The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Electron Spin Resonance (ESR) Spectroscopy
2.4. Measurement of Mitochondrial ROS
2.5. Intracellular Porphyrin Accumulation after ALA Treatment
2.6. Cellular Uptake of ALA
2.7. Cell Viability Assay after PDT
2.8. Statistical Analysis
3. Results
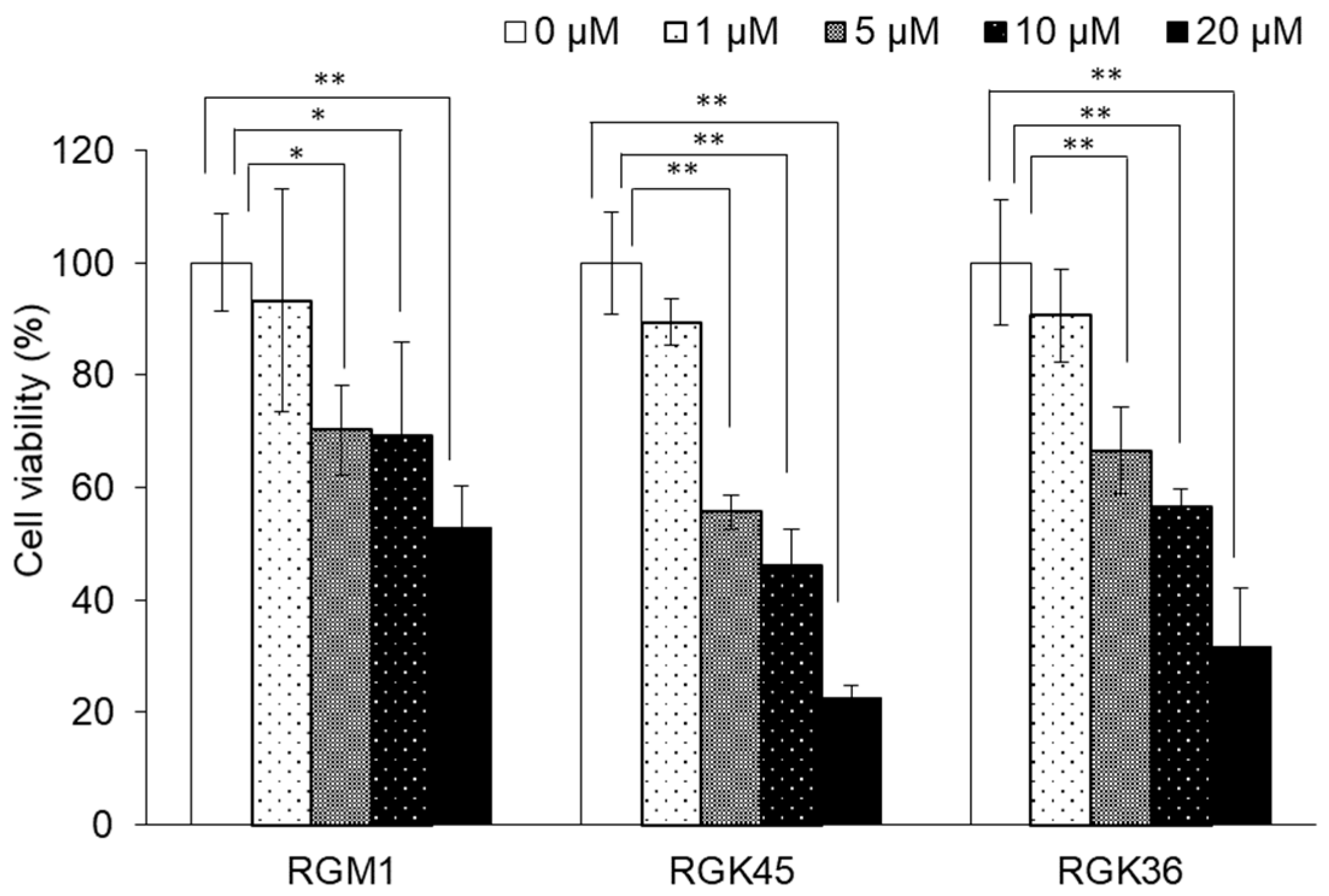
3.1. Cisplatin-Induced Cytotoxicity in RGK36, RGK45, and RGM1 Cells
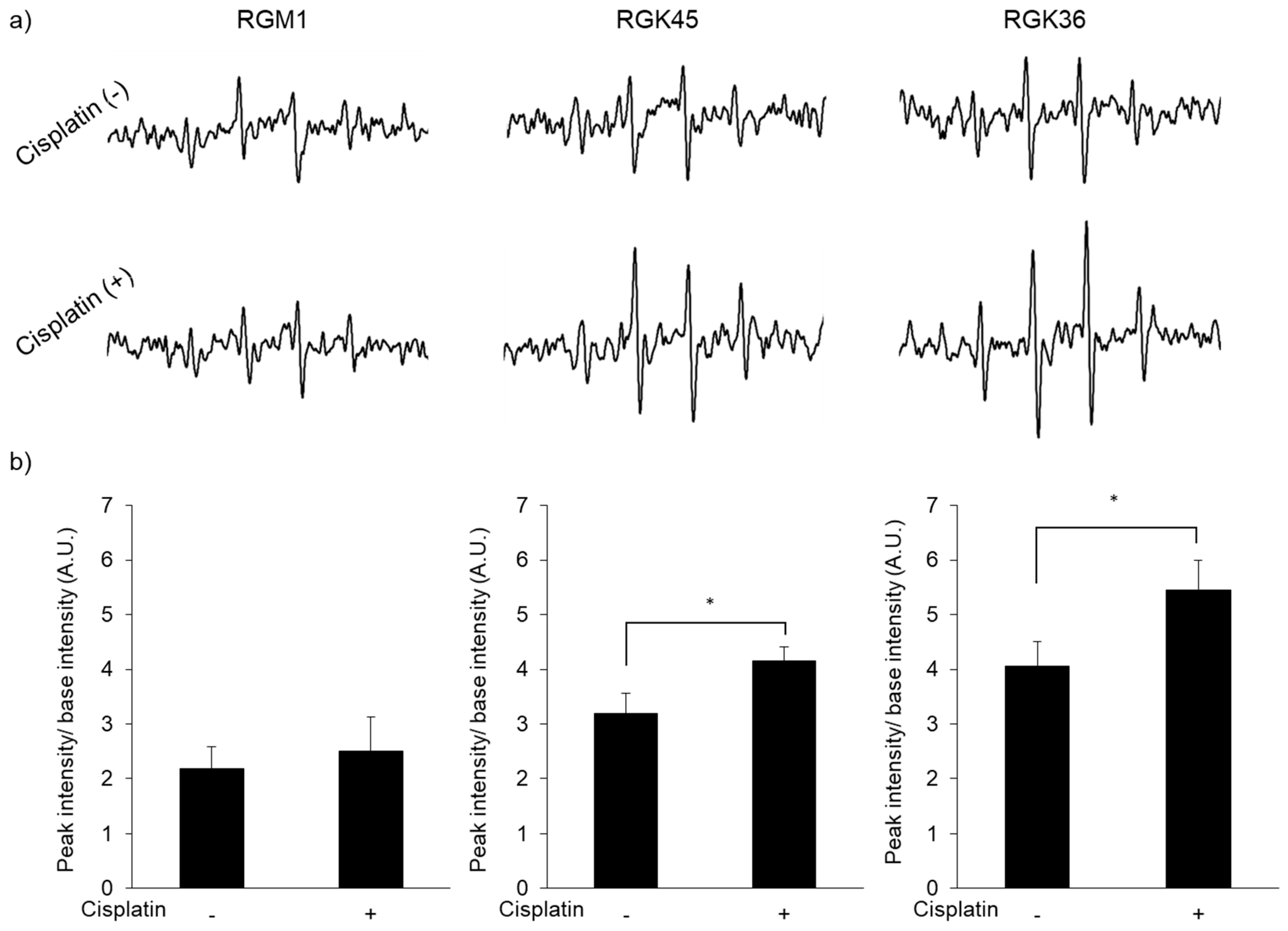
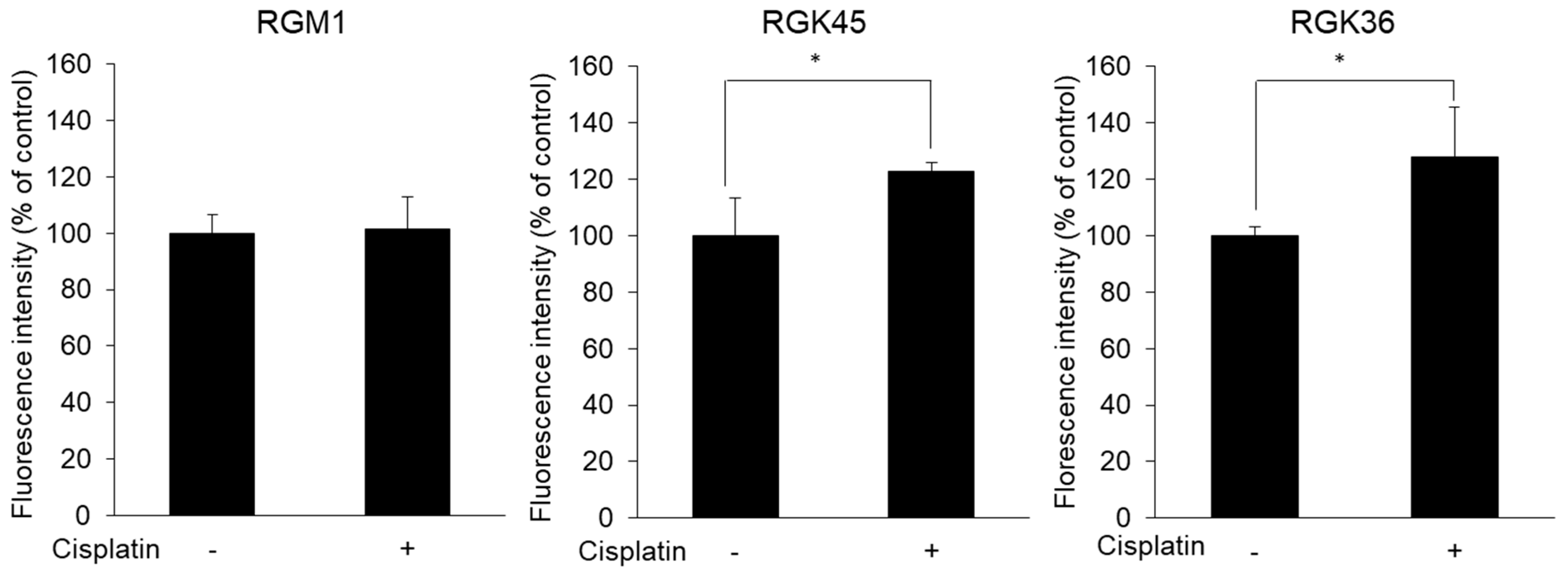
3.2. Intracellular ROS Production
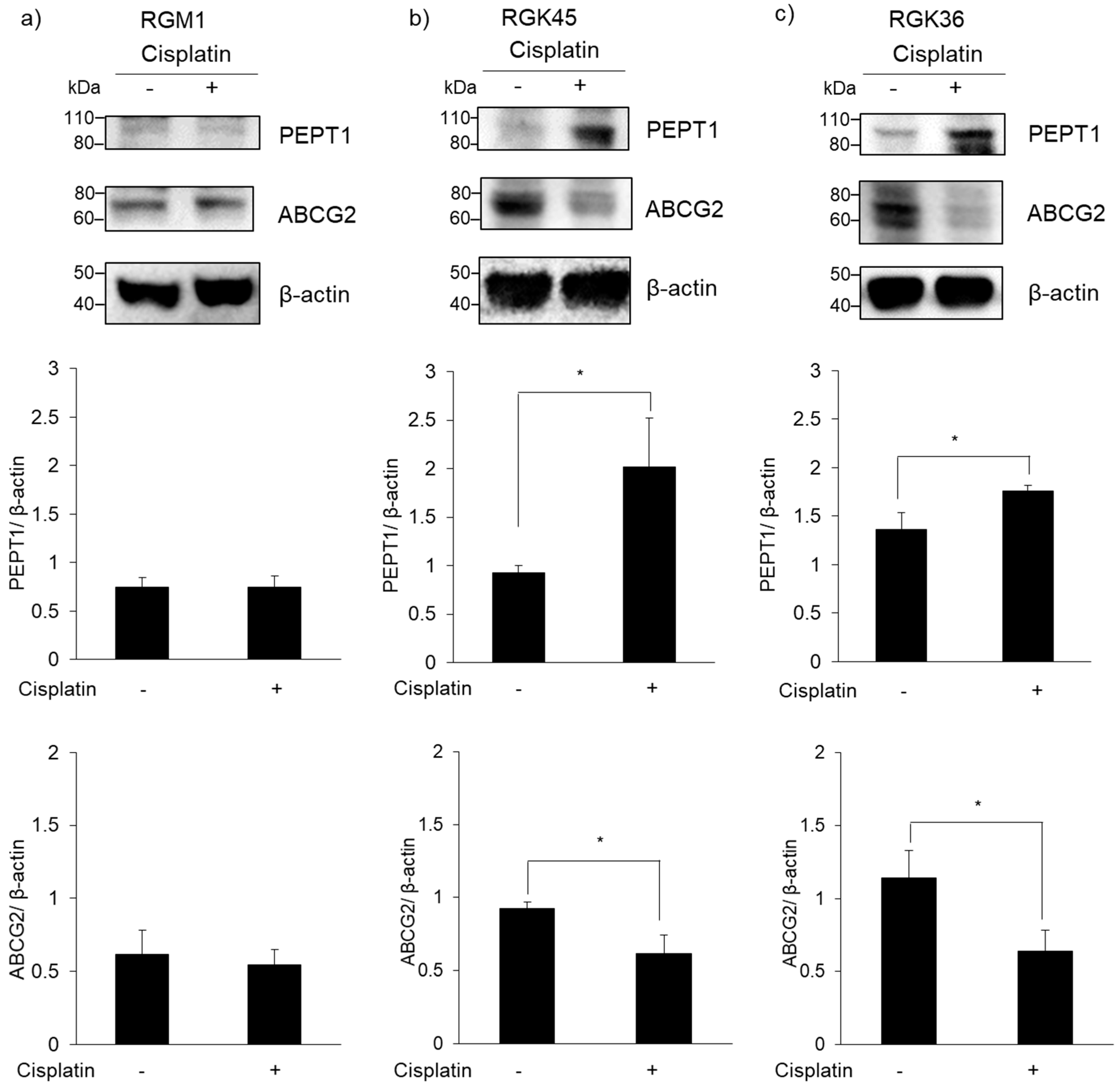
3.3. PEPT1 and ABCG2 Expression
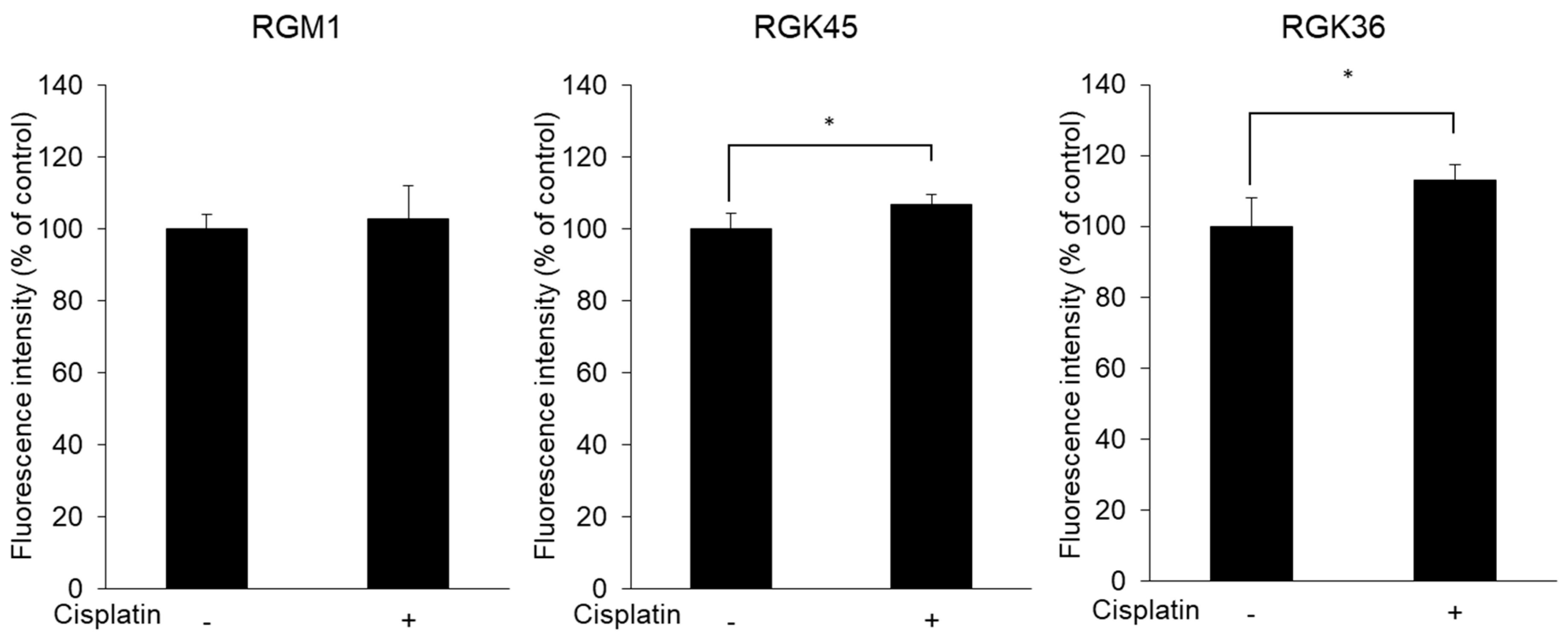
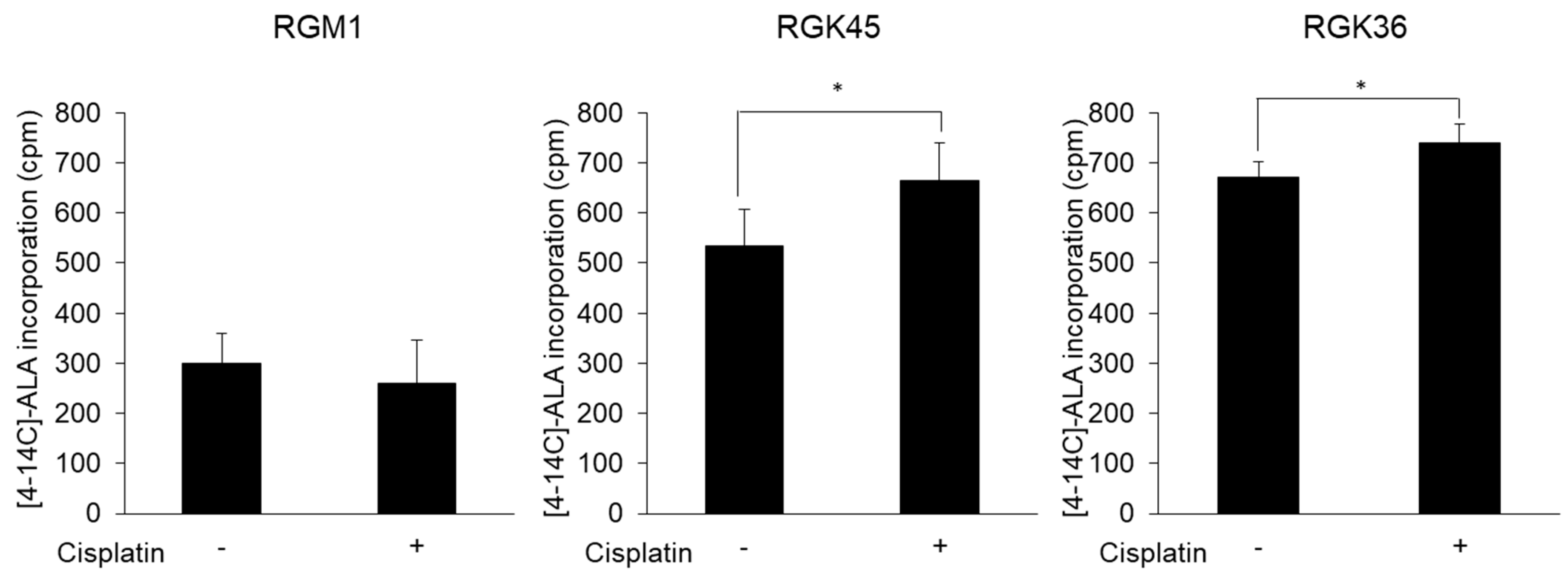
3.4. Intracellular Porphyrin Accumulation after ALA Addition
3.5. Cancer Cell-Specific Uptake of ALA
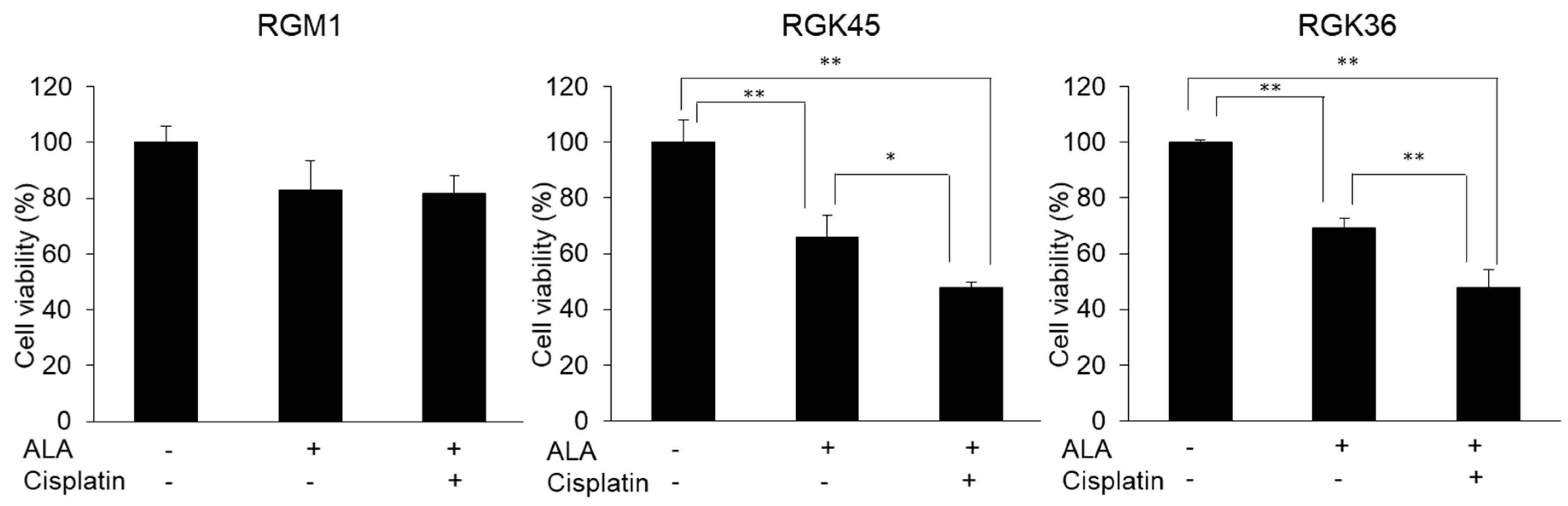
