MGL1 Receptor Plays a Key Role in the Control of T. cruzi Infection by Increasing Macrophage Activation through Modulation of ERK1/2, c-Jun, NF-κB and NLRP3 Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Parasites
2.3. Soluble T. cruzi Lysate Antigen (TcAg)
2.4. Cell preparations and T. cruzi Infection In Vitro
2.5. Reactive Oxygen Species Activity Assay
2.6. Detection of Nitric Oxide and Cytokines Production
2.7. Viability of Internalized T. cruzi
2.8. Flow Cytometry Analysis
2.9. Co-culture of Mφ and Splenic Cells
2.10. Protein Levels of NF-κB, P38, ERK1/2 and NLRP3 Signaling Pathways Detected by Western Blotting
2.11. Statistical Analysis
3. Results
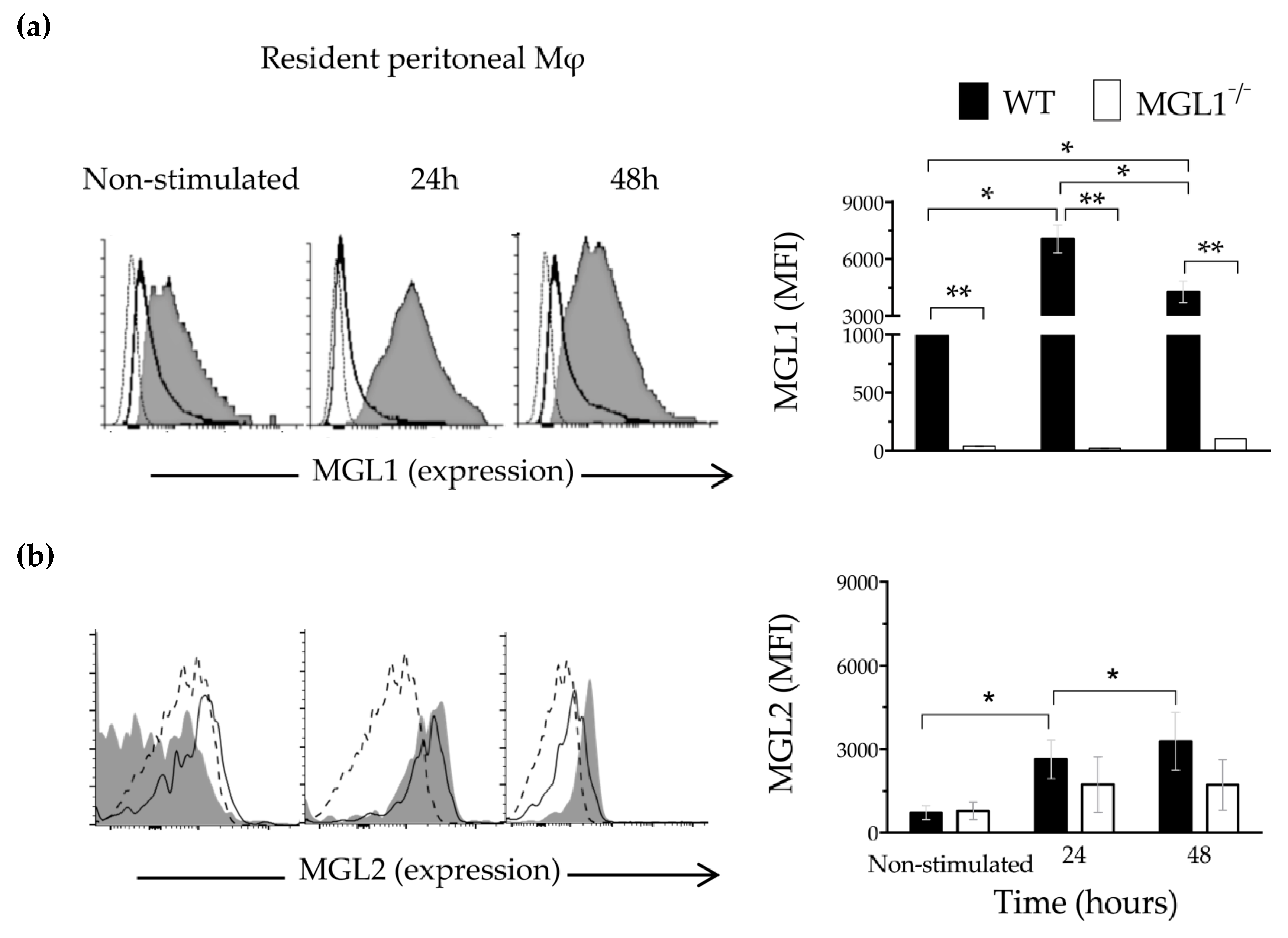
3.1. Trypanosoma cruzi Antigens Induce High Expression of MGL1 and Moderate Expression of MGL2 in PE-Mφ
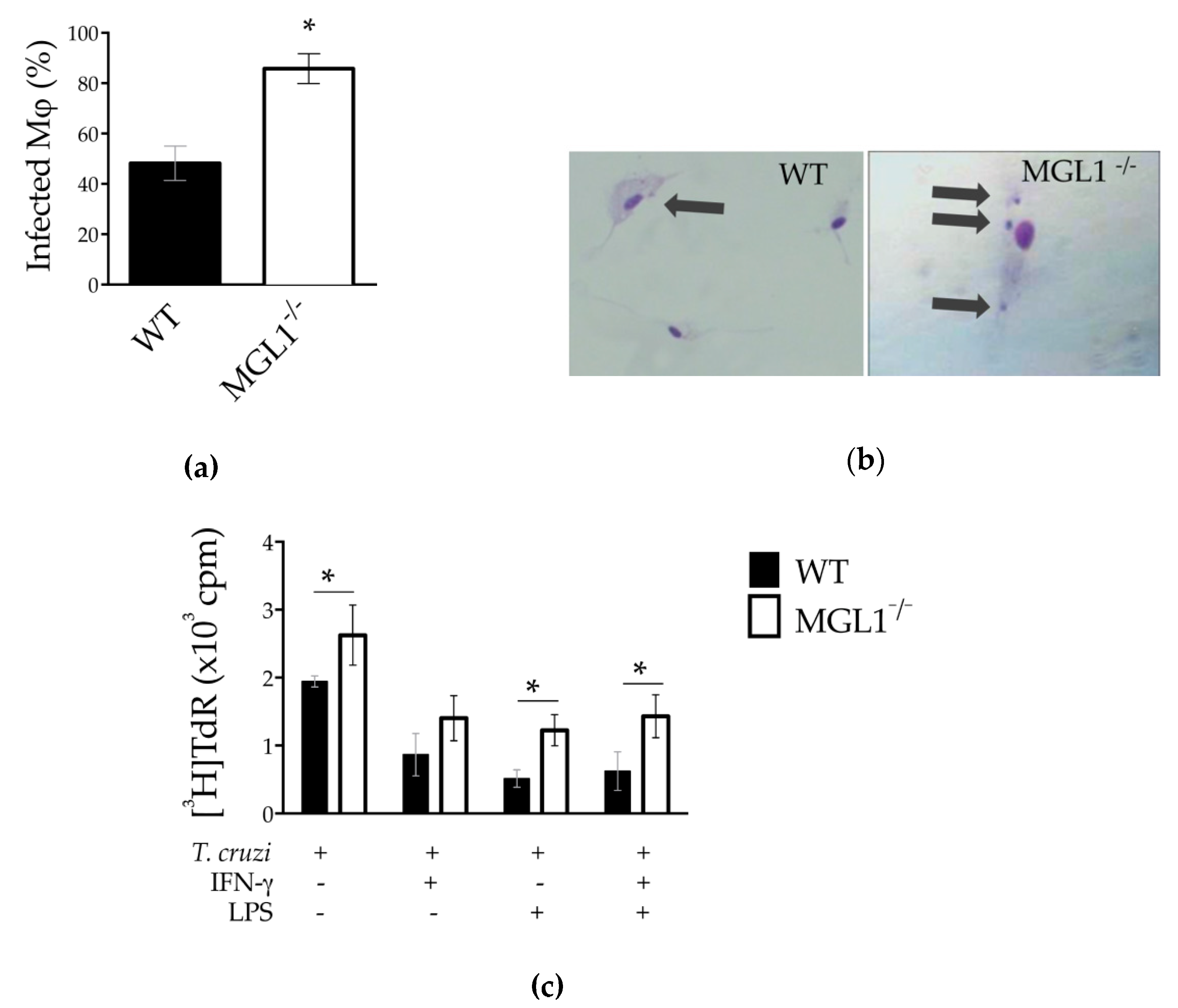
3.2. The MGL1 Receptor Plays a Role in Controlling T. cruzi PE-Mφ Infection
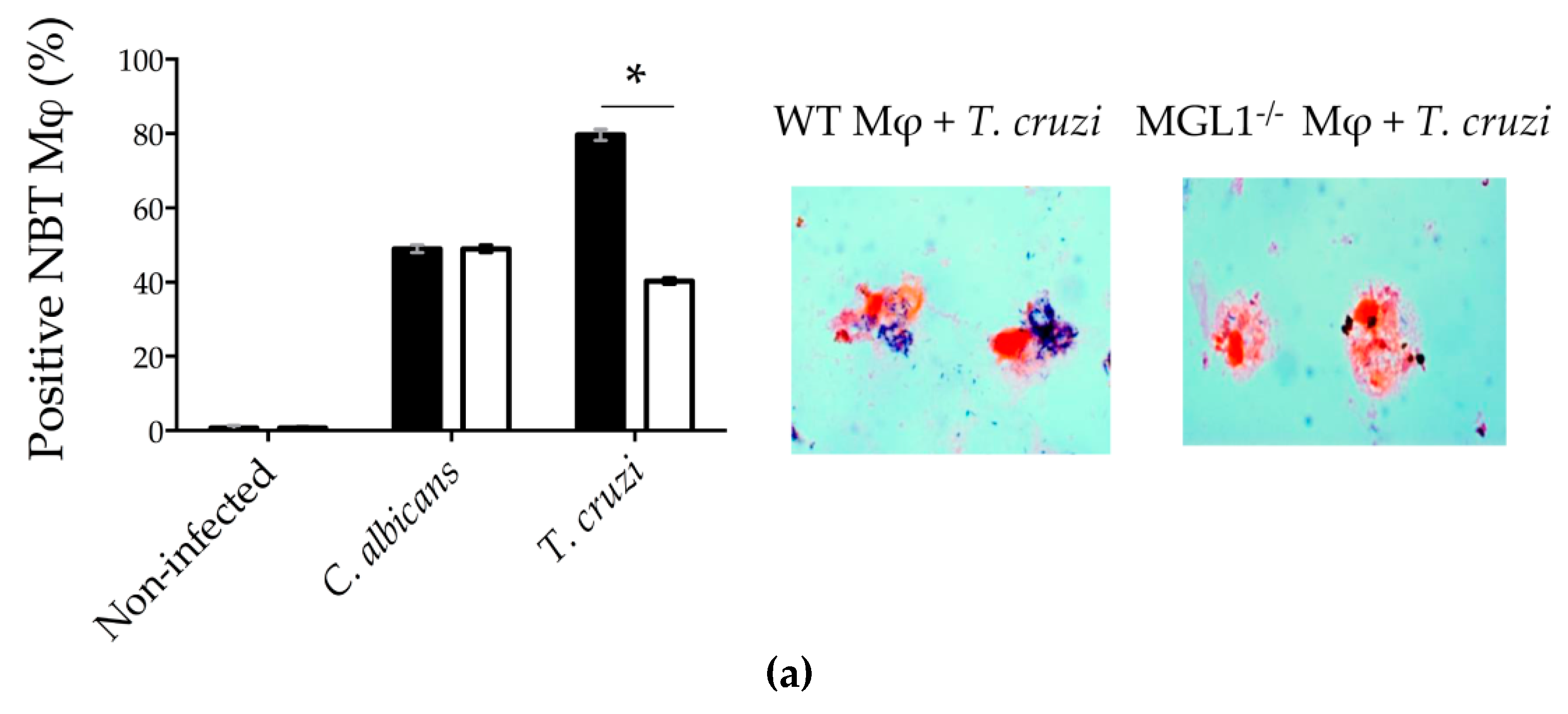
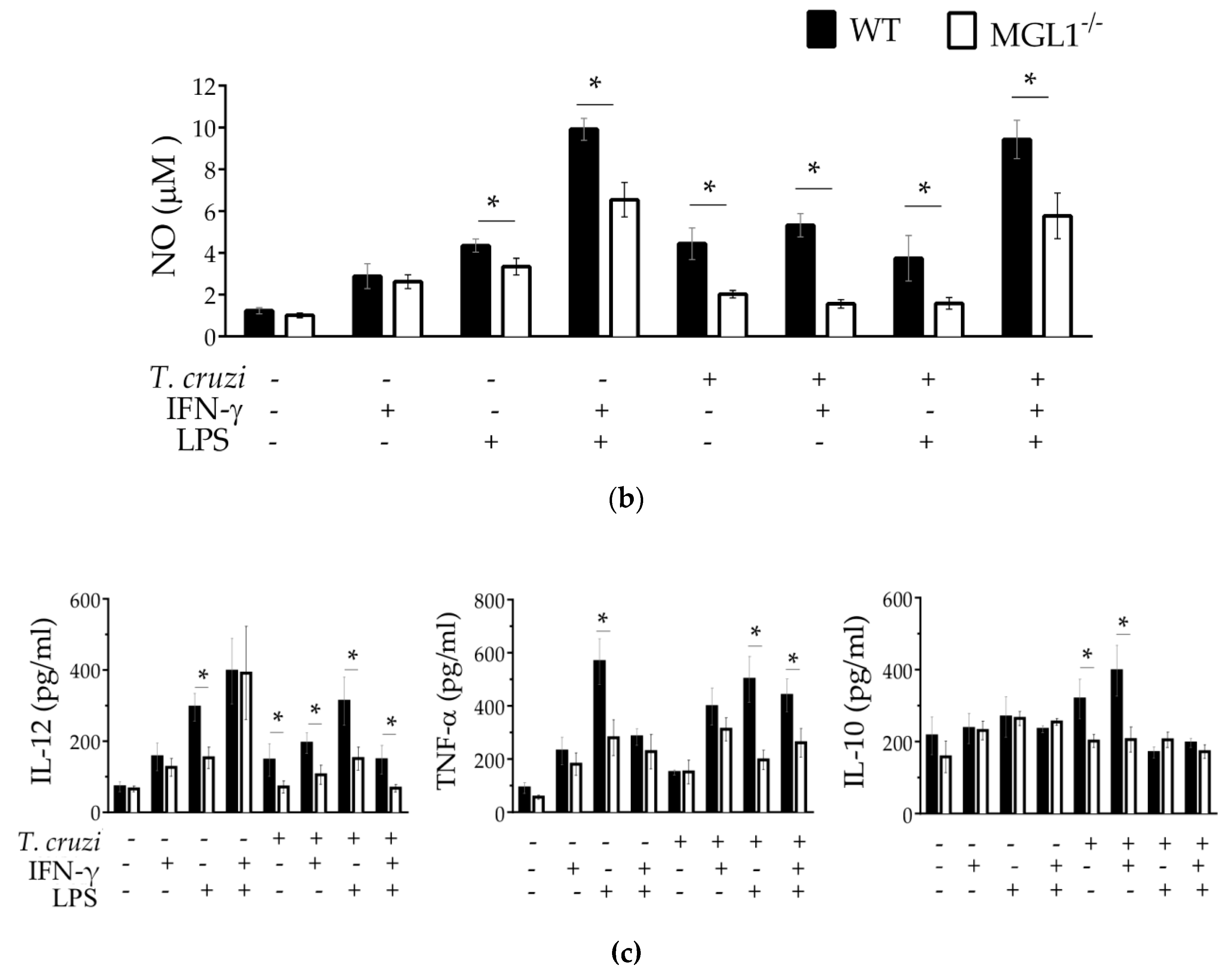
3.3. MGL1−/− PE-Mφ Have a Deficient Oxidative Burst, as well as Nitric Oxide and Proinflammatory Cytokine Production during T. cruzi Infection
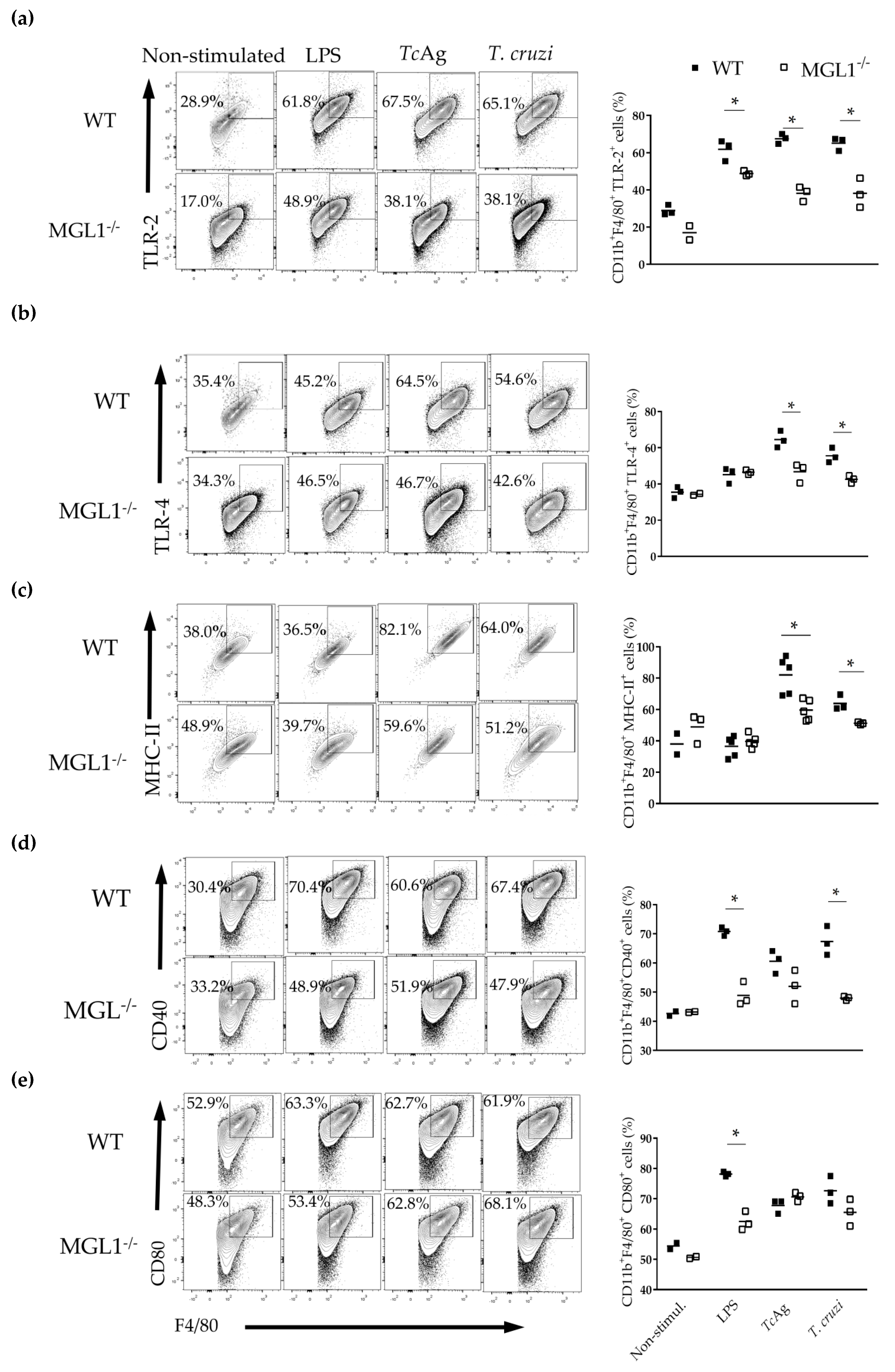
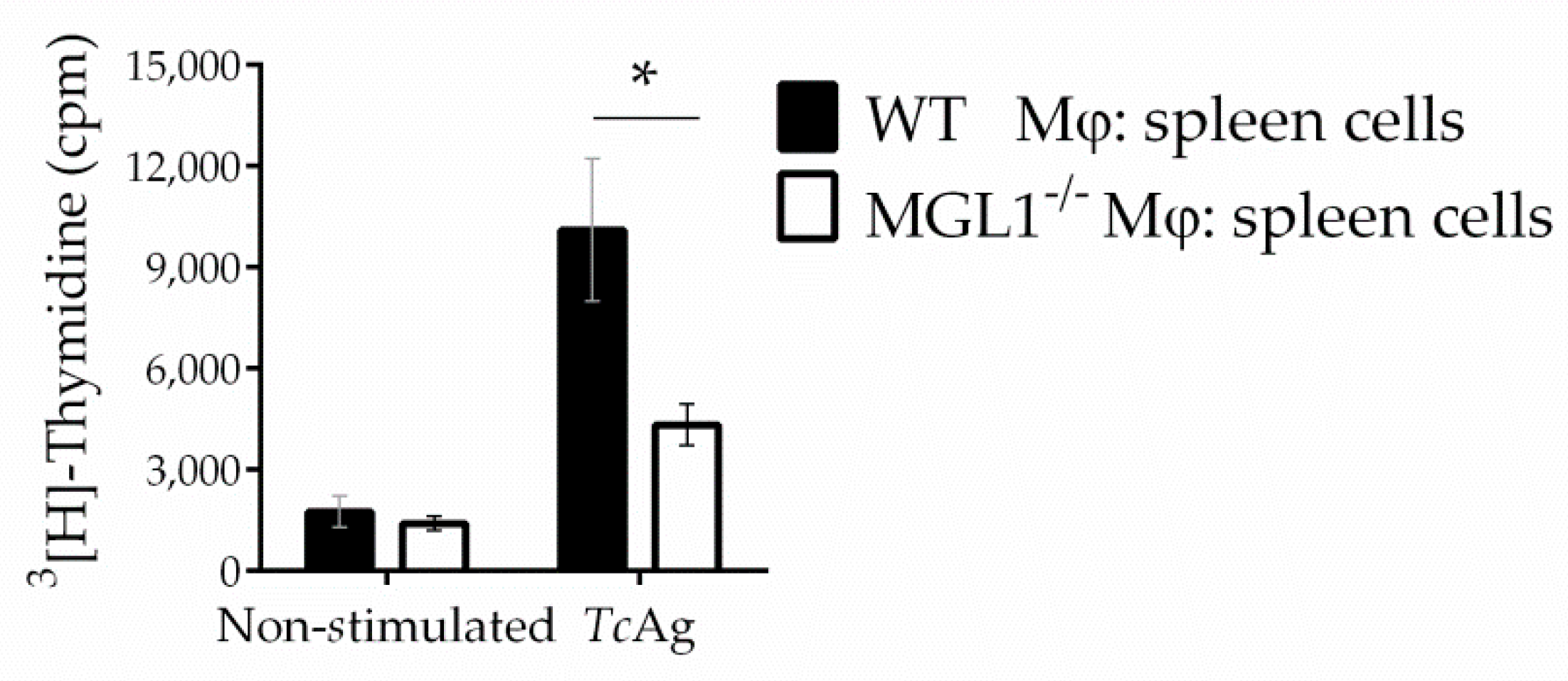
3.4. MGL1−/− PE-Mφ Exhibit Deficient Activation of T. cruzi Antigen-Specific Lymphocytes
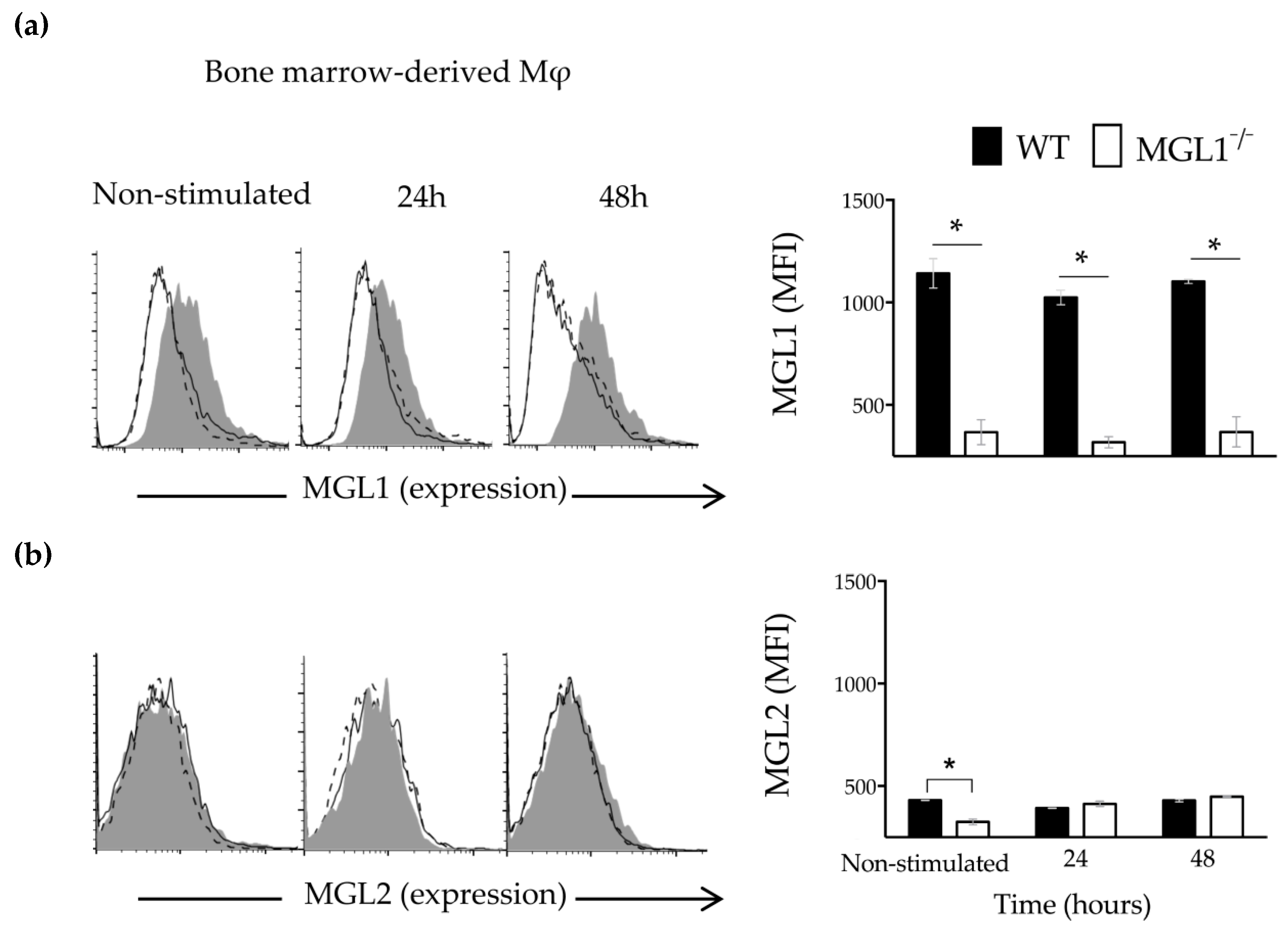
3.5. Trypanosoma cruzi Antigens Induce High Expression of MGL1 and Low Expression (Almost Absent) of MGL2 in BMMφ
3.6. MGL1 Deficiency in BMMφ Results in Reduced Activation of the NFκ-B and ERK1/2 Signaling Pathways in Response to T. cruzi Antigen
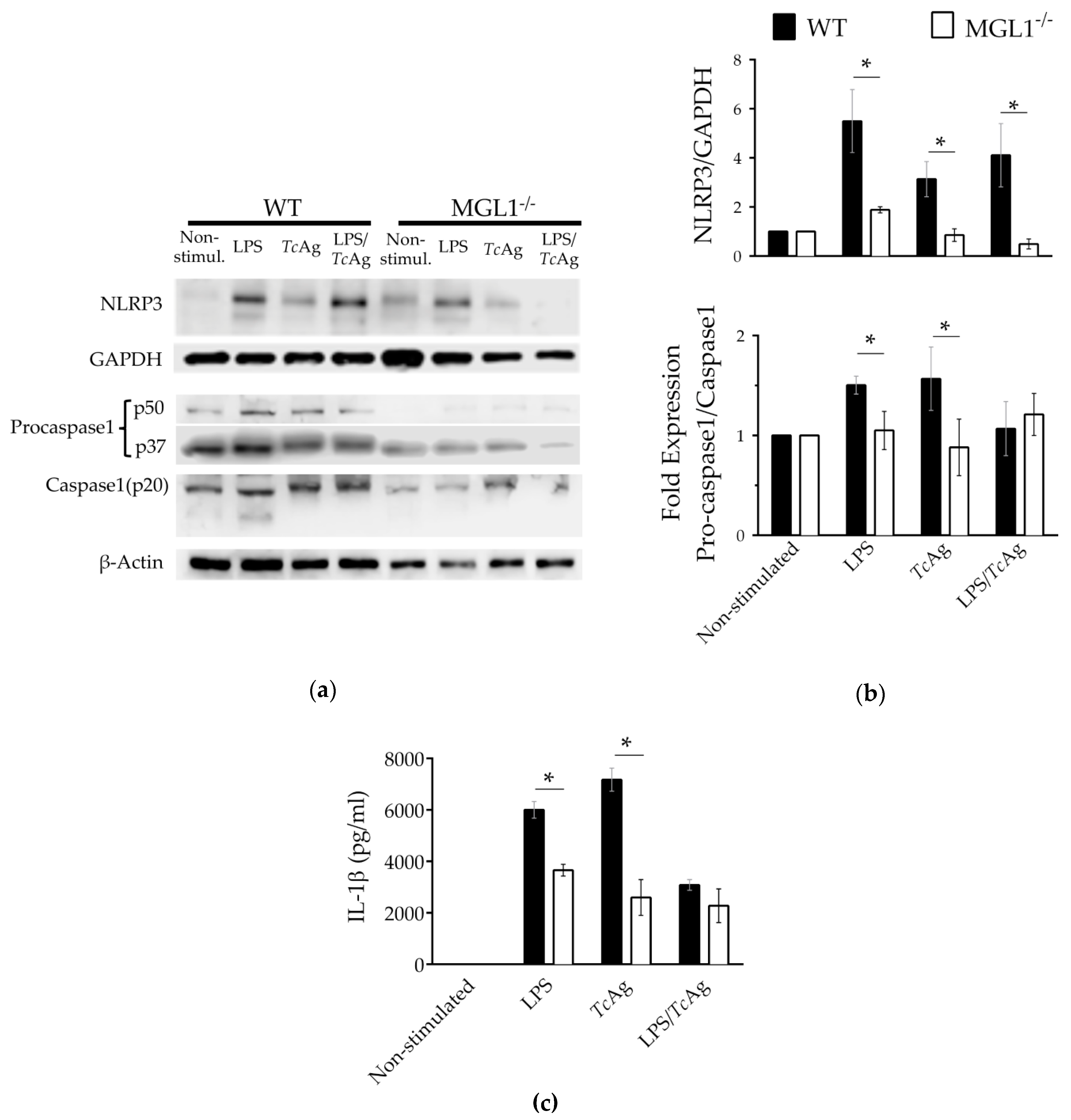
3.7. MGL1 Regulates the Expression of the NLP3 Sensor
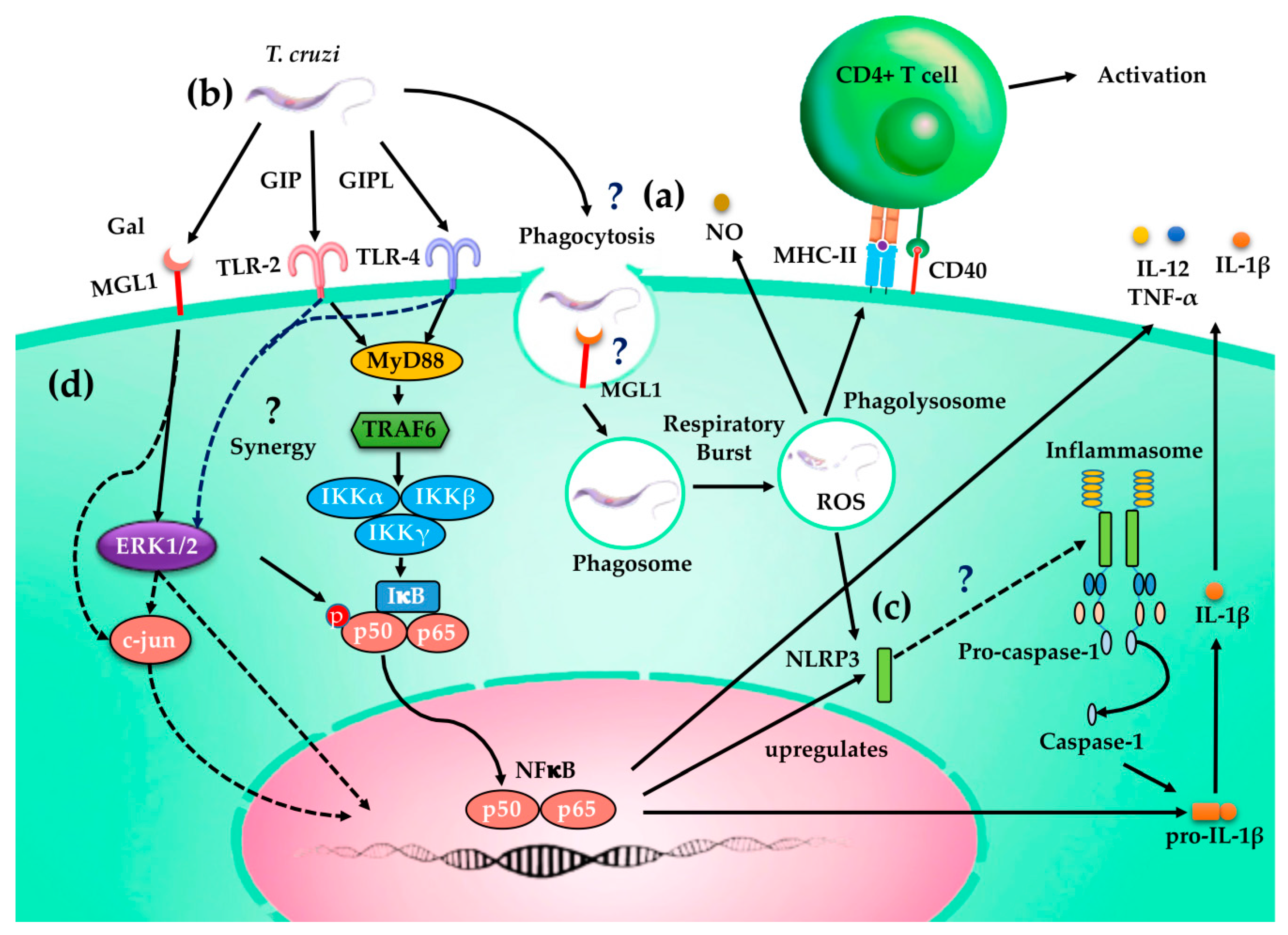
