TCTP is Essential for Cell Proliferation and Survival during CNS Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Conditional Knockout Mice, Breeding, and Genotyping
2.2. Tissue Processing, Histological Analysis, and Immunohistochemistry
2.3. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time Reverse Transcriptase PCR
2.4. Cell Proliferation Assay
2.5. TUNEL Assay
2.6. Primary Neuronal Cultures
2.7. Cortical Progenitor Cultures and Immunofluorescence
2.8. Cell Survival Assay and MTT Reduction Assay
2.9. Statistical Analysis of the Data
3. Results
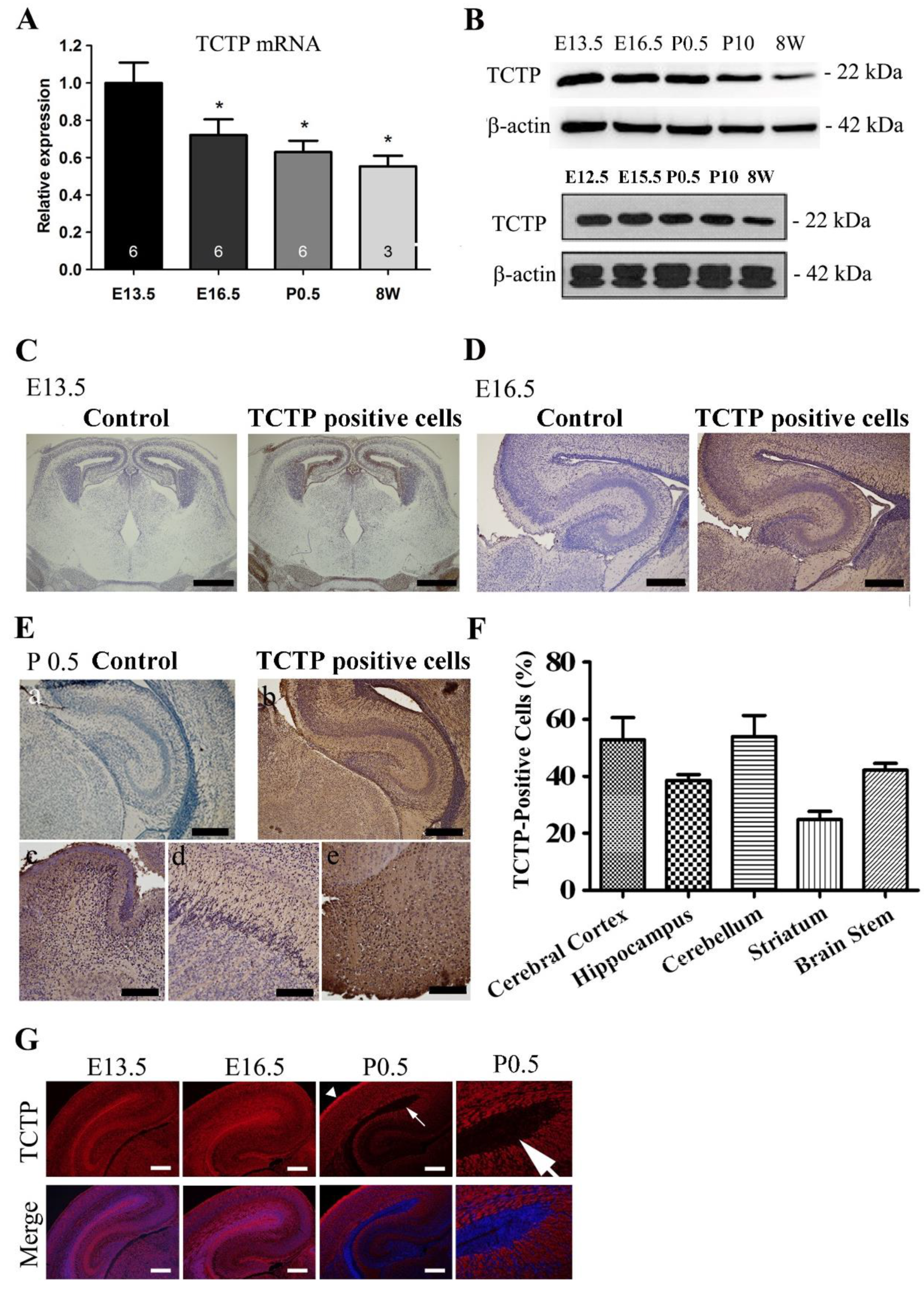
3.1. Expression Pattern of TCTP in the Mouse Brain during Development
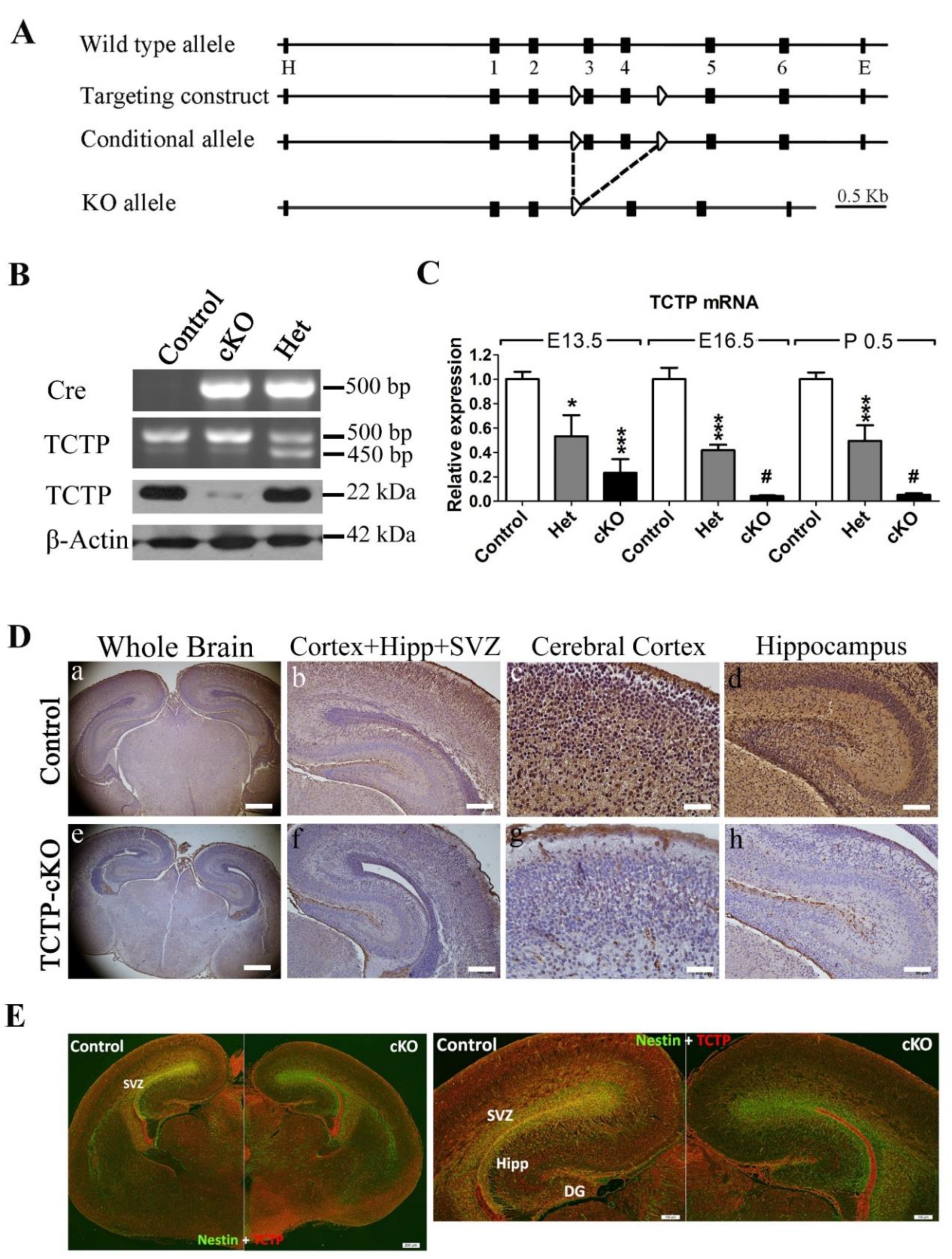
3.2. Generation of Conditional TCTP Knockout Mice
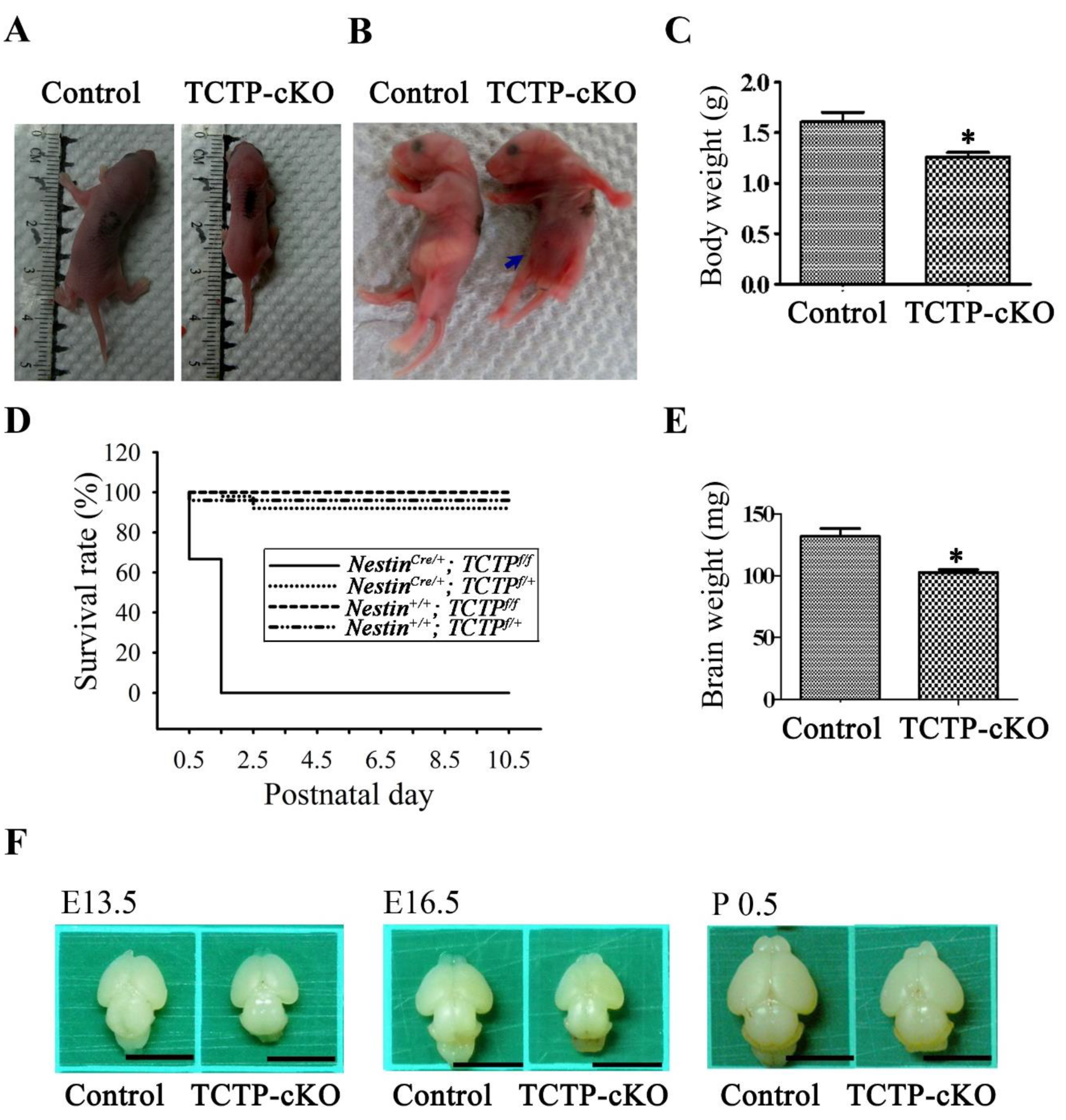
3.3. Loss of TCTP in Neuronal Progenitor Cells Resulted in Early Neonatal Death
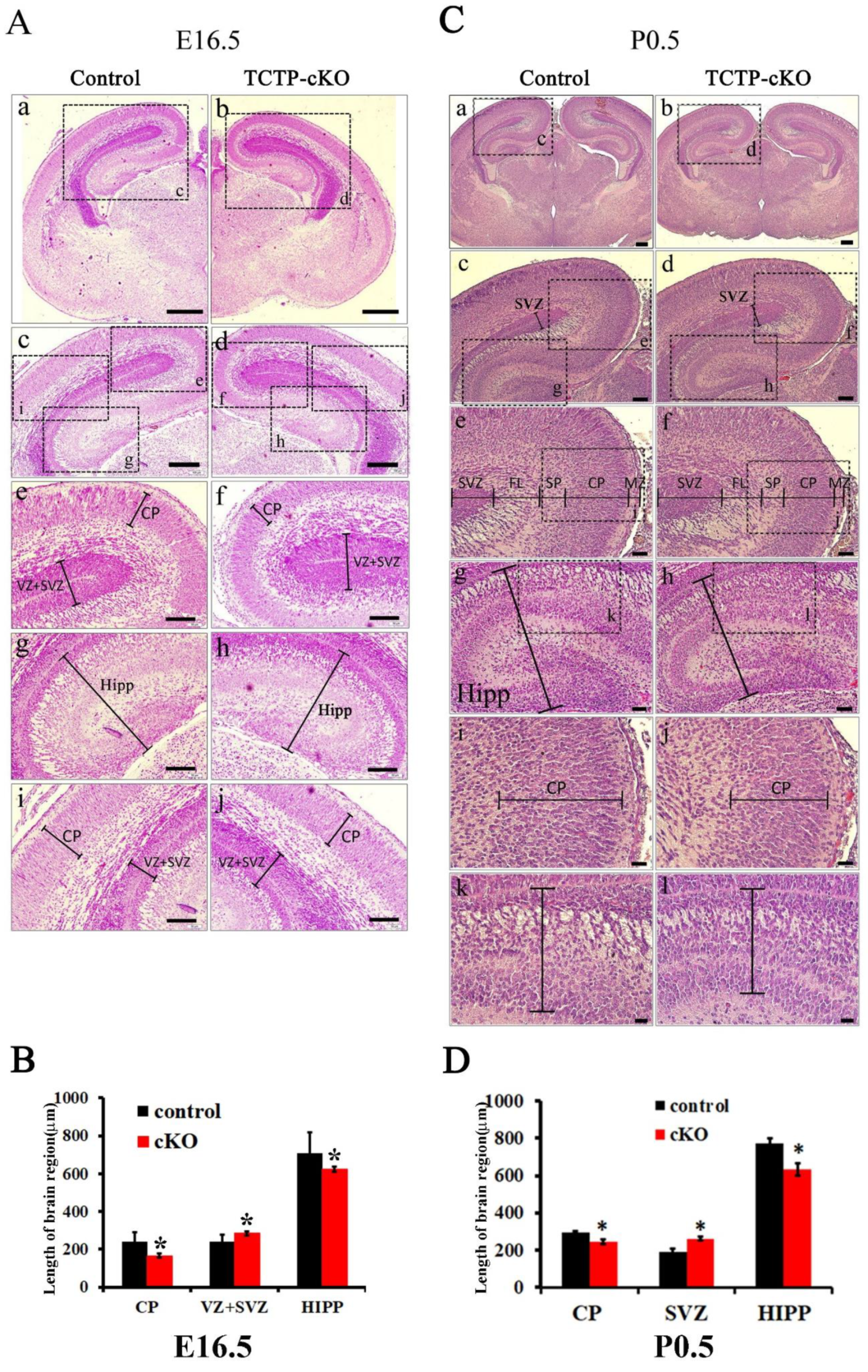
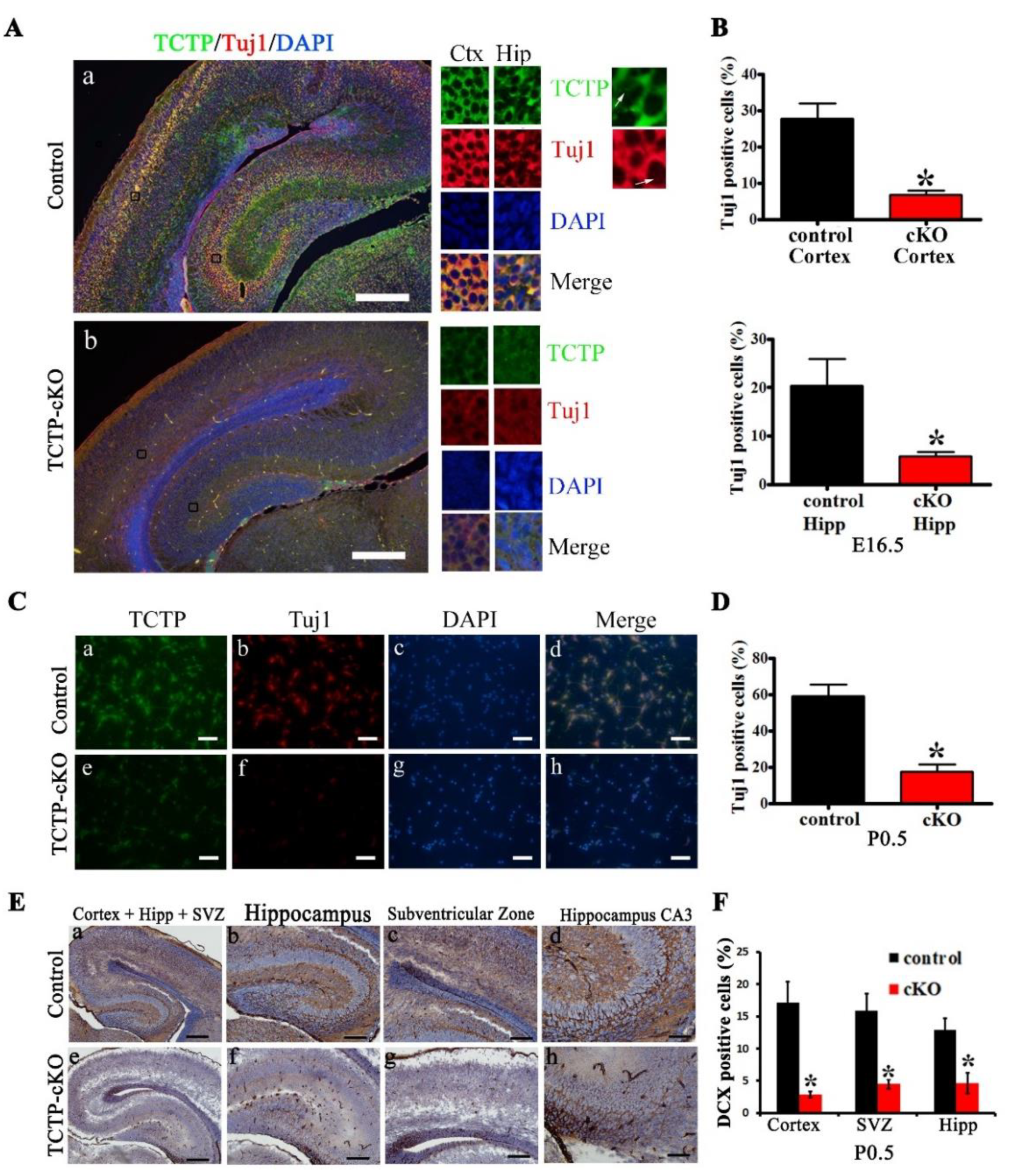
3.4. TCTP is Required for Cortical Neurogenesis
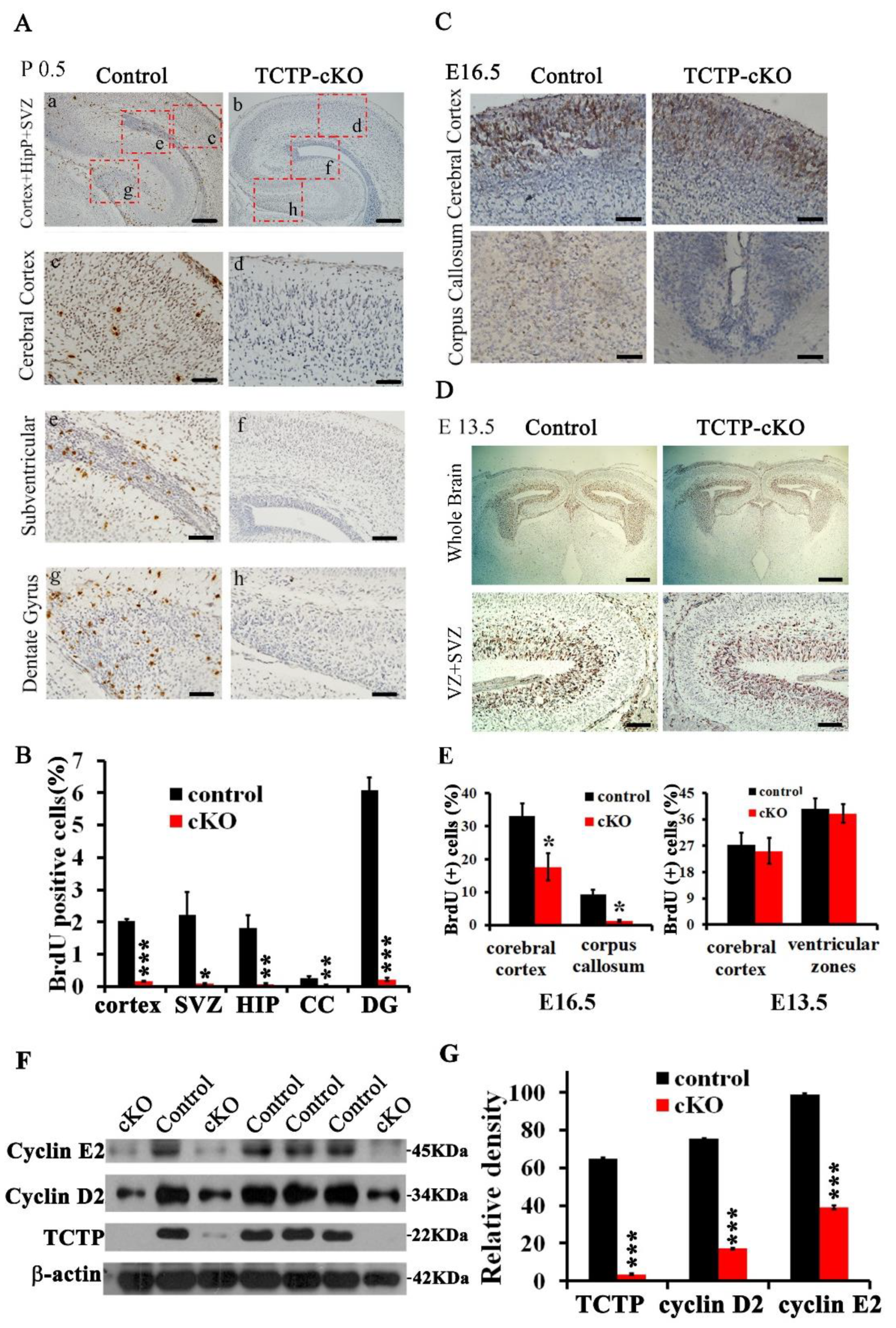
3.5. Decreased Cell Proliferation in Nestin-Cre-Derived TCTP-Deficient Mouse Brain
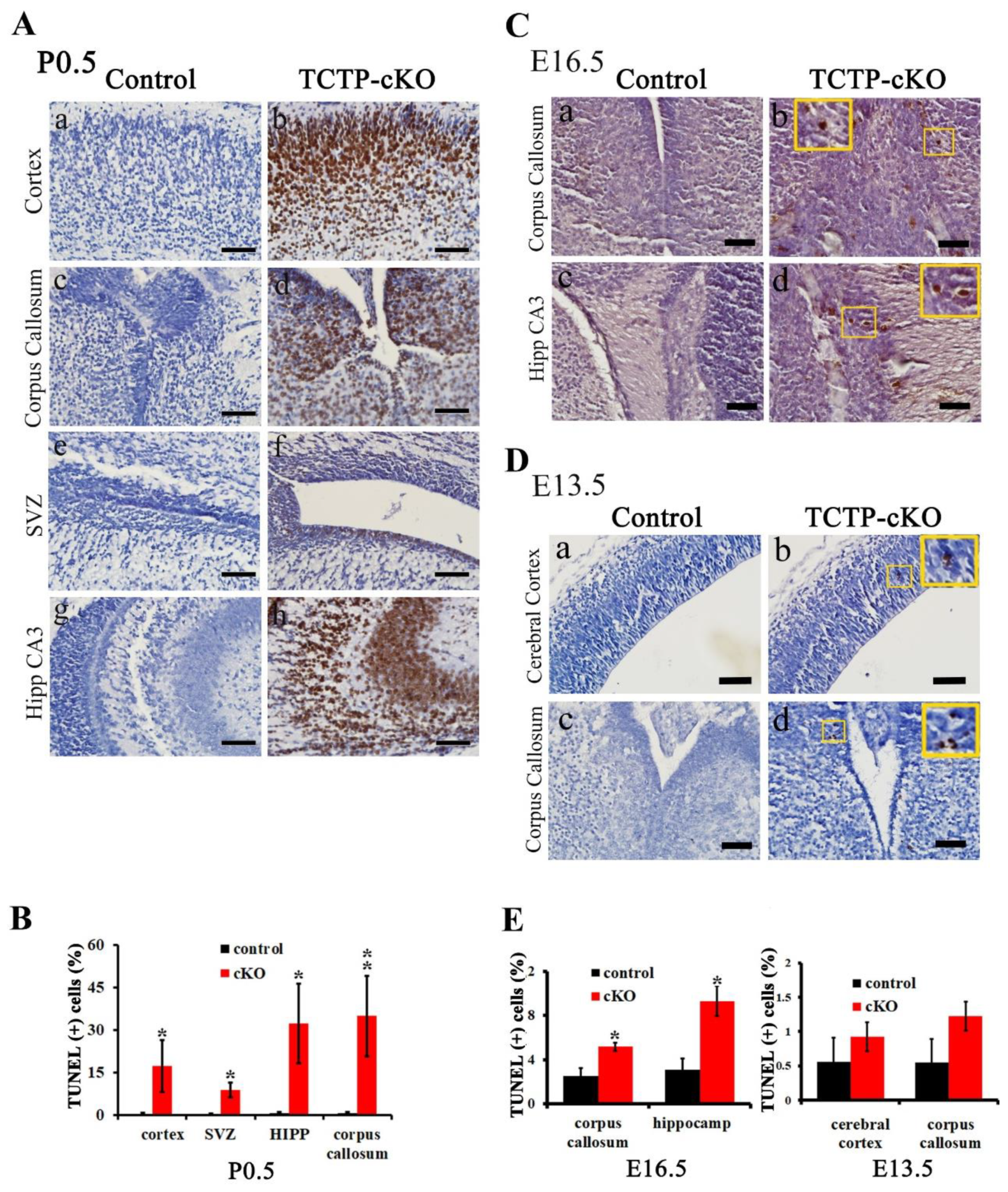
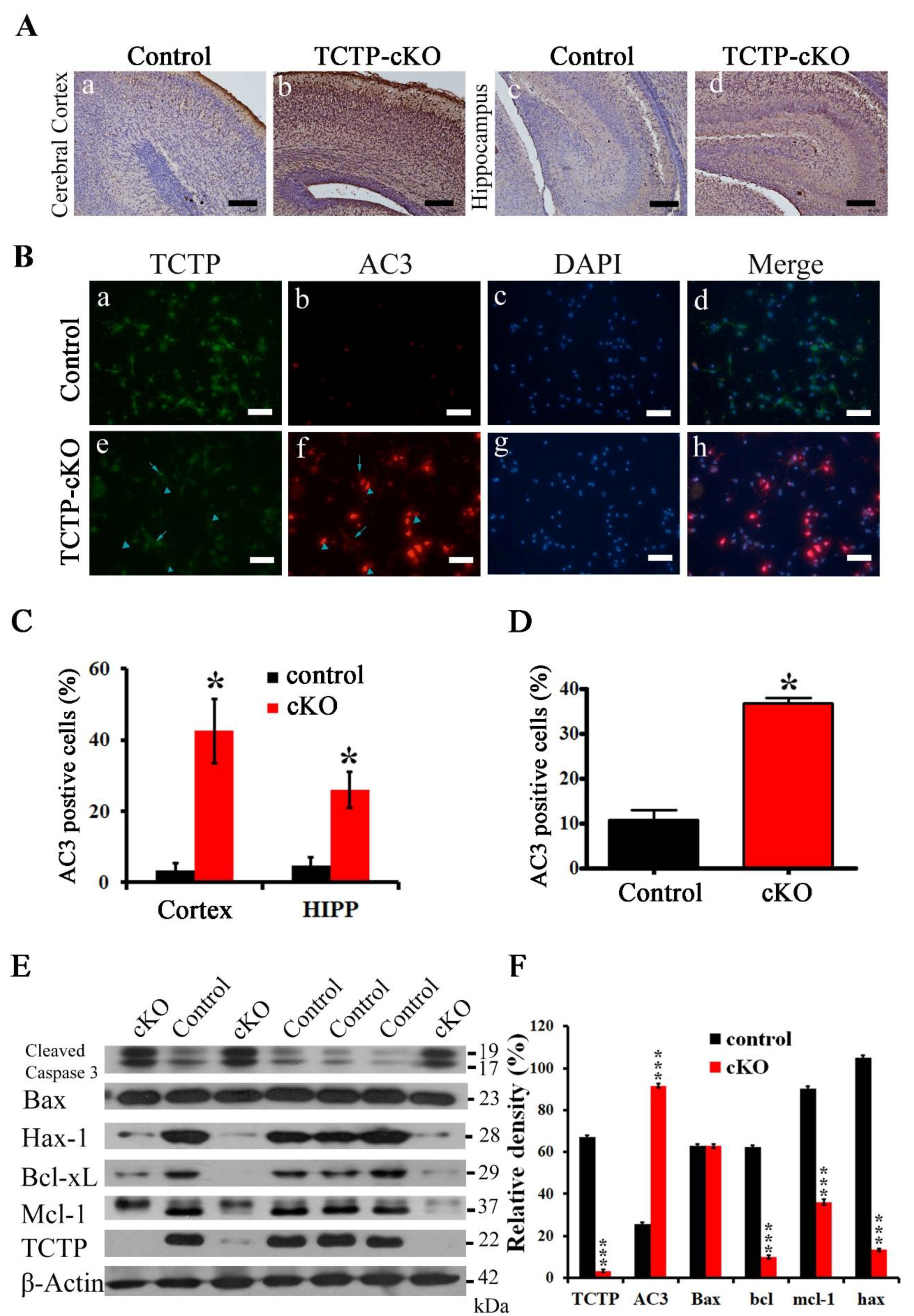
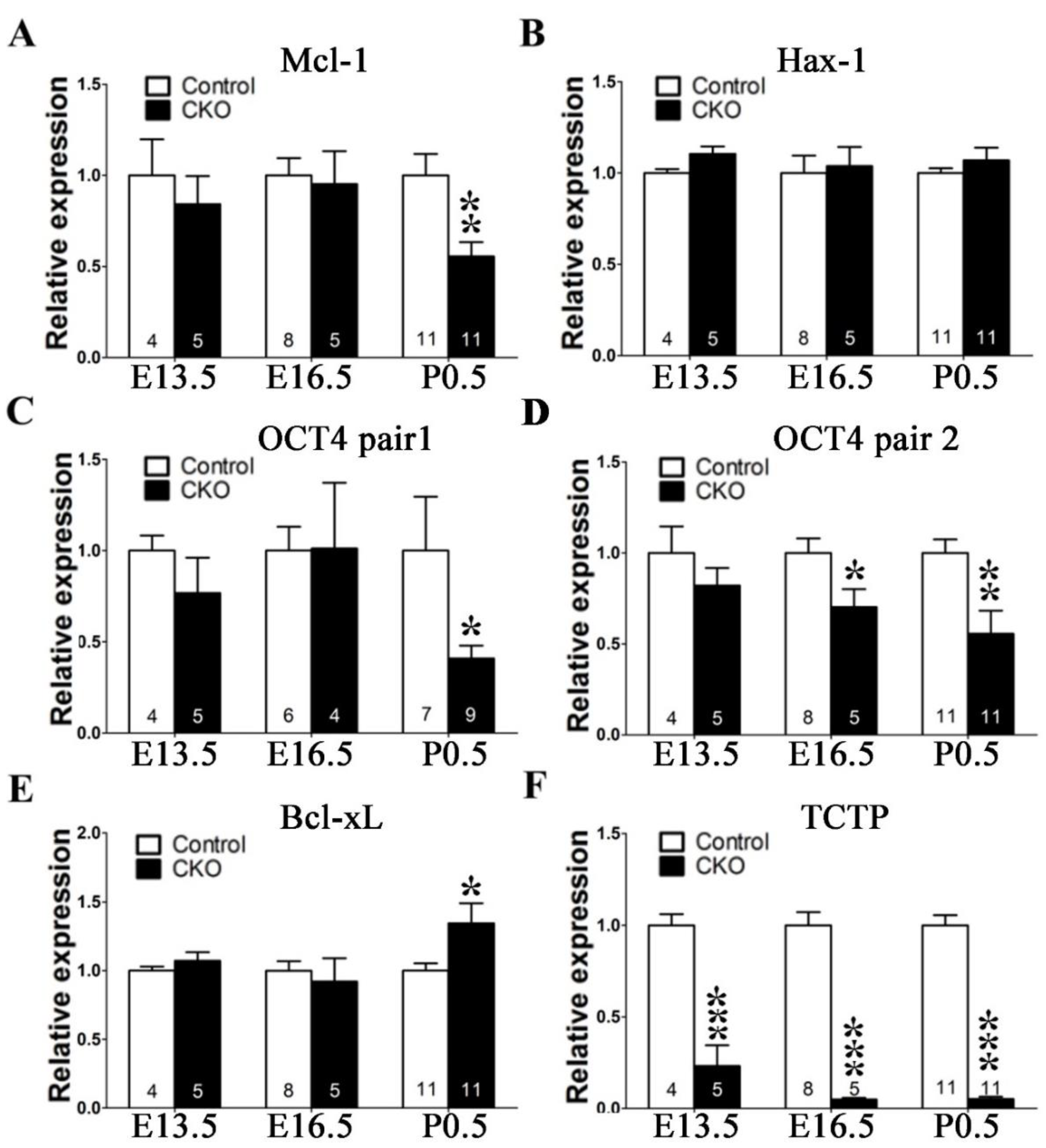
3.6. Disruption of TCTP-Induced Cell Apoptosis and Cell Autonomous Behavior
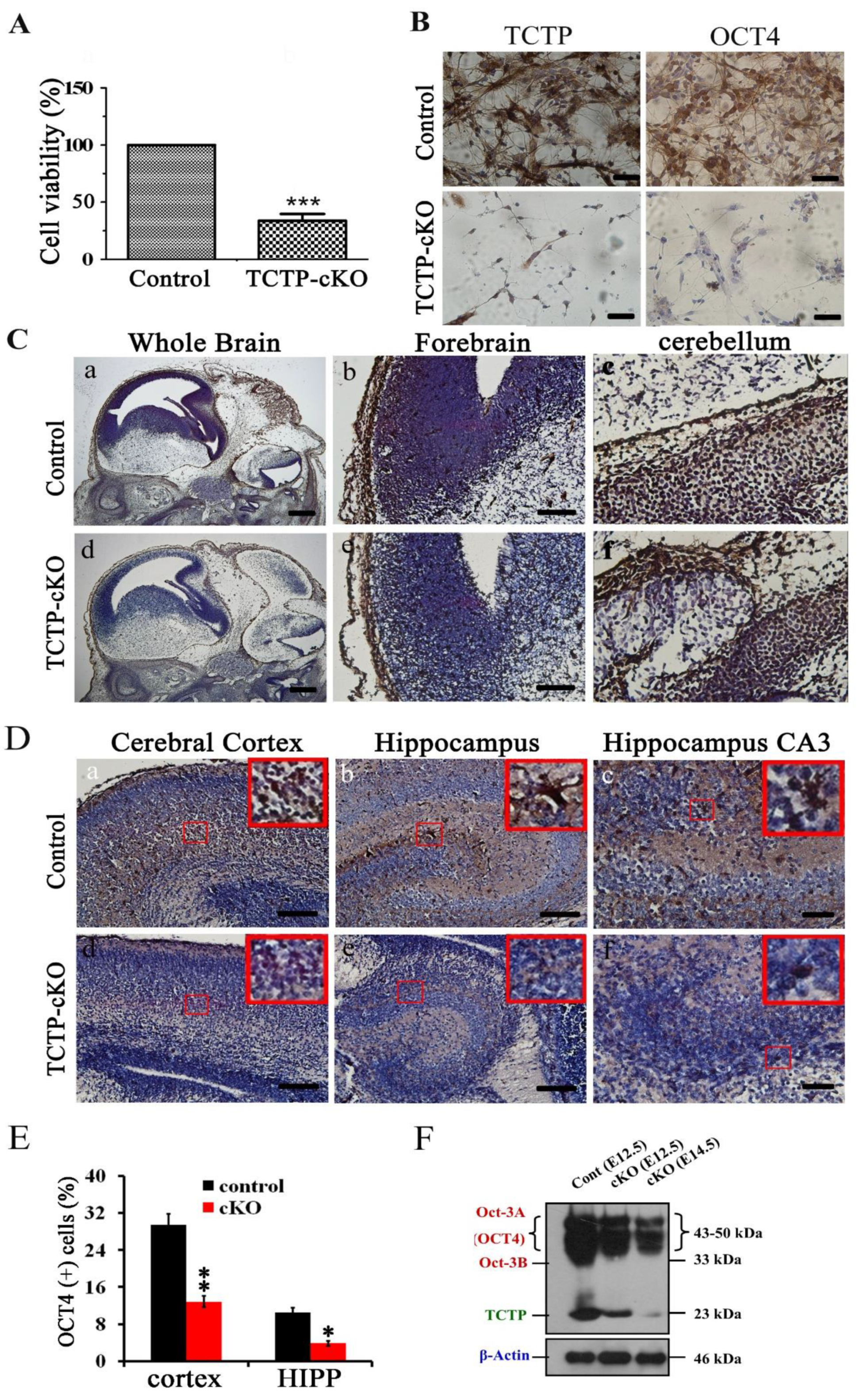
3.7. Conditional Deletion of TCTP in Neuron Progenitor Cells Resulted in Decreased Cell Survival and Suppression of Transcription Factor Oct4 Expression in Cortical Progenitor Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fiucci, G.; Lespagnol, A.; Stumptner-Cuvelette, P.; Beaucourt, S.; Duflaut, D.; Susini, L.; Amson, R.; Telerman, A. Genomic organization and expression of mouse Tpt1 gene. Genomics 2003, 81, 570–578. [Google Scholar] [CrossRef]