3.6. Cell Viability after PDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, H.Y.; Park, H.J. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, T.; Kajimoto, Y.; Sun, W.; Nakagawa, H.; Inoue, Y.; Ikegami, Y.; Miyatake, S.-I.; Kuroiwa, T. Role of Nrf2 in Cancer Photodynamic Therapy: Regulation of Human ABC Transporter ABCG2. J. Pharm. Sci. 2013, 102, 3058–3069. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, J.T. The porphyrias, appropriate test selection. Clin. Chim. Acta 2003, 333, 203–207. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Marcus, S.L.; Pottier, R.H. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J. Clin. Laser Med. Surg. 1996, 14, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Doring, F.; Walter, J.; Will, J.; Focking, M.; Boll, M.; Amasheh, S.; Clauss, W.; Daniel, H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J. Clin. Investig. 1998, 101, 2761–2767. [Google Scholar] [CrossRef]
- Daniel, H.; Herget, M. Cellular and molecular mechanisms of renal peptide transport. Am. J. Physiol. Physiol. 1997, 273, F1–F8. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tamura, A.; Saito, H.; Onishi, Y.; Ishikawa, T. Human ABC transporter ABCG2 in xenobiotic protection and redox biology. Drug Metab. Rev. 2006, 38, 371–391. [Google Scholar] [CrossRef]
- Ito, H.; Tamura, M.; Matsui, H.; Majima, H.J.; Indo, H.P.; Hyodo, I. Reactive oxygen species involved cancer cellular specific 5-aminolevulinic acid uptake in gastric epithelial cells. J. Clin. Biochem. Nutr. 2014, 54, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, H.; Ito, H.; Terasaki, M.; Matsui, H. Hyperthermia enhances photodynamic therapy by regulation of HCP1 and ABCG2 expressions via high level ROS generation. Sci. Rep. 2019, 9, 1638. [Google Scholar] [CrossRef]
