and implicated in cancer progression, metastasis and immune evasion [72]. The overall description of the mechanisms of DNA methylation and demethylation are summarized in Figure 5. Although the critical role of TNFR2 in immune evasion of tumor cells, no study to date has been investigated on DNA methylation/demethylation in the TNFR2 gene in cancer subjects. Whereas there were significant findings have been reported recently in studies investigated on methylation/demethylation in several immune checkpoint genes including PD-1, PD-L1, CTLA-4, TIM-3, LAG-3 and TIGIT [73,74]. Demethylation and many mechanisms are part of the epigenetics field, which is considered as a gate for promising trends in cancer research in the current era.
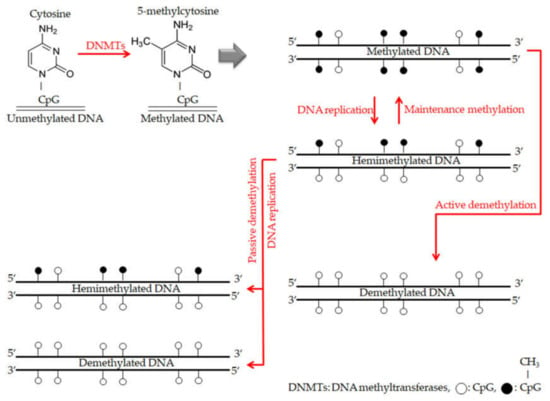
Figure 5.
The overall description for mechanisms of DNA methylation and demethylation. During DNA methylation, DNA methyltransferases (DNMTs) enzymes are responsible for adding methyl groups to specific CpG islands and establishing DNA methylation patterns. With each DNA replication round, there is a new strand of unmethylated DNA. Thus the new double strand DNA becomes hemimethylated. Furthermore, maintenance methylation process by DNMT1 enzyme leads to copy that DNA methylation pattern from the old strand onto the new strand. On the other hand, during DNA demethylation, maintenance methylation process finishing with remarkably failing, which leads to stopping DNA methylation patterns (passive demethylation). While, active demethylation achieving by specific enzymes have not been explicitly explored and results in modifying methylated cytosines to only cytosines without methyl groups and independently of DNA replication [75].
It has been reported that epigenetic regulation is one of the key mechanisms which facilitate cancer progression and immunosuppression, including the regulation of immune checkpoints expression in the tumor microenvironment [76]. For example, three important epigenetic modifications have been reported in colorectal cancer; DNA methylation, post-translational modifications in chromatin-protein interactions and expression of non-coding RNAs [77,78]. Silencing of tumor suppressor genes can occur as a result of hypermethylation of the CpG islands, which highly enriched within the promoter regions of tumor suppressor genes [79]. Alternatively, promoter demethylation and distribution of repressive histones can work together to induce the upregulation of many genes in cancers [80]. Besides, it has been suggested that the enrichment of repressive histones, histone 3 lysine 9 trimethylation (H3K9me3) and H3K27me3 in the promoter regions along with CpG hypermethylation are the common epigenetic modifications in breast cancer [74]. Therefore, we hypothesized that TNFR2 gene might be highly expressed and demethylated in cancer models. Thus using demethylation inhibitors as a potential approach will result in decrease of the relative expression of TNFR2 gene; consequently, downregulation of TNFR2 on cancer cells and immune suppressive cells. Furthermore, using the above hypotheses in the meanwhile with TNFR2 antibodies delivered via NPs is novel hypotheses for an effective cancer immunotherapy (Figure 6).
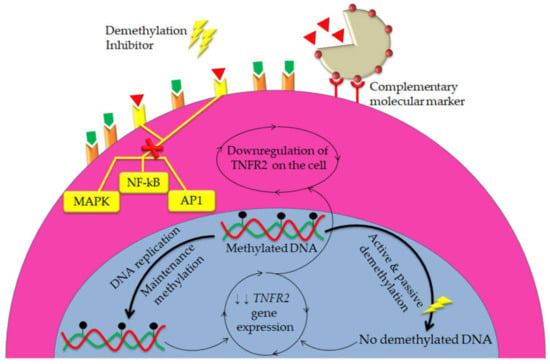
Figure 6.
NPs are hypothesized to be utilized synergistically with DNA demethylation inhibitors to stronger blockade of TNFR2 signals in both cancer cells and immunosuppressive cells. Owning to their ability to target specific cellular sites, NPs with specific ligands can deliver TNFR2 antibodies to cancer cells and immunosuppressive cells only, which in turn stop TNFR2 intracellular signal pathways. Meanwhile, using DNA demethylation inhibitors might lead to epigenetic alteration results in decrease the expression of TNFR2 gene, thus downregulation of TNFR2. Consequently, combination of these two strategies might extend the anti-tumor effects of TNFR2 antibodies with more precision in immunotherapy.
