How Does SUMO Participate in Spindle Organization?
Abstract
1. The Biological Context
2. SUMO Targets
2.1. SUMOylation Concerns One-Third of Human Proteins and Spindle Proteins are not an Exception
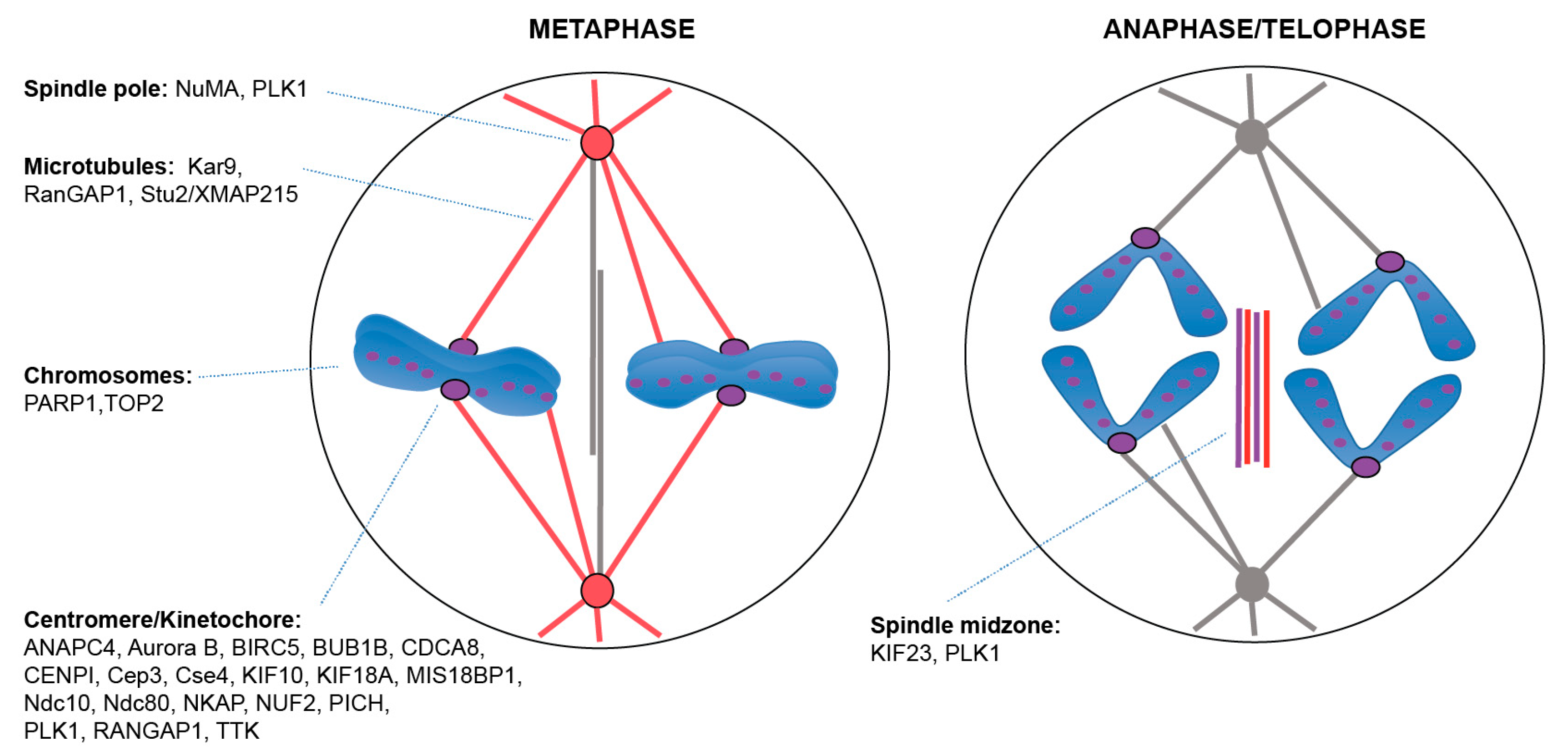
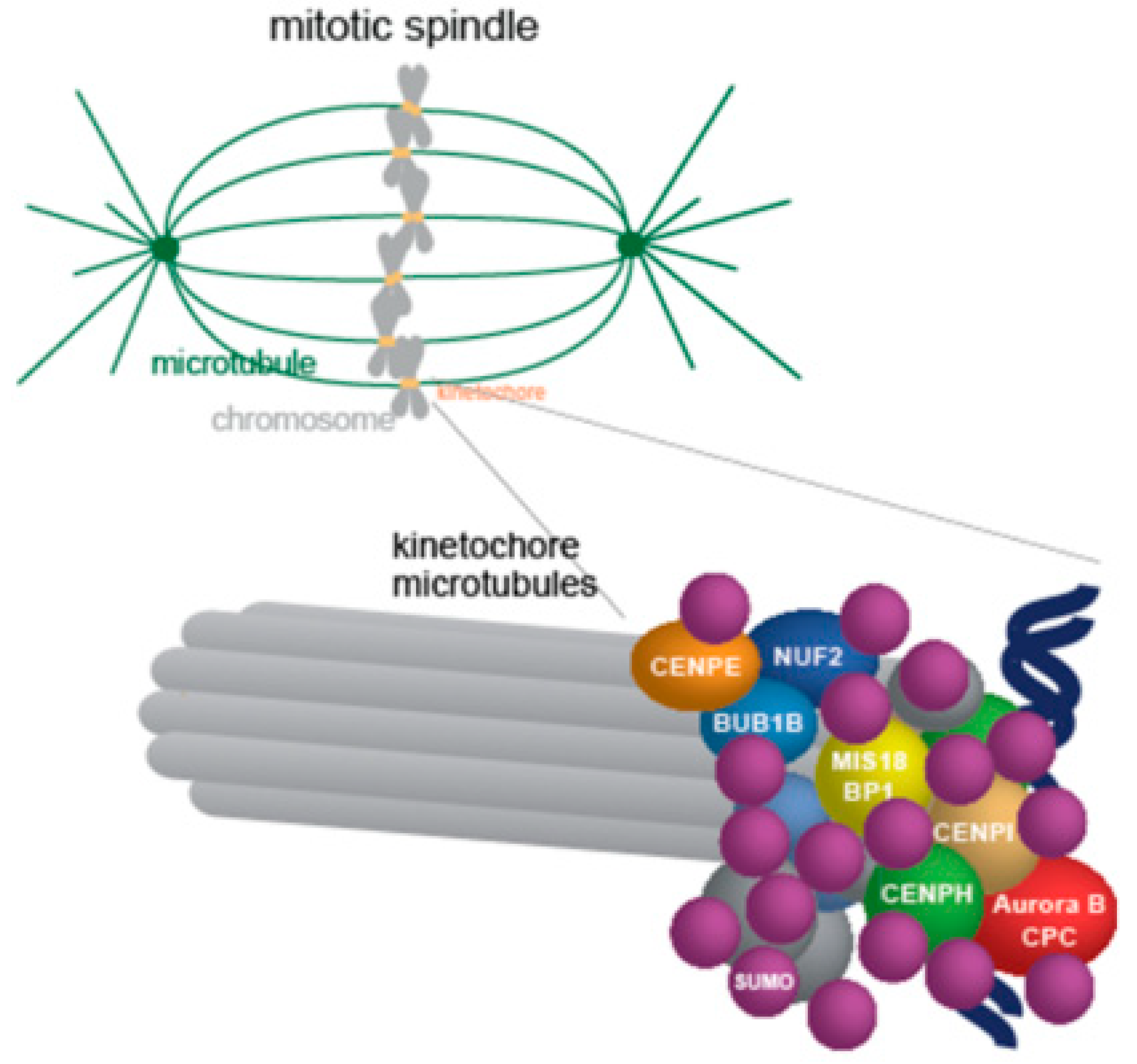
2.2. Centromere/Kinetochore and Chromosome Associated Proteins