- Chu, G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem. 1994, 269, 787–790. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, G.H.; Kim, J.W.; Kwon, S.S.; Sato, E.F.; Cho, K.H.; Shim, E.B. Computational modeling of apoptotic signaling pathways induced by cisplatin. BMC Syst. Biol. 2012, 6, 122. [Google Scholar] [CrossRef]
- He, G.; He, G.; Zhou, R.; Pi, Z.; Zhu, T.; Jiang, L.; Xie, Y. Enhancement of cisplatin-induced colon cancer cells apoptosis by shikonin, a natural inducer of ROS in vitro and in vivo. Biochem. Biophys. Res. Commun. 2016, 469, 1075–1082. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Matsui, H. Monascus purpureus induced apoptosis on gastric cancer cell by scavenging mitochondrial reactive oxygen species. J. Clin. Biochem. Nutr. 2017, 61, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Yoshihiro, K.; Hasko, G.; Pacher, P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem. Biophys. Res. Commun. 2007, 358, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, T.; Takahashi, K.; Ikeda, N.; Kajimoto, Y.; Hagiya, Y.; Ogura, S.I.; Miyatake, S.I.; Kuroiwa, T. Transporter-Mediated Drug Interaction Strategy for 5-Aminolevulinic Acid (ALA)-Based Photodynamic Diagnosis of Malignant Brain Tumor: Molecular Design of ABCG2 Inhibitors. Pharmaceutics 2011, 3, 615–635. [Google Scholar] [CrossRef]
- Ito, H.; Matsui, H.; Tamura, M.; Majima, H.J.; Indo, H.P.; Hyodo, I. Mitochondrial reactive oxygen species accelerate the expression of heme carrier protein 1 and enhance photodynamic cancer therapy effect. J. Clin. Biochem. Nutr. 2014, 55, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Matsui, H.; Hirayama, A.; Indo, H.P.; Majima, H.J.; Hyodo, I. Reactive oxygen species