Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis
Abstract
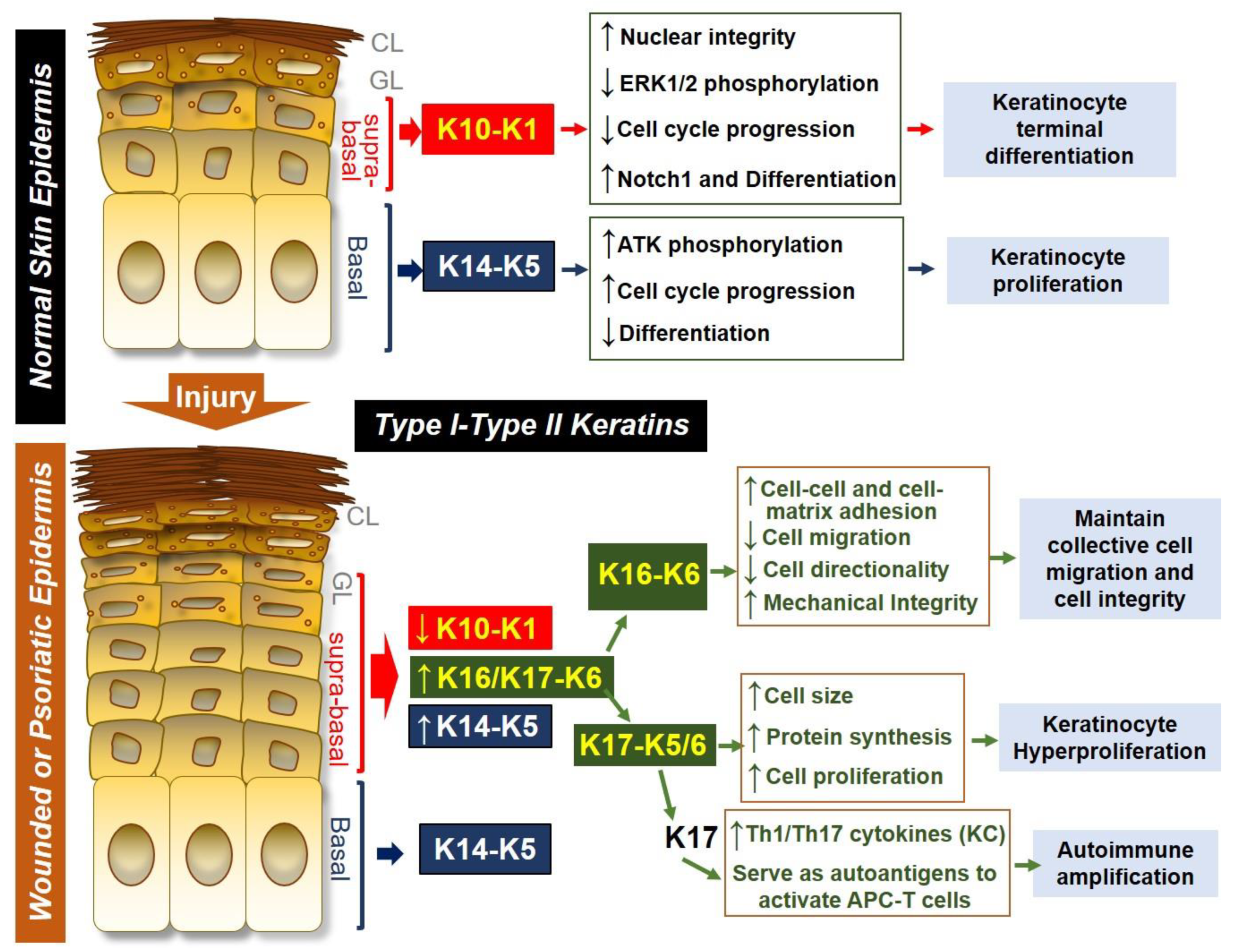
:1. Introduction:
2. Keratin Expression During Skin Development and in Skin Diseases
2.1. Keratin Expression During Epidermal Development
2.2. Keratin Expression During Skin Wounding
2.3. Keratin 6, 16 and 17 are Hallmarks of Psoriasis
2.4. Regulation of Keratin 6/16/17 Expression in Skin Wounds and in Psoriasis
3. Keratin 6, 16 and 17—Key Barrier Alarmins During Skin Wounding and/or Psoriasis Pathogenesis
3.1. Keratin 6 and 16 Maintain the Cell Adhesion and Optimal Cell Migration During Skin Wounding
3.2. Keratin 17 Promotes Cell Proliferation
3.3. Keratin 17 Promotes Th1/Th17 Cytokine Production from Keratinocytes
3.4. KRT17 Peptides Are Auto-Antigens for Psoriatic T Cells
4. Therapeutic Potential of Keratins in Skin Diseases
4.1. Therapeutic Effect of Keratins in Promoting Wound Repair
4.2. KRT17 as A Potential Therapeutic Target for Psoriasis
4.3. Targeting KRT6 for Pachyonychia Congenita
5. Conclusions
Funding
Conflicts of Interest
References
- Chen, S.X.; Zhang, L.J.; Gallo, R.L. Dermal White Adipose Tissue: A Newly Recognized Layer of Skin Innate Defense. J. Invest. Dermatol. 2019, 139, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Hooper, L.V. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Keymeulen, A.; Blanpain, C. Tracing epithelial stem cells during development, homeostasis, and repair. J. Cell Biol. 2012, 197, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.J. Isolation, Culture, and Characterization of Primary Mouse Epidermal Keratinocytes. In Mouse Cell Culture: Methods and Protocols; Bertoncello, I., Ed.; Springer, Humana Press: New York, NY, USA, 2019; Volume 1940, pp. 205–215. [Google Scholar]
- Zhang, L.-J. Keratins in Skin Epidermal Development and Diseases. In Keratin; Blumenberg, M., Ed.; IntechOpen: London, UK, 2018; Volume 79050, pp. 1–17. [Google Scholar]
- Coulombe, P.A. The Molecular Revolution in Cutaneous Biology: Keratin Genes and their Associated Disease: Diversity, Opportunities, and Challenges. J. Invest. Dermatol. 2017, 137, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.; Strelkov, S.V.; Burkhard, P.; Aebi, U. Intermediate filaments: Primary determinants of cell architecture and plasticity. J. Clin. Invest. 2009, 119, 1772–1783. [Google Scholar] [CrossRef]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The Catalog of Human Cytokeratins - Patterns of Expression in Normal Epithelia, Tumors and Cultured-Cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Franke, W.W.; Schiller, D.L.; Moll, R.; Winter, S.; Schmid, E.; Engelbrecht, I.; Denk, H.; Krepler, R.; Platzer, B. Diversity of Cytokeratins—Differentiation Specific Expression of Cytokeratin Polypeptides in Epithelial-Cells and Tissues. J. Mol. Biol. 1981, 153, 933–959. [Google Scholar] [CrossRef]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.; Rogers, M.A.; et al. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Hesse, M.; Zimek, A.; Weber, K.; Magin, T.M. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur. J. Cell Biol. 2004, 83, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.A.; Winter, H.; Langbein, L.; Bleiler, R.; Schweizer, J. The human type I keratin gene family: Characterization of new hair follicle specific members and evaluation of the chromosome 17q21.2 gene domain. Differentiation 2004, 72, 527–540. [Google Scholar] [CrossRef]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Toivola, D.M.; Boor, P.; Alam, C.; Strnad, P. Keratins in health and disease. Curr. Opin. Cell Biol. 2015, 32, 73–81. [Google Scholar] [CrossRef]