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Mendoza, A.; Carrero, J.C.; Rodriguez-Sosa, M. Parasitic infections: A role for C-type lectins receptors. Biomed. Res. Int. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Redelinghuys, P.; Brown, G.D. Inhibitory C-type lectin receptors in myeloid cells. Immunol. Lett. 2011, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.W.; van Beelen, A.J.; Bruijns, S.C.; Joshi, M.; Fehres, C.M.; van Bloois, L.; Verstege, M.I.; Ambrosini, M.; Kalay, H.; Nazmi, K. Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells. J. Control. Release 2012, 160, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, A.M.; Brown, G.D. C-type lectins and phagocytosis. Immunobiology 2009, 214, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.M.; Brown, G.D. The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine 2009, 48, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Willment, J.A.; Whitehead, L. C-type lectins in immunity and homeostasis. Nat. Rev. Immunol. 2018, 18, 374–389. [Google Scholar] [CrossRef]
- Montero-Barrera, D.; Valderrama-Carvajal, H.; Terrazas, C.A.; Rojas-Hernandez, S.; Ledesma-Soto, Y.; Vera-Arias, L.; Carrasco-Yepez, M.; Gomez-Garcia, L.; Martinez-Saucedo, D.; Becerra-Diaz, M.; et al. The macrophage galactose-type lectin-1 (MGL1) recognizes Taenia crassiceps antigens, triggers intracellular signaling, and is critical for resistance to this infection. Biomed. Res. Int. 2015, 2015, 615865. [Google Scholar] [CrossRef]
- Del Fresno, C.; Iborra, S.; Saz-Leal, P.; Martinez-Lopez, M.; Sancho, D. Flexible Signaling of Myeloid C-Type Lectin Receptors in Immunity and Inflammation. Front. Immunol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- van Kooyk, Y. C-type lectins on dendritic cells: Key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008, 36, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, L.; Lugo-Villarino, G.; Meunier, E.; Valentin, A.; Olagnier, D.; Authier, H.; Duval, C.; Dardenne, C.; Bernad, J.; Lemesre, J.L. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity 2013, 38, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; O’Sullivan, J.M.; Drakeford, C.; Aguila, S.; Jondle, C.N.; Sharma, J.; Fallon, P.G.; Brophy, T.M.; Preston, R.J.S.; Smyth, P.; et al. A novel role for the macrophage galactose-type lectin receptor in mediating von Willebrand factor clearance. Blood 2018, 131, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kawakami, K.; Osawa, T.; Toyoshima, S. Molecular cloning and expression of cDNA encoding a galactose/N-acetylgalactosamine-specific lectin on mouse tumoricidal macrophages. J. Biochem. 1992, 111, 331–336. [Google Scholar] [CrossRef]
- Tsuiji, M.; Fujimori, M.; Ohashi, Y.; Higashi, N.; Onami, T.M.; Hedrick, S.M.; Irimura, T. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J. Biol. Chem. 2002, 277, 28892–28901. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Aarnoudse, C.A.; Broks-van den Berg, V.C.; Boks, M.; Geijtenbeek, T.B.; van Kooyk, Y. MGL-mediated internalization and antigen presentation by dendritic cells: A role for tyrosine-5. Eur. J. Immunol. 2007, 37, 2075–2081. [Google Scholar] [CrossRef]
- Upham, J.P.; Pickett, D.; Irimura, T.; Anders, E.M.; Reading, P.C. Macrophage receptors for influenza A virus: Role of the macrophage galactose-type lectin and mannose receptor in viral entry. J. Virol. 2010, 84, 3730–3737. [Google Scholar] [CrossRef]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Weelij, D.R.; Garcia-Vallejo, J.J.; van Vliet, S.J.; Saeland, E.; van Kooyk, Y. Characterization of murine MGL1 and MGL2 C-type lectins: Distinct glycan specificities and tumor binding properties. Mol. Immunol. 2009, 46, 1240–1249. [Google Scholar] [CrossRef]
- Denda-Nagai, K.; Aida, S.; Saba, K.; Suzuki, K.; Moriyama, S.; Oo-Puthinan, S.; Tsuiji, M.; Morikawa, A.; Kumamoto, Y.; Sugiura, D.; et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): Efficient uptake and presentation of glycosylated antigens by dendritic cells. J. Biol. Chem. 2010, 285, 19193–19204. [Google Scholar] [CrossRef]