- Bommer, U.A.; Thiele, B.J. The translationally controlled tumour protein (TCTP). Int. J. Biochem. Cell Boil. 2004, 36, 379–385. [Google Scholar] [CrossRef]
- Gachet, Y.; Tournier, S.; Lee, M.; Lazaris-Karatzas, A.; Poulton, T.; Bommer, U.A. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J. Cell Sci. 1999, 112, 1257–1271. [Google Scholar]
- Jung, J.; Kim, M.; Kim, M.J.; Kim, J.; Moon, J.; Lim, J.S.; Kim, M.; Lee, K. Translationally controlled tumor protein interacts with the third cytoplasmic domain of Na,K-ATPase alpha subunit and inhibits the pump activity in HeLa cells. J. Boil. Chem. 2004, 279, 49868–49875. [Google Scholar] [CrossRef] [PubMed]
- Yarm, F.R. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell. Boil. 2002, 22, 6209–6221. [Google Scholar] [CrossRef] [PubMed]
- Cans, C.; Passer, B.J.; Shalak, V.; Nancy-Portebois, V.; Crible, V.; Amzallag, N.; Allanic, D.; Tufino, R.; Argentini, M.; Moras, D.; et al. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc. Natl. Acad. Sci. USA 2003, 100, 13892–13897. [Google Scholar] [CrossRef] [PubMed]
- Langdon, J.M.; Vonakis, B.M.; MacDonald, S.M. Identification of the interaction between the human recombinant histamine releasing factor/translationally controlled tumor protein and elongation factor-1 delta (also known as eElongation factor-1B beta). Biochim. Biophys. Acta 2004, 1688, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Amzallag, N.; Passer, B.J.; Allanic, D.; Segura, E.; Thery, C.; Goud, B.; Amson, R.; Telerman, A. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J. Boil. Chem. 2004, 279, 46104–46112. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.W.; Cheng, Y.S.; Yuan, H.S.; Yang-Yen, H.F. Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol. Cell. Boil. 2005, 25, 3117–3126. