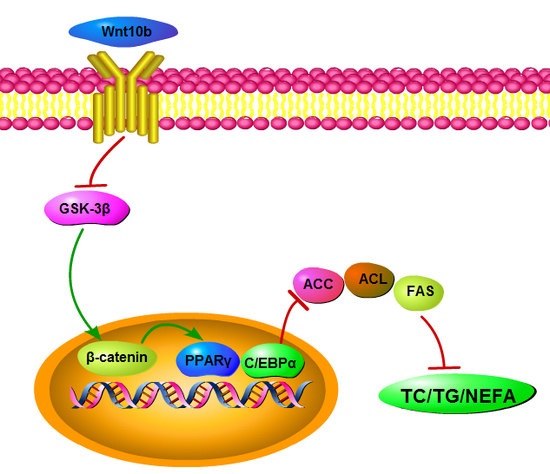
Wnt10b Participates in Regulating Fatty Acid Synthesis in the Muscle of Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Conditions
2.2. Construction of pEGFP-N1-Wnt10b Vector
2.3. Injection of the pEGFP-N1-Wnt10b Vector
2.4. Wnt10b RNA Interference
2.5. Real-Time Quantitative Polymerase Chain Reaction
2.6. Western-Blot Analysis
2.7. Biochemical Index Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of Wnt10b Gene Overexpression on the Gene Expression of Wnt10b, GSK-3β, β-catenin, C/EBPα, and PPARγ
3.2. Effect of Wnt10b Gene Overexpression on the Protein Expression of Wnt10b, GSK-3β, β-catenin, C/EBPα, and PPARγ
3.3. Effect of Wnt10b Gene Overexpression on the Gene Expression and Activity of Fatty Acid Synthetase (FAS), Acetyl-CoA Carboxylase (ACC) and ATP-Citrate Lyase (ACL),
3.4. Effect of Wnt10b Gene Overexpression on the Biochemical Index in the Muscle
3.5. Effect of Wnt10b Gene Interference on the Gene Expression of Wnt10b, GSK-3β, β-catenin, C/EBPα, and PPARγ
3.6. Effect of Wnt10b Gene Interference on the Protein Expression of Wnt10b, GSK-3β, β-catenin, C/EBPα, and PPARγ
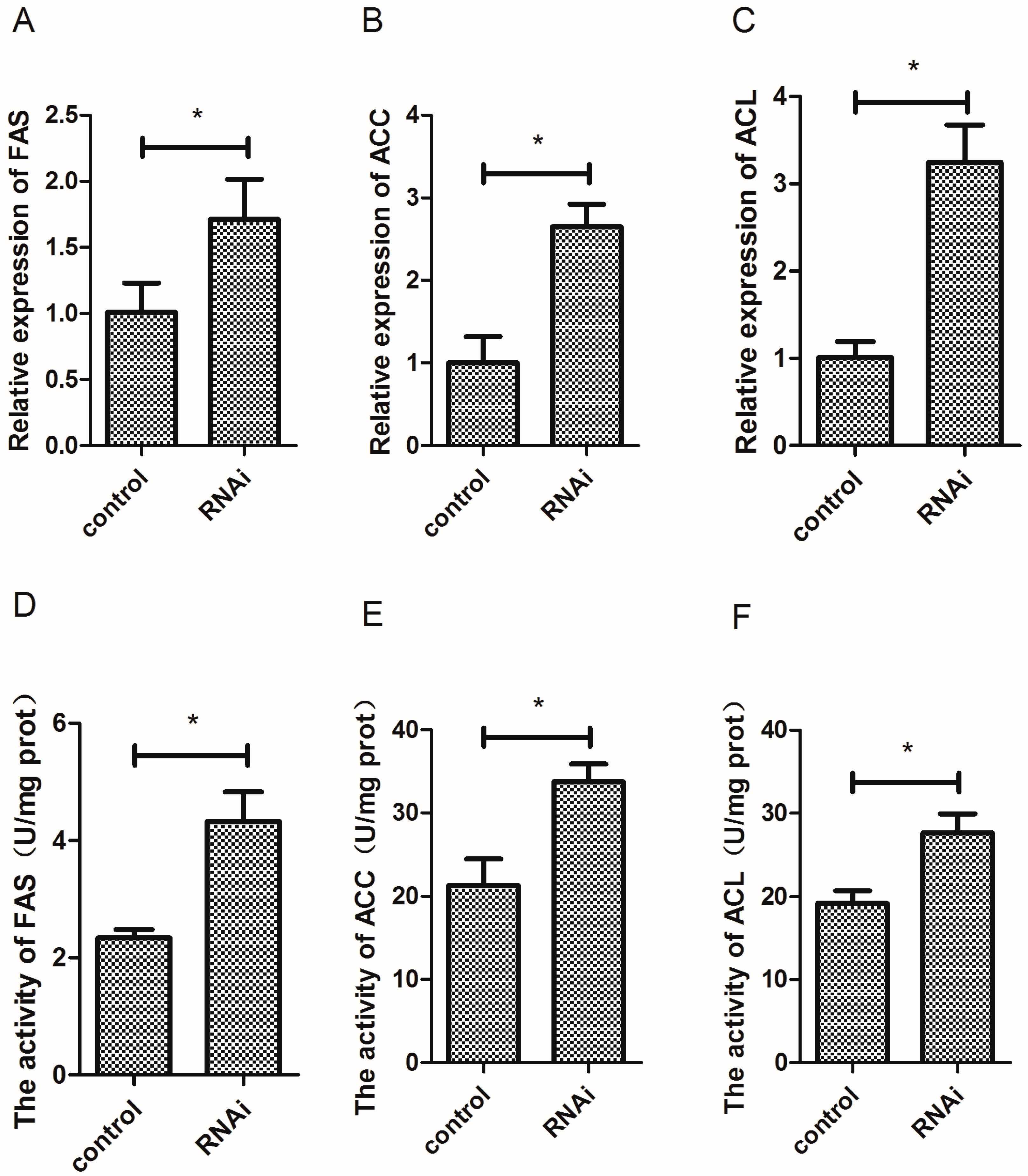
3.7. Effect of Wnt10b Gene Interference on the Gene Expression and Activity of FAS, ACC, and ACL
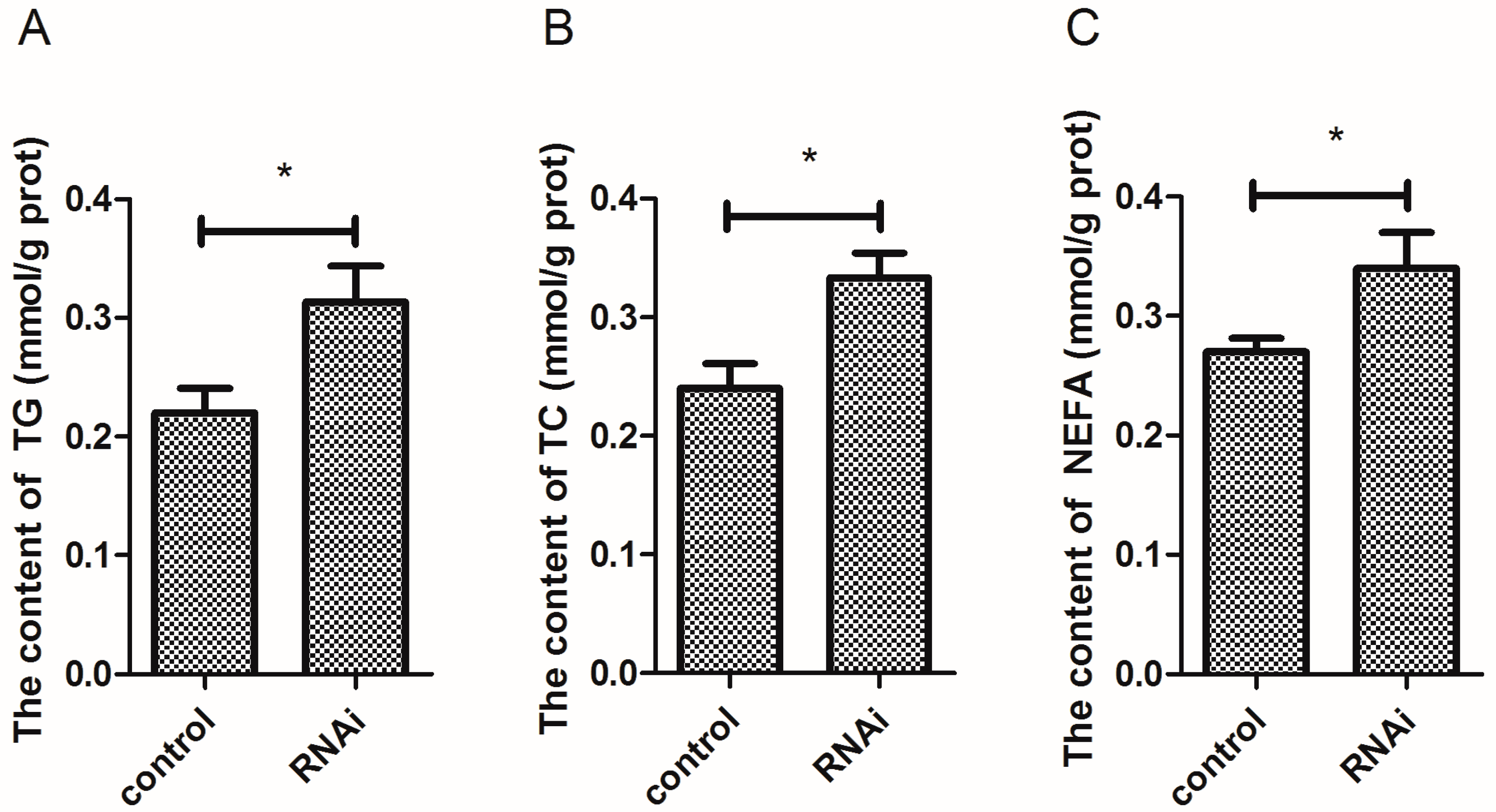
3.8. Effect of Wnt10b Gene Interference on the Biochemical Index in the Muscle
3.9. Effect of Wnt10b Gene Overexpression and Interference on the mRNA Expression of HMGCR in the Muscle of Zebrafish
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prud’homme, B.; Lartillot, N.; Balavoine, G.; Adoutte, A.; Vervoort, M. Phylogenetic Analysis of the Wnt Gene Family: Insights from Lophotrochozoan Members. Curr. Biol. 2002, 12, 1395–1400. [Google Scholar] [CrossRef]
- Nusse, R. An ancient cluster of Wnt paralogues. Trends Genet. 2001, 17, 443. [Google Scholar] [CrossRef]