Although the co-administration of TNFR2 antibodies conjugated with NPs used synergistically with DNA demethylation, would be a promising therapeutic approach, the clinical usage of these agents is likely to require further investigation to establish dosing, injection procedure and administration patterns. Although this is considered a relatively novel approach, we do not expect any significant side effects for TNFR2 targeting via this approach, as the receptor is preferentially expressed on cancer cells and immunosuppressive cells. Furthermore, previous studies aimed to modulate TNFRs by targeting TNF itself, which might have caused total inhibition of TNF and thus the onset of serious side effects including peripheral and central nervous system demyelinating disorders [81]. In addition, other well-known side effects could emerge, such as injection site reactions, hemocytopenia, congestive heart failure and T-cell lymphomas [82]. Therefore, we believe our perspective provides a relatively safer and more targeted approach towards an effective cancer treatment.
5. Conclusions and Future Directions
It is known that the TNF works as an anti-tumor cytokine, while its role may be negatively converted to be a tumor-promoting substance upon its conjugation with TNFR2, which is only expressed on some tumor cells and immunosuppressive cells. In both cell types, TNF-TNFR2 axis was implicated in increasing growth and development via three main pathways, including NF-kB, AP1 and MAPK pathways. Based on these pathways, TNF-TNFR2 axis was responsible for promoting carcinogenesis and cancer immune evasion. Consequently, there were several studies showing blockade of TNF-TNFR2 axis as a promising cancer treatment. Towards an efficient and precision immunotherapy, nanomedicine provided many effective approaches, especially for antibodies-based drugs delivery, not only to reach the targeted sites but also to restore the immune response to suppress the cancer cells. To date, no study has employed any nanomedicine in blockade TNFR2.
Moreover, based on the understanding that significant epigenetic modifications occur in cancer, recent epigenetic reports provided new insight to improve responses to immunotherapy targeted immune checkpoints via specific epigenetic drugs. One of the most common and significant modifications is DNA demethylation. However, no study to date has been investigated on DNA methylation/demethylation of TNFR2 gene. Therefore, future studies should investigate how to incorporate TNFR2 antibodies in nanoparticles with specific characterization to create a more efficient blockade for TNFR2. Studies should also focus on understanding the status of DNA methylation/demethylation pattern in the promoter region of TNFR2 gene, that will be helpful before using DNA demethylation inhibitors. In the end, providing a combination of nanomedicine-based immunotherapies (TNFR2 antibodies) at the same time with epigenetic drugs (DNA demethylation inhibitors) will be a novel strategy to improve the effectiveness of cancer immunotherapies.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Grant Scheme (FRGS) by Ministry of Higher Education (MOHE) Malaysia, grant number [203/PPSP/6171198], and Universiti Sains Malaysia (USM) Research Grant, grant number [1001/PPSP/8012294].
Acknowledgments
The second author (Engku Nur Syafirah) was financially supported by USM Fellowship Scheme.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants–past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 2572. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.; Fernandez, M.C.; D’Costa, Z.; Brodt, P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res. 2016, 11, 1–27. [Google Scholar] [PubMed]
- Hamilton, K.E.; Simmons, J.G.; Ding, S.; Van Landeghem, L.; Lund, P.K. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol. Cancer Res. 2011, 9, 1718–1731. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, M.; Nagaishi, T.; Kanai, T.; Nagano, K.; Oshima, S.; Nemoto, Y.; Yoshioka, A.; Totsuka, T.; Okamoto, R.; Nakamura, T.; et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G850–G859. [Google Scholar] [CrossRef]