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Xiong, Z.; Yan, Y.; Wang, X.; Nishino, M.; Mirkovic, D.; Nguyen, J.; Wang, H.; Yang, X.F. An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene 2005, 24, 4778–4788. [Google Scholar] [CrossRef]
- Rid, R.; Onder, K.; Trost, A.; Bauer, J.; Hintner, H.; Ritter, M.; Jakab, M.; Costa, I.; Reischl, W.; Richter, K.; et al. H2O2-dependent translocation of TCTP into the nucleus enables its interaction with VDR in human keratinocytes: TCTP as a further module in calcitriol signalling. J. Steroid Biochem. Mol. Boil. 2010, 118, 29–40. [Google Scholar] [CrossRef]
- Rho, S.B.; Lee, J.H.; Park, M.S.; Byun, H.J.; Kang, S.; Seo, S.S.; Kim, J.Y.; Park, S.Y. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011, 585, 29–35. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Rafnar, T.; Langdon, J.; Lichtenstein, L.M. Molecular identification of an IgE-dependent histamine-releasing factor. Science 1995, 269, 688–690. [Google Scholar] [CrossRef]
- Kim, S.H.; Cairns, N.; Fountoulakisc, M.; Lubec, G. Decreased brain histamine-releasing factor protein in patients with Down syndrome and Alzheimer’s disease. Neurosci. Lett. 2001, 300, 41–44. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, P.S.; Chou, C.H.; Yan, Y.T.; Liu, H.; Weng, S.Y.; Yang-Yen, H.F. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific manner. Mol. Boil. Cell 2007, 18, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Yang, C.Y.; Yen, J.J.; Chou, C.H.; Chen, S.H.; Wang, C.K.; Lai, Y.G.; Liao, N.S.; Yang-Yen, H.F. Critical roles of translationally controlled tumor protein in the homeostasis and TCR-mediated proliferation of peripheral T cells. J. Immunol. 