- Filardy, A.A.; Guimaraes-Pinto, K.; Nunes, M.P.; Zukeram, K.; Fliess, L.; Pereira, L.; Oliveira Nascimento, D.; Conde, L.; Morrot, A. Human Kinetoplastid Protozoan Infections: Where Are We Going Next? Front. Immunol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Acosta-Serrano, A.; Almeida, I.C.; Freitas-Junior, L.H.; Yoshida, N.; Schenkman, S. The mucin-like glycoprotein super-family of Trypanosoma cruzi: Structure and biological roles. Mol. Biochem. Parasitol. 2001, 114, 143–150. [Google Scholar] [CrossRef]
- Vazquez, A.; Ruiz-Rosado Jde, D.; Terrazas, L.I.; Juarez, I.; Gomez-Garcia, L.; Calleja, E.; Camacho, G.; Chavez, A.; Romero, M.; Rodriguez, T.; et al. Mouse macrophage galactose-type lectin (mMGL) is critical for host resistance against Trypanosoma cruzi infection. Int. J. Biol. Sci. 2014, 10, 909–920. [Google Scholar] [CrossRef]
- Onami, T.M.; Lin, M.Y.; Page, D.M.; Reynolds, S.A.; Katayama, C.D.; Marth, J.D.; Irimura, T.; Varki, A.; Varki, N.; Hedrick, S.M. Generation of mice deficient for macrophage galactose- and N-acetylgalactosamine-specific lectin: Limited role in lymphoid and erythroid homeostasis and evidence for multiple lectins. Mol. Cell. Biol. 2002, 22, 5173–5181. [Google Scholar] [CrossRef]
- Laird, P.W.; Zijderveld, A.; Linders, K.; Rudnicki, M.A.; Jaenisch, R.; Berns, A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991, 19, 4293. [Google Scholar] [CrossRef]
- Migliorini, P.; Corradin, G.; Corradin, S.B. Macrophage NO2-production as a sensitive and rapid assay for the quantitation of murine IFN-gamma. J. Immunol. Methods 1991, 139, 107–114. [Google Scholar] [CrossRef]
- Mylonas, K.J.; Nair, M.G.; Prieto-Lafuente, L.; Paape, D.; Allen, J.E. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J. Immunol. 2009, 182, 3084–3094. [Google Scholar] [CrossRef]