- Fuchs, E.; Cleveland, D.W. A structural scaffolding of intermediate filaments in health and disease. Science 1998, 279, 514–519. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Tong, X.; Mazzalupo, S.; Wang, Z.; Wong, P. Great promises yet to be fulfilled: Defining keratin intermediate filament function in vivo. Eur. J. Cell Biol. 2004, 83, 735–746. [Google Scholar] [CrossRef]
- Kirfel, J.; Magin, T.M.; Reichelt, J. Keratins: A structural scaffold with emerging functions. Cell. Mol. Life Sci. 2003, 60, 56–71. [Google Scholar] [CrossRef]
- Jackson, B.W.; Grund, C.; Schmid, E.; Burki, K.; Franke, W.W.; Illmensee, K. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation 1980, 17, 161–179. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Liu, H.B.; Parent, C.A.; Coulombe, P.A. Keratin 6 regulates collective keratinocyte migration by altering cell-cell and cell-matrix adhesion. J. Cell Biol. 2018, 217, 4314–4330. [Google Scholar] [CrossRef]
- Sun, T.T.; Eichner, R.; Nelson, W.G.; Tseng, S.C.; Weiss, R.A.; Jarvinen, M.; Woodcock-Mitchell, J. Keratin classes: Molecular markers for different types of epithelial differentiation. J. Invest. Dermatol. 1983, 81, 109s–115s. [Google Scholar] [CrossRef]
- Sun, T.T.; Eichner, R.; Nelson, W.G.; Vidrich, A.; Woodcock-Mitchell, J. Keratin expression during normal epidermal differentiation. Curr. Probl. Dermatol. 1983, 11, 277–291. [Google Scholar]
- Koch, P.J.; Roop, D.R. The role of keratins in epidermal development and homeostasis--going beyond the obvious. J. Invest. Dermatol. 2004, 123, x. [Google Scholar] [CrossRef]
- Nagarajan, P.; Romano, R.A.; Sinha, S. Transcriptional control of the differentiation program of interfollicular epidermal keratinocytes. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 57–79. [Google Scholar]
- Romano, R.A.; Smalley, K.; Magraw, C.; Serna, V.A.; Kurita, T.; Raghavan, S.; Sinha, S. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 2012, 139, 772–782. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Blanpain, C. Development and homeostasis of the skin epidermis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008383. [Google Scholar] [CrossRef]
- Fuchs, E.; Tyner, A.L.; Giudice, G.J.; Marchuk, D.; RayChaudhury, A.; Rosenberg, M. The human keratin genes and their differential expression. Curr. Top Dev. Biol. 1987, 22, 5–34. [Google Scholar]
- Cooper, D.; Schermer, A.; Sun, T.T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: Strategies, applications, and limitations. Lab. Invest. 1985, 52, 243–256. [Google Scholar]
- Fuchs, E.; Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef]
- Alam, H.; Sehgal, L.; Kundu, S.T.; Dalal, S.N.; Vaidya, M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Biol. Cell 2011, 22, 4068–4078. [Google Scholar] [CrossRef]
- Reichelt, J.; Breiden, B.; Sandhoff, K.; Magin, T.M. Loss of keratin 10 is accompanied by increased sebocyte proliferation and differentiation. Eur. J. Cell Biol. 2004, 83, 747–759. [Google Scholar] [CrossRef]