2009, 183, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Yang-Yen, H.F.; Chiang, M.K.; Wang, M.J.; Wu, S.S.; Chen, S.H. TCTP is essential for beta-cell proliferation and mass expansion during development and beta-cell adaptation in response to insulin resistance. Endocrinology 2014, 155, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Vanderluit, J.L.; Le Grand, J.N.; Jahani-Asl, A.; Ruzhynsky, V.A.; Cheung, E.C.; Kelly, M.A.; MacKenzie, A.E.; Park, D.S.; Opferman, J.T.; et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 6068–6078. [Google Scholar] [CrossRef]
- Motoyama, N.; Wang, F.; Roth, K.A.; Sawa, H.; Nakayama, K.; Negishi, I.; Senju, S.; Zhang, Q.; Fujii, S.; Loh, D.Y. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 1995, 267, 1506–1510. [Google Scholar] [CrossRef]
- Savitt, J.M.; Jang, S.S.; Mu, W.; Dawson, V.L.; Dawson, T.M. Bcl-x is required for proper development of the mouse substantia nigra. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 6721–6728. [Google Scholar] [CrossRef]
- Roque, C.G.; Wong, H.H.; Lin, J.Q.; Holt, C.E. Tumor protein Tctp regulates axon development in the embryonic visual system. Development (Camb. Engl.) 2016, 143, 1134–1148. [Google Scholar] [CrossRef]
- Berube, N.G.; Mangelsdorf, M.; Jagla, M.; Vanderluit, J.; Garrick, D.; Gibbons, R.J.; Higgs, D.R.; Slack, R.S.; Picketts, D.J. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J. Clin. Investig. 2005, 115, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Tronche, F.; Kellendonk, C.; Kretz, O.; Gass, P.; Anlag, K.; Orban, P.C.; Bock, R.; Klein, R.; Schutz, G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999, 23, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Magaud, J.P.; Sargent, I.; Clarke, P.J.; Ffrench, M.; Rimokh, R.; Mason, D.Y. Double immunocytochemical labeling of cell and tissue samples with monoclonal anti-bromodeoxyuridine. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1989, 37, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Mar, M.H.; Meeker, R.B.; Fernandes, A.; Zeisel, S.H. Choline deficiency induces apoptosis in primary cultures of fetal neurons. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, D.A.; Dong, L.; Callaghan, S.M.; Hou, Y.X.; Dagnino, L.; Slack, R.S. Neural precursor cells differentiating in the absence of Rb exhibit delayed terminal mitosis and deregulated E2F 1 and 3 activity. Dev. Biol. 1999, 207, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Gilyarov, A.V. Nestin in central nervous system cells. Neurosci. Behave. Physiol. 2008, 38, 165–169. [Google Scholar] [CrossRef]
- Und Halbach, O.V. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007, 329, 409–420. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Liang, H.; Hippenmeyer, S.; Ghashghaei, H.T. A Nestin-cre transgenic mouse is insufficient for recombination in early embryonic neural progenitors. Biol. Open 2012, 1, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Susini, L.; Besse, S.; Duflaut, D.; Lespagnol, A.; Beekman, C.; Fiucci, G.; Atkinson, A.R.; Busso, D.; Poussin, P.; Marine, J.C.; et al. TCTP protects from apoptotic cell death by antagonizing bax function. Cell Death Differ. 2008, 15, 1211–1220. [Google Scholar] [CrossRef]
- Niwa, H.; Miyazaki, J.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Koziol, M.J.; Garrett, N.; Gurdon, J.B. Tpt1 activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr. Boil. CB 2007, 17, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, D.; Fujise, K. Characterization of fortilin, a novel antiapoptotic protein. J. Boil. Chem. 2001, 276, 47542–47549. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Buchan, H.L.; Townsend, K.J.; Craig, R.W. MCL-1, a member of the BLC-2 family, is induced rapidly in response to signals for cell differentiation or death, but not to signals for cell proliferation. J. Cell. Physiol. 1996, 166, 523–536. [Google Scholar] [CrossRef]