- Manzanero, S. Generation of mouse bone marrow-derived macrophages. Methods Mol. Biol. 2012, 844, 177–181. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Paiva, C.N.; Feijó, D.F.; Dutra, F.F.; Carneiro, V.C.; Freitas, G.B.; Alves, L.S.; Mesquita, J.; Fortes, G.B.; Figueiredo, R.T.; Souza, H.S. Oxidative stress fuels Trypanosoma cruzi infection in mice. J. Clin. Investig. 2012, 122, 2531–2542. [Google Scholar] [CrossRef]
- Abrahamsohn, I.A.; Coffman, R.L. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 1996, 84, 231–244. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Bay, S.; Vuist, I.M.; Kalay, H.; García-Vallejo, J.J.; Leclerc, C.; van Kooyk, Y. MGL signaling augments TLR2-mediated responses for enhanced IL-10 and TNF-α secretion. J. Leukoc. Biol. 2013, 94, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.D.; Miller, H.R.; Yu, G.; Tallarico, J.A.; Sorger, P.K.; Wang, Y.; Feng, Y.; Thomas, J.R.; Ross, N.T.; Mitchison, T. A High Content Screen in Macrophages Identifies Small Molecule Modulators of STING-IRF3 and NFkB Signaling. ACS Chem. Biol. 2018, 13, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Dlugosz, E.; Basalaj, K.; Zawistowska-Deniziak, A. Cytokine production and signalling in human THP-1 macrophages is dependent on Toxocara canis glycans. Parasitol. Res. 2019, 118, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.; Fernandez-Troy, N.; Espel, E. Post-transcriptional regulation of TNF-alpha during in vitro differentiation of human monocytes/macrophages in primary culture. J. Leukoc. Biol. 2002, 71, 1026–1032. [Google Scholar] [PubMed]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 2016, 13, 51. [Google Scholar] [CrossRef]
- Lawlor, K.E.; Vince, J.E. Ambiguities in NLRP3 inflammasome regulation: Is there a role for mitochondria? Biochim. Biophys. Acta 2014, 1840, 1433–1440. [Google Scholar] [CrossRef]
- Ng, W.C.; Liong, S.; Tate, M.D.; Irimura, T.; Denda-Nagai, K.; Brooks, A.G.; Londrigan, S.L.; Reading, P.C. The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J. Virol. 2014, 88, 1659–1672. [Google Scholar] [CrossRef]