- Cai, X.; Cao, C.; Li, J.; Chen, F.; Zhang, S.; Liu, B.; Zhang, W.; Zhang, X.; Ye, L. Inflammatory factor TNF-alpha promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-kappaB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017, 8, 58338–58352. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Z.; Zhao, N. Clinical implications of tumor necrosis factor receptor 2 in breast cancer. Oncol. Lett. 2017, 14, 2393–2398. [Google Scholar] [CrossRef]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 2017, 10, eaaf8608. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Min, Y.; Rodgers, Z.; Zhang, L.; Wang, A.Z. Nanomedicine approaches to improve cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1456. [Google Scholar] [CrossRef] [PubMed]
- Mohamud, R.; Xiang, S.D.; Selomulya, C.; Rolland, J.M.; O’Hehir, R.E.; Hardy, C.L.; Plebanski, M. The effects of engineered nanoparticles on pulmonary immune homeostasis. Drug Metab. Rev. 2014, 46, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Mohamud, R.; LeMasurier, J.S.; Boer, J.C.; Sieow, J.L.; Rolland, J.M.; O’Hehir, R.E.; Hardy, C.L.; Plebanski, M. Synthetic Nanoparticles That Promote Tumor Necrosis Factor Receptor 2 Expressing Regulatory T Cells in the Lung and Resistance to Allergic Airways Inflammation. Front. Immunol. 2017, 8, 1812. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.L.; Lemasurier, J.S.; Mohamud, R.; Yao, J.; Xiang, S.D.; Rolland, J.M.; O’ Hehir, R.E.; Plebanski, M. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J. Immunol. 2013, 191, 5278–5290. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.; Ahmad, S.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. A Perspective Review on the Role of Nanomedicine in the Modulation of TNF-TNFR2 Axis in Breast Cancer Immunotherapy. J. Oncol. 2019, 2019, 13. [Google Scholar] [CrossRef]
- Calvanese, V.; Fernandez, A.F.; Urdinguio, R.G.; Suarez-Alvarez, B.; Mangas, C.; Perez-Garcia, V.; Bueno, C.; Montes, R.; Ramos-Mejia, V.; Martinez-Camblor, P.; et al. A promoter DNA demethylation landscape of human hematopoietic differentiation. Nucleic Acids Res. 2012, 40, 116–131. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ohkura, N.; Sakaguchi, S. Molecular determinants of regulatory T cell development: The essential roles of epigenetic changes. Front. Immunol. 2013, 4, 106. [Google Scholar] [CrossRef]
- Wieczorek, G.; Asemissen, A.; Model, F.; Turbachova, I.; Floess, S.; Liebenberg, V.; Baron, U.; Stauch, D.; Kotsch, K.; Pratschke, J.; et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009, 69, 599–608. [Google Scholar] [CrossRef]
- Punit, S.; Dube, P.E.; Liu, C.Y.; Girish, N.; Washington, M.K.; Polk, D.B. Tumor Necrosis Factor Receptor 2 Restricts the Pathogenicity of CD8(+) T Cells in Mice with Colitis. Gastroenterology 2015, 149, 993–1005. [Google Scholar] [CrossRef]
- Jamieson, N.B.; Maker, A.V. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther. 2017, 24, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Pennica, D.; Goeddel, D.V. Ligand passing: The 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J. Biol. Chem. 1993, 268, 18542–18548. [Google Scholar] [PubMed]
- Tartaglia, L.A.; Goeddel, D.V. Two TNF receptors. Immunol. Today 1992, 13, 151–153. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Hsu, H.; Goeddel, D.V.; Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 1996, 87, 565–576. [Google Scholar] [CrossRef]
- Yan, F.; Du, R.; Wei, F.; Zhao, H.; Yu, J.; Wang, C.; Zhan, Z.; Ding, T.; Ren, X.; Chen, X. Expression of TNFR2 by regulatory T cells in peripheral blood is correlated with clinical pathology of lung cancer patients. Cancer Immunol. Immunother. 2015, 64, 1475–1485. [Google Scholar] [CrossRef]
- Vanamee, E.S.; Faustman, D.L. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef]
- Zhang, T.; Jiao, J.; Jiao, X.; Zhao, L.; Tian, X.; Zhang, Q.; Ma, D.; Cui, B. Aberrant frequency of TNFR2(+) Treg and related cytokines in patients with CIN and cervical cancer. Oncotarget 2017, 9, 5073–5083. [Google Scholar] [CrossRef]
- Nie, Y.; He, J.; Shirota, H.; Trivett, A.L.; Yang, D.; Klinman, D.M.; Oppenheim, J.J.; Chen, X. Blockade of TNFR2 signaling enhances the immunotherapeutic effect of CpG ODN in a mouse model of colon cancer. Sci. Signal 2018, 11, 1937–9145. [Google Scholar] [CrossRef]
- Wang, J.; Al-Lamki, R.S. Tumor necrosis factor receptor 2: Its contribution to acute cellular rejection and clear cell renal carcinoma. BioMed Res. Int. 2013, 2013, 82131. [Google Scholar] [CrossRef]