- Suzuki, Y.; Demoliere, C.; Kitamura, D.; Takeshita, H.; Deuschle, U.; Watanabe, T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J. Immunol. (Baltim. Md. 1950) 1997, 158, 2736–2744. [Google Scholar]
- Amson, R.; Pece, S.; Lespagnol, A.; Vyas, R.; Mazzarol, G.; Tosoni, D.; Colaluca, I.; Viale, G.; Rodrigues-Ferreira, S.; Wynendaele, J.; et al. Reciprocal repression between P53 and TCTP. Nat. Med. 2011, 18, 91–99. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chern, J.J.; Cai, Y.; Liu, M.; Choi, K.W. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 2007, 445, 785–788. [Google Scholar] [CrossRef]
- Hao, S.; Qin, Y.; Yin, S.; He, J.; He, D.; Wang, C. Serum translationally controlled tumor protein is involved in rat liver regeneration after hepatectomy. Hepatol. Res. 2016, 46, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Chen, D.; Luo, S.; Hao, W.; Jing, F.; Liu, T.; Wang, S.; Geng, Y.; Li, L.; Xu, W.; et al. Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/ MMP9 signaling. Oncotarget 2016, 7, 50057–50073. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Zaehres, H.; Lensch, M.W.; Daheron, L.; Stewart, S.A.; Itskovitz-Eldor, J.; Daley, G.Q. High-efficiency RNA interference in human embryonic stem cells. Stem Cells (Dayt. Ohio) 2005, 23, 299–305. [Google Scholar] [CrossRef]
- Rodda, D.J.; Chew, J.L.; Lim, L.H.; Loh, Y.H.; Wang, B.; Ng, H.H.; Robson, P. Transcriptional regulation of nanog by OCT4 and SOX2. J. Boil. Chem. 2005, 280, 24731–24737. [Google Scholar] [CrossRef]