- Ding, D.; Yao, Y.; Zhang, S.; Su, C.; Zhang, Y. C-type lectins facilitate tumor metastasis. Oncol. Lett. 2017, 13, 13–21. [Google Scholar] [CrossRef][Green Version]
- Singh, S.K.; Streng-Ouwehand, I.; Litjens, M.; Kalay, H.; Saeland, E.; van Kooyk, Y. Tumour-associated glycan modifications of antigen enhance MGL2 dependent uptake and MHC class I restricted CD8 T cell responses. Int. J. Cancer 2011, 128, 1371–1383. [Google Scholar] [CrossRef]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Freire, T.; Zhang, X.; Deriaud, E.; Ganneau, C.; Vichier-Guerre, S.; Azria, E.; Launay, O.; Lo-Man, R.; Bay, S.; Leclerc, C. Glycosidic Tn-based vaccines targeting dermal dendritic cells favor germinal center B-cell development and potent antibody response in the absence of adjuvant. Blood 2010, 116, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gutierrez, E.; Perez-Cervera, Y.; Camoin, L.; Zenteno, E.; Aquino-Gil, M.O.; Lefebvre, T.; Cabrera-Bravo, M.; Reynoso-Ducoing, O.; Bucio-Torres, M.I.; Salazar-Schettino, P.M. Identification of O-Glcnacylated Proteins in Trypanosoma cruzi. Front. Endocrinol. 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Closel, M.; Valente, E.P.; Cardoso, J.E.; Akira, S.; Alvarez-Leite, J.I.; Ropert, C.; Gazzinelli, R.T. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J. Immunol. 2004, 172, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.J.; Chowdhury, I.H.; Szczesny, B.; Wan, X.; Garg, N.J. Macrophages Promote Oxidative Metabolism To Drive Nitric Oxide Generation in Response to Trypanosoma cruzi. Infect. Immun. 2016, 84, 3527–3541. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.E.; de Lederkremer, R.M. Trans-sialidase and mucins of Trypanosoma cruzi: An important interplay for the parasite. Carbohydr. Res. 2011, 346, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Postan, M.; Mensa-Wilmot, K.; Tarleton, R.L. Glycosylphosphatidylinositols are required for the development of Trypanosoma cruzi amastigotes. Infect. Immun. 1997, 65, 4055–4060. [Google Scholar]