- Paramio, J.M.; Casanova, M.L.; Segrelles, C.; Mittnacht, S.; Lane, E.B.; Jorcano, J.L. Modulation of cell proliferation by cytokeratins K10 and K16. Mol. Cell. Biol. 1999, 19, 3086–3094. [Google Scholar] [CrossRef]
- Wallace, L.; Roberts-Thompson, L.; Reichelt, J. Deletion of K1/K10 does not impair epidermal stratification but affects desmosomal structure and nuclear integrity. J. Cell Sci. 2012, 125, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Bunick, C.G.; Milstone, L.M. The X-Ray Crystal Structure of the Keratin 1-Keratin 10 Helix 2B Heterodimer Reveals Molecular Surface Properties and Biochemical Insights into Human Skin Disease. J. Invest. Dermatol. 2017, 137, 142–150. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, M.S.; Chung, B.M.; Leahy, D.J.; Coulombe, P.A. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat. Struct. Mol. Biol. 2012, 19, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care (New Rochelle) 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Paladini, R.D.; Takahashi, K.; Bravo, N.S.; Coulombe, P.A. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: Defining a potential role for keratin 16. J. Cell Biol. 1996, 132, 381–397. [Google Scholar] [CrossRef]
- McGowan, K.M.; Coulombe, P.A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J. Cell Biol. 1998, 143, 469–486. [Google Scholar] [CrossRef]
- Takahashi, K.; Yan, B.; Yamanishi, K.; Imamura, S.; Coulombe, P.A. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics 1998, 53, 170–183. [Google Scholar] [CrossRef]
- Hobbs, R.P.; Lessard, J.C.; Coulombe, P.A. Keratin intermediate filament proteins—novel regulators of inflammation and immunity in skin. J. Cell Sci. 2012, 125, 5257–5258. [Google Scholar] [CrossRef]
- Jacinto, A.; Martinez-Arias, A.; Martin, P. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 2001, 3, E117–E123. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Yin, X.; Low, H.Q.; Wang, L.; Li, Y.; Ellinghaus, E.; Han, J.; Estivill, X.; Sun, L.; Zuo, X.; Shen, C.; et al. Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat. Commun. 2015, 6, 6916. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-J. Type1 Interferons Potential Initiating Factors Linking Skin Wounds with Psoriasis Pathogenesis. Front. Immunol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Conrad, C.; Gilliet, M. Psoriasis: From Pathogenesis to Targeted Therapies. Clin. Rev. Allergy Immunol. 2018, 54, 102–113. [Google Scholar] [CrossRef]
- Zhang, L.J.; Sen, G.L.; Ward, N.L.; Johnston, A.; Chun, K.; Chen, Y.; Adase, C.; Sanford, J.A.; Gao, N.; Chensee, M.; et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-beta Production by Epidermal Keratinocytes during Skin Injury. Immunity 2016, 45, 119–130. [Google Scholar] [CrossRef]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Weiss, G.; Shemer, A.; Trau, H. The Koebner phenomenon: Review of the literature. J. Eur. Acad Dermatol. Venereol. 2002, 16, 241–248. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Bonish, B.K.; Marble, D.J.; Schriedel, K.A.; DiPietro, L.A.; Gordon, K.B.; Lingen, M.W. Lessons learned from psoriatic plaques concerning mechanisms of tissue repair, remodeling, and inflammation. J. Invest. Dermatol. Symp. Proc. 2006, 11, 16–29. [Google Scholar] [CrossRef]
- Jin, L.; Wang, G. Keratin 17: A Critical Player in the Pathogenesis of Psoriasis. Med. Res. Rev. 2014, 34, 438–454. [Google Scholar] [CrossRef]