- Radzisheuskaya, A.; Chia Gle, B.; dos Santos, R.L.; Theunissen, T.W.; Castro, L.F.; Nichols, J.; Silva, J.C. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat. Cell Biol. 2013, 15, 579–590. [Google Scholar] [CrossRef]
- Jing, Y.; He, L.L.; Mei, C.L. Translationally-controlled tumor protein activates the transcription of Oct-4 in kidney-derived stem cells. Exp. Ther. Med. 2017, 13, 280–284. [Google Scholar] [CrossRef][Green Version]
- Cheng, X.; Li, J.; Deng, J.; Li, Z.; Meng, S.; Wang, H. Translationally controlled tumor protein (TCTP) downregulates Oct4 expression in mouse pluripotent cells. BMB Rep. 2012, 45, 20–25. [Google Scholar] [CrossRef]
- Mizushima, N.; Yamamoto, A.; Hatano, M.; Kobayashi, Y.; Kabeya, Y.; Suzuki, K.; Tokuhisa, T.; Ohsumi, Y.; Yoshimori, T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Boil. 2001, 152, 657–668. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Vejsada, R.; Poueymirou, W.T.; Acheson, A.; Suri, C.; Conover, J.C.; Friedman, B.; McClain, J.; Pan, L.; Stahl, N.; et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell 1995, 83, 313–322. [Google Scholar] [CrossRef]
- Segre, J.A.; Bauer, C.; Fuchs, E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet. 1999, 22, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.M.; Teicher, M.H. Suckling. Science 1980, 210, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Westneat, M.W.; Hall, W.G. Ontogeny of feeding motor patterns in infant rats: An electromyographic analysis of suckling and chewing. Behav. Neurosci. 1992, 106, 539–554. [Google Scholar] [CrossRef] [PubMed]
| Stage | Embryonic Time Point | |||||||
|---|---|---|---|---|---|---|---|---|
| E9.5~E10.5 | E12.5 | E14.5 | E16.5 | P0.5 | P1.5 | P3.5 | P10.5 | |
| Litter | 6 | 4 | 5 | 4 | 11 | 5 | 8 | 11 |
| Total number of Offspring | 37 | 22 | 31 | 33 | 73 | 38 | 48 | 71 |