- Rodrigues, M.M.; Oliveira, A.C.; Bellio, M. The Immune Response to Trypanosoma cruzi: Role of Toll-Like Receptors and Perspectives for Vaccine Development. J. Parasitol. Res. 2012, 2012, 507874. [Google Scholar] [CrossRef]
- Campos, M.A.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procopio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef]
- Oliveira, A.-C.; Peixoto, J.R.; de Arruda, L.B.; Campos, M.A.; Gazzinelli, R.T.; Golenbock, D.T.; Akira, S.; Previato, J.O.; Mendonça-Previato, L.; Nobrega, A. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 2004, 173, 5688–5696. [Google Scholar] [CrossRef]
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate Defense against Fungal Pathogens. Cold Spring Harb. Perspect. Med. 2014, 5, a019620. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yang, G.; Chen, H.Y.; Hsu, D.K.; Tomilov, A.; Olson, K.A.; Dehnad, A.; Fish, S.R.; Cortopassi, G.; Zhao, B.; et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016, 30, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.M.; Matteucci, K.C.; Buzzo, C.L.; Miollo, B.H.; Ferrante, D.; Torrecilhas, A.C.; Rodrigues, M.M.; Alvarez, J.M.; Bortoluci, K.R. NLRP3 controls Trypanosoma cruzi infection through a caspase-1-dependent IL-1R-independent NO production. PLoS Negl. Trop. Dis. 2013, 7, e2469. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Sinha, M.; Gupta, S.; Gonzalez, M.N.; Fang, R.; Endsley, J.J.; Luxon, B.A.; Garg, N.J. Caspase-1/ASC inflammasome-mediated activation of IL-1β–ROS–NF-κB pathway for control of Trypanosoma cruzi replication and survival is dispensable in NLRP3−/−macrophages. PLoS ONE 2014, 9, e111539. [Google Scholar] [CrossRef]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, S.F.; Weng, I.C.; Hong, M.H.; Lo, T.H.; Jan, J.T.; Hsu, L.C.; Chen, H.Y.; Liu, F.T. Galectin-3 Enhances Avian H5N1 Influenza A Virus-Induced Pulmonary Inflammation by Promoting NLRP3 Inflammasome Activation. Am. J. Pathol. 2018, 188, 1031–1042. [Google Scholar] [CrossRef]