- Mommers, J.M.; van Rossum, M.M.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Changes in keratin 6 and keratin 10 (co-)expression in lesional and symptomless skin of spreading psoriasis. Dermatology 2000, 201, 15–20. [Google Scholar] [CrossRef]
- Jiang, S.; Hinchliffe, T.E.; Wu, T. Biomarkers of An Autoimmune Skin Disease—Psoriasis. Genom. Proteom. Bioinform. 2015, 13, 224–233. [Google Scholar] [CrossRef]
- Elango, T.; Sun, J.; Zhu, C.; Zhou, F.; Zhang, Y.; Sun, L.; Yang, S.; Zhang, X. Mutational analysis of epidermal and hyperproliferative type I keratins in mild and moderate psoriasis vulgaris patients: A possible role in the pathogenesis of psoriasis along with disease severity. Hum. Genomics 2018, 12, 27. [Google Scholar] [CrossRef]
- Jiang, M.; Li, B.; Zhang, J.; Hu, L.; Dang, E.; Wang, G. Vascular endothelial growth factor driving aberrant keratin expression pattern contributes to the pathogenesis of psoriasis. Exp. Cell Res. 2017, 360, 310–319. [Google Scholar] [CrossRef]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Kalali, B.N.; Kollisch, G.; Mages, J.; Muller, T.; Bauer, S.; Wagner, H.; Ring, J.; Lang, R.; Mempel, M.; Ollert, M. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. (Baltimore, Md. 1950) 2008, 181, 2694–2704. [Google Scholar] [CrossRef]
- Nelson, A.M.; Reddy, S.K.; Ratliff, T.S.; Hossain, M.Z.; Katseff, A.S.; Zhu, A.S.; Chang, E.; Resnik, S.R.; Page, C.; Kim, D.; et al. dsRNA Released by Tissue Damage Activates TLR3 to Drive Skin Regeneration. Cell Stem Cell 2015, 17, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Borkowski, A.W.; Kuo, I.H.; Bernard, J.J.; Yoshida, T.; Williams, M.R.; Hung, N.J.; Yu, B.D.; Beck, L.A.; Gallo, R.L. Toll-Like Receptor 3 Activation Is Required for Normal Skin Barrier Repair Following UV Damage. J. Invest. Dermatol. 2015, 135, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Gallo, R.L.; Bernard, J.J. Innate immune sensors stimulate inflammatory and immunosuppressive responses to UVB radiation. J. Invest. Dermatol. 2014, 134, 1508–1511. [Google Scholar] [CrossRef]
- Kulkarni, N.N.; Adase, C.A.; Zhang, L.J.; Borkowski, A.W.; Li, F.; Sanford, J.A.; Coleman, D.J.; Aguilera, C.; Indra, A.K.; Gallo, R.L. IL-1 Receptor-Knockout Mice Develop Epidermal Cysts and Show an Altered Innate Immune Response after Exposure to UVB Radiation. J. Invest. Dermatol. 2017, 137, 2417–2426. [Google Scholar] [CrossRef]
- Komine, M.; Rao, L.S.; Freedberg, I.M.; Simon, M.; Milisavljevic, V.; Blumenberg, M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J. Invest. Dermatol. 2001, 116, 330–338. [Google Scholar] [CrossRef]
- Ma, S.; Rao, L.; Freedberg, I.M.; Blumenberg, M. Transcriptional control of K5, K6, K14, and K17 keratin genes by AP-1 and NF-kappaB family members. Gene Expr. 1997, 6, 361–370. [Google Scholar]
- Chung, B.M.; Murray, C.I.; Van Eyk, J.E.; Coulombe, P.A. Identification of Novel Interaction between Annexin A2 and Keratin 17 EVIDENCE FOR RECIPROCAL REGULATION. J. Biol. Chem. 2012, 287, 7573–7581. [Google Scholar] [CrossRef]