| NestinCre/+; TCTPf/f | 10 (27%) | 5 (23%) | 8 (27%) | 7 (21%) | 25 (34%) | 7 (18%) | 0 (0%) | 0 (0%) |
| NestinCre/+; TCTPf/+ | 12 (32%) | 5 (23%) | 10 (32%) | 9 (27%) | 25 (34%) | 16 (42%) | 16 (33%) | 28 (39%) |
| Nestin+/+; TCTPf/f | 5 (14%) | 8 (36%) | 6 (19%) | 9 (27%) | 14 (19%) | 6 (16%) | 19 (40%) | 22 (31%) |
| Nestin+/+; TCTPf/w | 10 (27%) | 4 (18%) | 6 (19%) | 8 (24%) | 9 (12%) | 9 (24%) | 13 (27%) | 21 (30%) |
| Sequence | ||
|---|---|---|
| Gene | 5′-Sense-3′ | 5′-Anti-Sense-3′ |
| Gapdh | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT |
| Mcl-1 | GGAAGTCCTCGCCTGCGTCA | AAACATGGTCGGACGCCGCA |
| Bcl-xL | AGGCAGGCGATGAGTTTGAA | CGGCTCTCGGCTGCTGCATT |
| Hax-1 | GACCTTGCCTTCCCACTCTCCTGA | GTCCCTGCGACCCCCAATCTG |
| OCT4 pair1 * | GTGAGCCGTCTTTCCACCAGG | GGGTGAGAAGGCGAAGTCTG |
| OCT4 pair2# | CCCTCCCTGGGGATGCTGTGAG | GAGTGACAGACAGGCCAGGCTCC |
| Nanog | TGCGGCTCACTTCCTTCTGACTTC | GGCCCTTGTCAGCCTCAGGAC |
| TCTP | TATATGAGGTTGGGGAGCGCCCG | CCTCCGGACCTTCAGCGGAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Lu, C.-H.; Tsai, M.-J. TCTP is Essential for Cell Proliferation and Survival during CNS Development. Cells 2020, 9, 133. https://doi.org/10.3390/cells9010133
Chen S-H, Lu C-H, Tsai M-J. TCTP is Essential for Cell Proliferation and Survival during CNS Development. Cells. 2020; 9(1):133. https://doi.org/10.3390/cells9010133
Chicago/Turabian StyleChen, Sung-Ho, Chin-Hung Lu, and Ming-Jen Tsai. 2020. "TCTP is Essential for Cell Proliferation and Survival during CNS Development" Cells 9, no. 1: 133. https://doi.org/10.3390/cells9010133
APA StyleChen, S.-H., Lu, C.-H., & Tsai, M.-J. (2020). TCTP is Essential for Cell Proliferation and Survival during CNS Development. Cells, 9(1), 133. https://doi.org/10.3390/cells9010133