| Number of Amastigotes Per Macrophage | ||||||
|---|---|---|---|---|---|---|
| Mφ Infected/100 cells | 1 | 2–3 | 4 | 5 | ≥6 | |
| WT | 48% | 29% | 14% | 4% | 1% | 0% |
| MGL1−/− | 86%* | 22%* | 33%* | 21%* | 7%* | 3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, T.; Pacheco-Fernández, T.; Vázquez-Mendoza, A.; Nieto-Yañez, O.; Juárez-Avelar, I.; Reyes, J.L.; Terrazas, L.I.; Rodriguez-Sosa, M. MGL1 Receptor Plays a Key Role in the Control of T. cruzi Infection by Increasing Macrophage Activation through Modulation of ERK1/2, c-Jun, NF-κB and NLRP3 Pathways. Cells 2020, 9, 108. https://doi.org/10.3390/cells9010108
Rodriguez T, Pacheco-Fernández T, Vázquez-Mendoza A, Nieto-Yañez O, Juárez-Avelar I, Reyes JL, Terrazas LI, Rodriguez-Sosa M. MGL1 Receptor Plays a Key Role in the Control of T. cruzi Infection by Increasing Macrophage Activation through Modulation of ERK1/2, c-Jun, NF-κB and NLRP3 Pathways. Cells. 2020; 9(1):108. https://doi.org/10.3390/cells9010108
Chicago/Turabian StyleRodriguez, Tonathiu, Thalia Pacheco-Fernández, Alicia Vázquez-Mendoza, Oscar Nieto-Yañez, Imelda Juárez-Avelar, José L. Reyes, Luis I. Terrazas, and Miriam Rodriguez-Sosa. 2020. "MGL1 Receptor Plays a Key Role in the Control of T. cruzi Infection by Increasing Macrophage Activation through Modulation of ERK1/2, c-Jun, NF-κB and NLRP3 Pathways" Cells 9, no. 1: 108. https://doi.org/10.3390/cells9010108
APA StyleRodriguez, T., Pacheco-Fernández, T., Vázquez-Mendoza, A., Nieto-Yañez, O., Juárez-Avelar, I., Reyes, J. L., Terrazas, L. I., & Rodriguez-Sosa, M. (2020). MGL1 Receptor Plays a Key Role in the Control of T. cruzi Infection by Increasing Macrophage Activation through Modulation of ERK1/2, c-Jun, NF-κB and NLRP3 Pathways. Cells, 9(1), 108. https://doi.org/10.3390/cells9010108