- Watanabe, H.; Gaide, O.; Petrilli, V.; Martinon, F.; Contassot, E.; Roques, S.; Kummer, J.A.; Tschopp, J.; French, L.E. Activation of the IL-1 beta-Processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 2007, 127, 1956–1963. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hoh, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-Induced activation and secretion of interleukin-1 beta by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular toll-like receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef]
- Zhang, W.; Dang, E.; Shi, X.; Jin, L.; Feng, Z.; Hu, L.; Wu, Y.; Wang, G. The pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2. PLoS ONE 2012, 7, e40797. [Google Scholar] [CrossRef]
- Shi, X.; Jin, L.; Dang, E.; Chang, T.; Feng, Z.; Liu, Y.; Wang, G. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J. Invest. Dermatol. 2011, 131, 2401–2408. [Google Scholar] [CrossRef]
- Chang, T.; Sun, L.C.; Wang, Y.; Wang, D.T.; Li, W.; Li, C.Y.; Gao, T.W.; Liu, Y.F.; Wang, G. Inhibition of keratin 17 expression with antisense and RNAi strategies: Exploring novel therapy for psoriasis. Exp. Dermatol. 2011, 20, 555–560. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Cui, T.; Dang, E.; Wang, G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Invest. Dermatol. 2017, 137, 2168–2176. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Sun, Z.; Dang, E.; Li, B.; Fang, H.; Li, J.; Gao, L.; Zhang, K.; Wang, G. TGFbeta/SMAD/microRNA-486-3p Signaling Axis Mediates Keratin 17 Expression and Keratinocyte Hyperproliferation in Psoriasis. J. Invest. Dermatol. 2017, 137, 2177–2186. [Google Scholar] [CrossRef]
- Zhang, J.W.; Li, X.; Wei, J.A.; Chen, H.M.; Lu, Y.; Li, L.; Han, L.; Lu, C.J. Gallic acid inhibits the expression of keratin 16 and keratin 17 through Nrf2 in psoriasis-like skin disease. Int. Immunopharmacol. 2018, 65, 84–95. [Google Scholar] [CrossRef]
- Wong, P.; Colucci-Guyon, E.; Takahashi, K.; Gu, C.H.; Babinet, C.; Coulombe, P.A. Introducing a null mutation in the mouse K6 alpha and K6 beta genes reveals their essential structural role in the oral mucosa. J. Cell Biol. 2000, 150, 921–928. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Bravo, N.S.; Paladini, R.D.; Nguyen, D.; Takahashi, K. Overexpression of human keratin 16 produces a distinct skin phenotype in transgenic mouse skin. Biochem. Cell Biol. 1995, 73, 611–618. [Google Scholar] [CrossRef]
- Takahashi, K.; Folmer, J.; Coulombe, P.A. Increased expression of keratin 16 causes anomalies in cytoarchitecture and keratinization in transgenic mouse skin. J. Cell Biol. 1994, 127, 505–520. [Google Scholar] [CrossRef]
- Wong, P.; Coulombe, P.A. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 2003, 163, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Wawersik, M.J.; Mazzalupo, S.; Nguyen, D.; Coulombe, P.A. Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Mol. Biol. Cell 2001, 12, 3439–3450. [Google Scholar] [CrossRef]
- Rotty, J.D.; Coulombe, P.A. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J. Cell Biol. 2012, 197, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Mazzalupo, S.; Wong, P.; Martin, P.; Coulombe, P.A. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev. Dynam. 2003, 226, 356–365. [Google Scholar] [CrossRef]
- Kim, S.; Wong, P.; Coulombe, P.A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 2006, 441, 362–365. [Google Scholar] [CrossRef]
- Yang, L.; Jin, L.; Ke, Y.; Fan, X.; Zhang, T.; Zhang, C.; Bian, H.; Wang, G. E3 Ligase Trim21 Ubiquitylates and Stabilizes Keratin 17 to Induce STAT3 Activation in Psoriasis. J. Invest. Dermatol. 2018, 138, 2568–2577. [Google Scholar] [CrossRef] [Green Version]