induced by non-steroidal anti-inflammatory drugs enhance the effects of photodynamic therapy in gastric cancer cells. J. Clin. Biochem. Nutr. 2016, 58, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.C.; Biswas, R.; Mondal, A.; Lee, Y.K.; Chung, P.S. Cisplatin enhances the efficacy of 5-aminolevulinic acid mediated photodynamic therapy in human head and neck squamous cell carcinoma. Gen. Physiol. Biophys. 2014, 33, 53–62. [Google Scholar] [CrossRef]
- Wei, X.Q.; Ma, H.Q.; Liu, A.H.; Zhang, Y.Z. Synergistic anticancer activity of 5-aminolevulinic acid photodynamic therapy in combination with low-dose cisplatin on Hela cells. Asian Pac. J. Cancer Prev. 2013, 14, 3023–3028. [Google Scholar] [CrossRef]
- Yu, C.H.; Yu, C.C. Photodynamic therapy with 5-aminolevulinic acid (ALA) impairs tumor initiating and chemo-resistance property in head and neck cancer-derived cancer stem cells. PLoS ONE 2014, 9, e87129. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, H.; Ito, H.; Matsui, H. The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation. Cells 2019, 8, 918. https://doi.org/10.3390/cells8080918
Kurokawa H, Ito H, Matsui H. The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation. Cells. 2019; 8(8):918. https://doi.org/10.3390/cells8080918
Chicago/Turabian StyleKurokawa, Hiromi, Hiromu Ito, and Hirofumi Matsui. 2019. "The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation" Cells 8, no. 8: 918. https://doi.org/10.3390/cells8080918
APA StyleKurokawa, H., Ito, H., & Matsui, H. (2019). The Cisplatin-Derived Increase of Mitochondrial Reactive Oxygen Species Enhances the Effectiveness of Photodynamic Therapy via Transporter Regulation. Cells, 8(8), 918. https://doi.org/10.3390/cells8080918