- Kusserow, A.; Pang, K.; Sturm, C.; Hrouda, M.; Lentfer, J.; Schmidt, H.A.; Technau, U.; von Haeseler, A.; Hobmayer, B.; Martindale, M.Q.; et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 2005, 433, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Scarda, A.; Granzotto, M.; Milan, G.; Dalla Nora, E.; Keogh, J.; De Pergola, G.; Stirling, H.; Pannacciulli, N.; Sethi, J.K.; et al. WNT10B mutations in human obesity. Diabetologia 2006, 49, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Xiong, D.H.; Shen, H.; Zhao, L.J.; Xiao, P.; Guo, Y.; Wang, W.; Yang, T.L.; Recker, R.R.; Deng, H.W. Polymorphisms of the lowdensity lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J. Med. Genet. 2006, 43, 798–803. [Google Scholar] [CrossRef]
- Kanazawa, A.; Tsukada, S.; Sekine, A.; Tsunoda, T.; Takahashi, A.; Kashiwagi, A.; Tanaka, Y.; Babazono, T.; Matsuda, M.; Kaku, K.; et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am. J. Hum. Genet. 2004, 75, 832–843. [Google Scholar] [CrossRef]
- Prestwich, T.C.; Macdougald, O.A. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Behari, J.; Li, H.; Liu, S.; Stefanovic-Racic, M.; Alonso, L.; O’Donnell, C.P.; Shiva, S.; Singamsetty, S.; Watanabe, Y.; Singh, V.P.; et al. β-catenin links hepatic metabolic zonation with lipid metabolism and diet-induced obesity in mice. Am. J. Pathol. 2014, 184, 3284–3298. [Google Scholar] [CrossRef]
- Hansen, E.; Fernandes, K.; Goldspink, G.; Butterworth, P.; Umeda, P.K.; Chang, K.C. Strong expression of foreign genes following direct injection into fish muscle. FEBS Lett. 1991, 290, 73–76. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Palanisamy, R.; Bhatt, P.; Kumaresan, V.; Gnanam, A.J.; Pasupuleti, M.; Kasi, M. A novel murrel Channa striatus mitochondrial manganese superoxide dismutase: Gene silencing, SOD activity, superoxide anion production and expression. Fish Physiol. Biochem. 2014, 40, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Borello, U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999, 18, 6867–6872. [Google Scholar] [CrossRef] [PubMed]