- Depianto, D.; Kerns, M.L.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.M.; Arutyunov, A.; Ilagan, E.; Yao, N.; Wills-Karp, M.; Coulombe, P.A. Regulation of C-X-C chemokine gene expression by keratin 17 and hnRNP K in skin tumor keratinocytes. J. Cell Biol. 2015, 208, 613–627. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, R.P.; Jacob, J.T.; Coulombe, P.A. Keratins Are Going Nuclear. Dev. Cell 2016, 38, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, R.P.; DePianto, D.J.; Jacob, J.T.; Han, M.C.; Chung, B.M.; Batazzi, A.S.; Poll, B.G.; Guo, Y.; Han, J.; Ong, S.; et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat. Genet. 2015, 47, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Wang, G.; Fan, J.Y.; Li, W.; Liu, Y.F. HLA DR B1*04,*07-restricted epitopes on Keratin 17 for autoreactive T cells in psoriasis. J. Dermatol. Sci. 2005, 38, 25–39. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.S.; Sigmundsdottir, H.; Sigurgeirsson, B.; Good, M.F.; Valdimarsson, H.; Jonsdottir, I. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin. Exp. Immunol. 1999, 117, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Charles, C.A.; Tomic-Canic, M.; Vincek, V.; Nassiri, M.; Stojadinovic, O.; Eaglstein, W.H.; Kirsner, R.S. A gene signature of nonhealing venous ulcers: Potential diagnostic markers. J. Am. Acad Dermatol. 2008, 59, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Pechter, P.M.; Gil, J.; Valdes, J.; Tomic-Canic, M.; Pastar, I.; Stojadinovic, O.; Kirsner, R.S.; Davis, S.C. Keratin dressings speed epithelialization of deep partial-thickness wounds. Wound Repair Regen. 2012, 20, 236–242. [Google Scholar] [CrossRef]
- Konop, M.; Sulejczak, D.; Czuwara, J.; Kosson, P.; Misicka, A.; Lipkowski, A.W.; Rudnicka, L. The role of allogenic keratin-derived dressing in wound healing in a mouse model. Wound Repair Regen. 2017, 25, 62–74. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.K.; Tang, K.C.; Yang, K.C.; Cheng, N.C.; Yu, J. Keratin Scaffolds with Human Adipose Stem Cells: Physical and Biological Effects toward Wound Healing. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef]
- Tzu, J.; Kerdel, F. From conventional to cutting edge: The new era of biologics in treatment of psoriasis. Dermatol. Ther. 2008, 21, 131–141. [Google Scholar] [CrossRef]
- Leachman, S.A.; Hickerson, R.P.; Schwartz, M.E.; Bullough, E.E.; Hutcherson, S.L.; Boucher, K.M.; Hansen, C.D.; Eliason, M.J.; Srivatsa, G.S.; Kornbrust, D.J.; et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol. Ther. 2010, 18, 442–446. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yin, M.; Zhang, L.-j. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. https://doi.org/10.3390/cells8080807
Zhang X, Yin M, Zhang L-j. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells. 2019; 8(8):807. https://doi.org/10.3390/cells8080807
Chicago/Turabian StyleZhang, Xiaowei, Meimei Yin, and Ling-juan Zhang. 2019. "Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis" Cells 8, no. 8: 807. https://doi.org/10.3390/cells8080807
APA StyleZhang, X., Yin, M., & Zhang, L.-j. (2019). Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells, 8(8), 807. https://doi.org/10.3390/cells8080807