- Cohen, J.L.; Wood, K.J. TNFR2: The new Treg switch? Oncoimmunology 2018, 7, e1373236. [Google Scholar] [CrossRef]
- Arcaro, A.; Guerreiro, A.S. The phosphoinositide 3-kinase pathway in human cancer: Genetic alterations and therapeutic implications. Curr. Genom. 2007, 8, 271–306. [Google Scholar] [CrossRef] [PubMed]
- Nur Husna, S.M.; Tan, H.T.; Mohamud, R.; Dyhl-Polk, A.; Wong, K.K. Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: A review. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Murphy, J.J. STAT5 in Cancer and Immunity. J. Interferon Cytokine Res. 2016, 36, 226–237. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumor necrosis factor signaling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Pan, S.; An, P.; Zhang, R.; He, X.; Yin, G.; Min, W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: Role in endothelial cell migration and angiogenesis. Mol. Cell. Biol. 2002, 22, 7512–7523. [Google Scholar] [CrossRef]
- Chen, X.; Subleski, J.J.; Kopf, H.; Howard, O.M.; Mannel, D.N.; Oppenheim, J.J. Cutting edge: Expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: Applicability to tumor-infiltrating T regulatory cells. J. Immunol. 2008, 180, 6467–6471. [Google Scholar] [CrossRef]
- Williams, G.S.; Mistry, B.; Guillard, S.; Ulrichsen, J.C.; Sandercock, A.M.; Wang, J.; Gonzalez-Munoz, A.; Parmentier, J.; Black, C.; Soden, J.; et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget 2016, 7, 68278–68291. [Google Scholar] [CrossRef]
- Nikalje, A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015, 5, 81–89. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Nayak, S.; Lyon, L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef]
- Ferji, K.; Nouvel, C.C.; Babin, J.; Li, M.-H.; Gaillard, C.D.; Nicol, E.; Chassenieux, C.; Six, J.-L. Polymersomes from amphiphilic glycopolymers containing polymeric liquid crystal grafts. ACS Macro Lett. 2015, 4, 1119–1122. [Google Scholar] [CrossRef]
- Six, J.-L.; Ferji, K. Polymerization induced self-assembly: An opportunity toward the self-assembly of polysaccharide-containing copolymers into high-order morphologies. Polym. Chem. 2019, 10, 45–53. [Google Scholar] [CrossRef]
- Sugano, M.; Egilmez, N.K.; Yokota, S.J.; Chen, F.A.; Harding, J.; Huang, S.K.; Bankert, R.B. Antibody targeting of doxorubicin-loaded liposomes suppresses the growth and metastatic spread of established human lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2000, 60, 6942–6949. [Google Scholar]
- Zhong, Q.; Bielski, E.R.; Rodrigues, L.S.; Brown, M.R.; Reineke, J.J.; da Rocha, S.R. Conjugation to Poly(amidoamine) Dendrimers and Pulmonary Delivery Reduce Cardiac Accumulation and Enhance Antitumor Activity of Doxorubicin in Lung Metastasis. Mol. Pharm. 2016, 13, 2363–2375. [Google Scholar] [CrossRef]
- Poon, C.; He, C.; Liu, D.; Lu, K.; Lin, W. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J. Control. Release 2015, 201, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Gener, P.; Andrade, F.; Seras-Franzoso, J.; Montero, S.; Fernandez, Y.; Hidalgo, M.; Arango, D.; Sayos, J.; Florindo, H.F.; et al. AKT2 siRNA delivery with amphiphilic-based polymeric micelles show efficacy against cancer stem cells. Drug Deliv. 2018, 25, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wen, A.M.; Commandeur, U.; Steinmetz, N.F. Presentation of HER2 epitopes using a filamentous plant virus-based vaccination platform. J. Mater. Chem. B 2014, 2, 6249–6258. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, W.; Meng, J.; Chen, S.; Xu, H.; Yang, X.D. Multi-walled carbon nanotubes conjugated to tumor protein enhance the uptake of tumor antigens by human dendritic cells in vitro. Cell Res. 2010, 20, 1170–1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, X.; Wei, X.; Jing, Y.; Zhou, S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv. Mater. 2015, 27, 6450–6456. [Google Scholar] [CrossRef]
- Seyfoori, A.; Sarfarazijami, S.; Seyyed Ebrahimi, S.A. pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1437–1443. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Sehgal, K.; Dhodapkar, K.M.; Dhodapkar, M.V. Targeting human dendritic cells in situ to improve vaccines. Immunol. Lett. 2014, 162, 59–67. [Google Scholar] [CrossRef]
- Ahmad, S.; Zamry, A.A.; Tan, H.T.; Wong, K.K.; Lim, J.; Mohamud, R. Targeting dendritic cells through gold nanoparticles: A review on the cellular uptake and subsequent immunological properties. Mol. Immunol. 2017, 91, 123–133. [Google Scholar] [CrossRef]