- Ridgeway, A.G.; Petropoulos, H.; Wilton, S.; Skerjanc, I.S. Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 2000, 275, 32398–32405. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multitasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef]
- Huelsken, J.; Behrens, J. The Wnt signalling pathway. J. Cell Sci. 2002, 115, 3977–3978. [Google Scholar] [CrossRef]
- Sheridan, M.A. Lipid dynamics in fish: Aspects of absorption, transportation, deposition and mobilization. Comp. Biochem. Physiol. B 1988, 90, 679–690. [Google Scholar] [CrossRef]
- Robinson, J.S.; Mead, J.F. Lipid absorption and deposition in rainbow trout (Salmo gairdnerii). Can. J. Biochem. 1973, 51, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Kaushik, S.; Larroquet, L.; Panserat, S.; Corraze, G. Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2006, 96, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Lazar, M.A. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 1997, 272, 21473–21478. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Mushiake, S.; Bessho, K.; Murakami, M.; Namba, N.; Kokubu, C.; Michigami, T.; Ozono, K. Wnt/Lrp/beta-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARgamma and C/EBPalpha. Biochem. Biophys. Res. Commun. 2007, 363, 276–282. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Mandrup, S. Adipogenesis: Forces that tip the scales. Trends Endocrinol. Metab. 2002, 13, 5–11. [Google Scholar] [CrossRef]







| Primer | Sequence (5′-3′) | Direction |
|---|---|---|
| Wnt10b-F1 | CAATGACATCCTCGGCCTGAAG | Forward |
| Wnt10b-R1 | TCACTTGCACACATTAACCCACTC | Reverse |
| Wnt10b-F2 | CCCAAGCTTGCCACCATGAAGGTGGCAGGAGAGCCAGTGC | Forward |
| Wnt10b-R2 | CGGGGTACCCCCTTGCACACATTAACCCACTCTG | Reverse |
| Wnt10b-F3 | GATCACTAATACGACTCACTATAGGGGAGACCAGCGCTGGAACTGCTC | Forward |
| Wnt10b-R3 | GATCACTAATACGACTCACTATAGGGCCACTCTGTT ATTACGAATCC | Reverse |
| Target Gene | Forward (5′-3′) | Reverse (5′-3′) | GenBank |
|---|---|---|---|
| Wnt10b | TCCTGAAACAGGCTCGAAGT | GCTGCTCACTTGCACACATT | AY182171.1 |
| GSK-3β | TCTGCTCACCGTTTCCTTTC | CTCCGACCCACTTAACTCCA | NM_131381.1 |
| β-catenin | GGAGCTCACCAGCTCTCTGT | TAGCTTGGGTCGTCCTGTCT | NM_001001889.1 |
| C/EBPα | CACAACAGCTCCAAGCAAGA | AATCCATGTAGCCGTTCAGG | BC063934.1 |
| PPARγ | CTGGACATCAAGCCCTTCTC | AGCTGTACATGTGCGTCAGG | NM_131467.1 |
| FAS | ACAATGCTGGTGACAGTGGA | TACGTGTGGGCAGTCTCAAG | XM_009306806.2 |
| ACC | AGGTGGTACGGATGGCTGCTC | GACGGTGCTGGACGCTGTTG | NM_001271308.1 |
| ACL | AGACCTGATCTCCAGCCTCACATC | ATGCCACTGTCGAATGCCTTACTG | BC076484.1 |
| HMGCR | ACGTCATCGGTTACATGCCAGTTC | GCCTTCAGTTGTCGCCATCGG | NM_001079977.2 |
| β-actin | CCGTGACATCAAGGAGAAGC | TACCGCAAGATTCCATACCC | AF057040.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Pang, Q.; Han, Q.; Shi, Q.; Zhang, Q.; Yu, H. Wnt10b Participates in Regulating Fatty Acid Synthesis in the Muscle of Zebrafish. Cells 2019, 8, 1011. https://doi.org/10.3390/cells8091011
Liu D, Pang Q, Han Q, Shi Q, Zhang Q, Yu H. Wnt10b Participates in Regulating Fatty Acid Synthesis in the Muscle of Zebrafish. Cells. 2019; 8(9):1011. https://doi.org/10.3390/cells8091011
Chicago/Turabian StyleLiu, Dongwu, Qiuxiang Pang, Qiang Han, Qilong Shi, Qin Zhang, and Hairui Yu. 2019. "Wnt10b Participates in Regulating Fatty Acid Synthesis in the Muscle of Zebrafish" Cells 8, no. 9: 1011. https://doi.org/10.3390/cells8091011
APA StyleLiu, D., Pang, Q., Han, Q., Shi, Q., Zhang, Q., & Yu, H. (2019). Wnt10b Participates in Regulating Fatty Acid Synthesis in the Muscle of Zebrafish. Cells, 8(9), 1011. https://doi.org/10.3390/cells8091011