- Sehgal, K.; Ragheb, R.; Fahmy, T.M.; Dhodapkar, M.V.; Dhodapkar, K.M. Nanoparticle-mediated combinatorial targeting of multiple human dendritic cell (DC) subsets leads to enhanced T cell activation via IL-15-dependent DC crosstalk. J. Immunol. 2014, 193, 2297–2305. [Google Scholar] [CrossRef]
- Lu, Y.; Miao, L.; Wang, Y.; Xu, Z.; Zhao, Y.; Shen, Y.; Xiang, G.; Huang, L. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Mol. Ther. 2016, 24, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Liu, Y.; Xu, C.F.; Shen, S.; Sun, R.; Du, X.J.; Xia, J.X.; Zhu, Y.H.; Wang, J. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 2016, 231, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Zhang, L.; Huang, L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 2014, 8, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Roeven, M.W.; Hobo, W.; van der Voort, R.; Fredrix, H.; Norde, W.J.; Teijgeler, K.; Ruiters, M.H.; Schaap, N.; Dolstra, H. Efficient nontoxic delivery of PD-L1 and PD-L2 siRNA into dendritic cell vaccines using the cationic lipid SAINT-18. J. Immunother. 2015, 38, 145–154. [Google Scholar] [CrossRef]
- Nicodemus, C.F. Antibody-based immunotherapy of solid cancers: Progress and possibilities. Immunotherapy 2015, 7, 923–939. [Google Scholar] [CrossRef]
- Conniot, J.; Silva, J.M.; Fernandes, J.G.; Silva, L.C.; Gaspar, R.; Brocchini, S.; Florindo, H.F.; Barata, T.S. Cancer immunotherapy: Nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2014, 2, 105. [Google Scholar] [CrossRef]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z.; et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2016, 5, e1074374. [Google Scholar] [CrossRef]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Kwong, B.; Gai, S.A.; Elkhader, J.; Wittrup, K.D.; Irvine, D.J. Localized immunotherapy via liposome-anchored Anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 2013, 73, 1547–1558. [Google Scholar] [CrossRef]
- Lei, C.; Liu, P.; Chen, B.; Mao, Y.; Engelmann, H.; Shin, Y.; Jaffar, J.; Hellstrom, I.; Liu, J.; Hellstrom, K.E. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am. Chem. Soc. 2010, 132, 6906–6907. [Google Scholar] [CrossRef]
- Kisseljova, N.P.; Kisseljov, F.L. DNA demethylation and carcinogenesis. Biochemistry 2005, 70, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Szyf, M. The role of DNA hypermethylation and demethylation in cancer and cancer therapy. Curr. Oncol. 2008, 15, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Elashi, A.A.; Sasidharan Nair, V.; Taha, R.Z.; Shaath, H.; Elkord, E. DNA methylation of immune checkpoints in the peripheral blood of breast and colorectal cancer patients. Oncoimmunology 2019, 8, e1542918. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Nair, V.; El Salhat, H.; Taha, R.Z.; John, A.; Ali, B.R.; Elkord, E. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT and PD-L1 genes in human primary breast cancer. Clin. Epigenet. 2018, 10, 78. [Google Scholar] [CrossRef]
- Chen, Z.X.; Riggs, A.D. DNA methylation and demethylation in mammals. J. Biol. Chem. 2011, 286, 18347–18353. [Google Scholar] [CrossRef]
- Heninger, E.; Krueger, T.E.; Lang, J.M. Augmenting antitumor immune responses with epigenetic modifying agents. Front. Immunol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, M. Epigenetic changes in colorectal cancer. Chin. J. Cancer 2013, 32, 21–30. [Google Scholar] [CrossRef]
- Lao, V.V.; Grady, W.M. Epigenetics and colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 686–700. [Google Scholar] [CrossRef]
- Issa, J.P. CpG island methylator phenotype in cancer. Nat. Rev. Cancer 2004, 4, 988–993. [Google Scholar] [CrossRef]
- Schlesinger, Y.; Straussman, R.; Keshet, I.; Farkash, S.; Hecht, M.; Zimmerman, J.; Eden, E.; Yakhini, Z.; Ben-Shushan, E.; Reubinoff, B.E.; et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007, 39, 232–236. [Google Scholar] [CrossRef]
- Mohan, N.; Edwards, E.T.; Cupps, T.R.; Oliverio, P.J.; Sandberg, G.; Crayton, H.; Richert, J.R.; Siegel, J.N. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheumatol. 2001, 44, 2862–2869. [Google Scholar] [CrossRef]
- Kemanetzoglou, E.; Andreadou, E. CNS Demyelination with TNF-alpha Blockers. Curr. Neurol. Neurosci. Rep. 2017, 17, 36. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).