2.3. Spindle-Associated Proteins
3. SUMO Functions in Spindle Organization
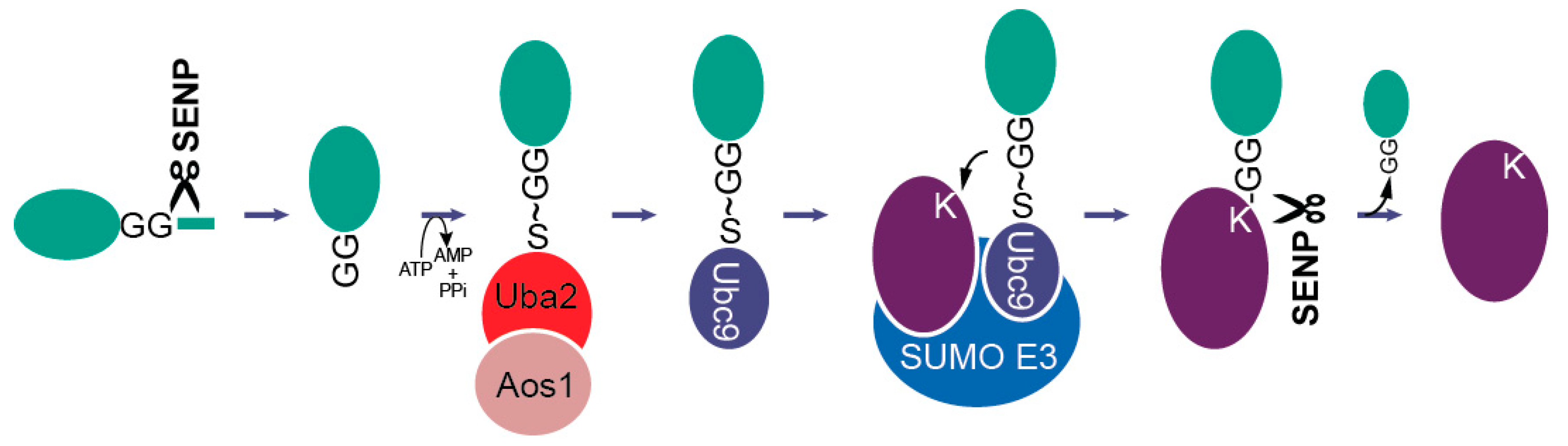
3.1. SUMO and SUMO Interacting Motifs (SIMs)
3.2. SUMO Functions: Direct and Indirect Regulation of Protein Localization within the Mitotic Spindle
3.3. SUMO Functions: Group SUMOylation and Phase Transition
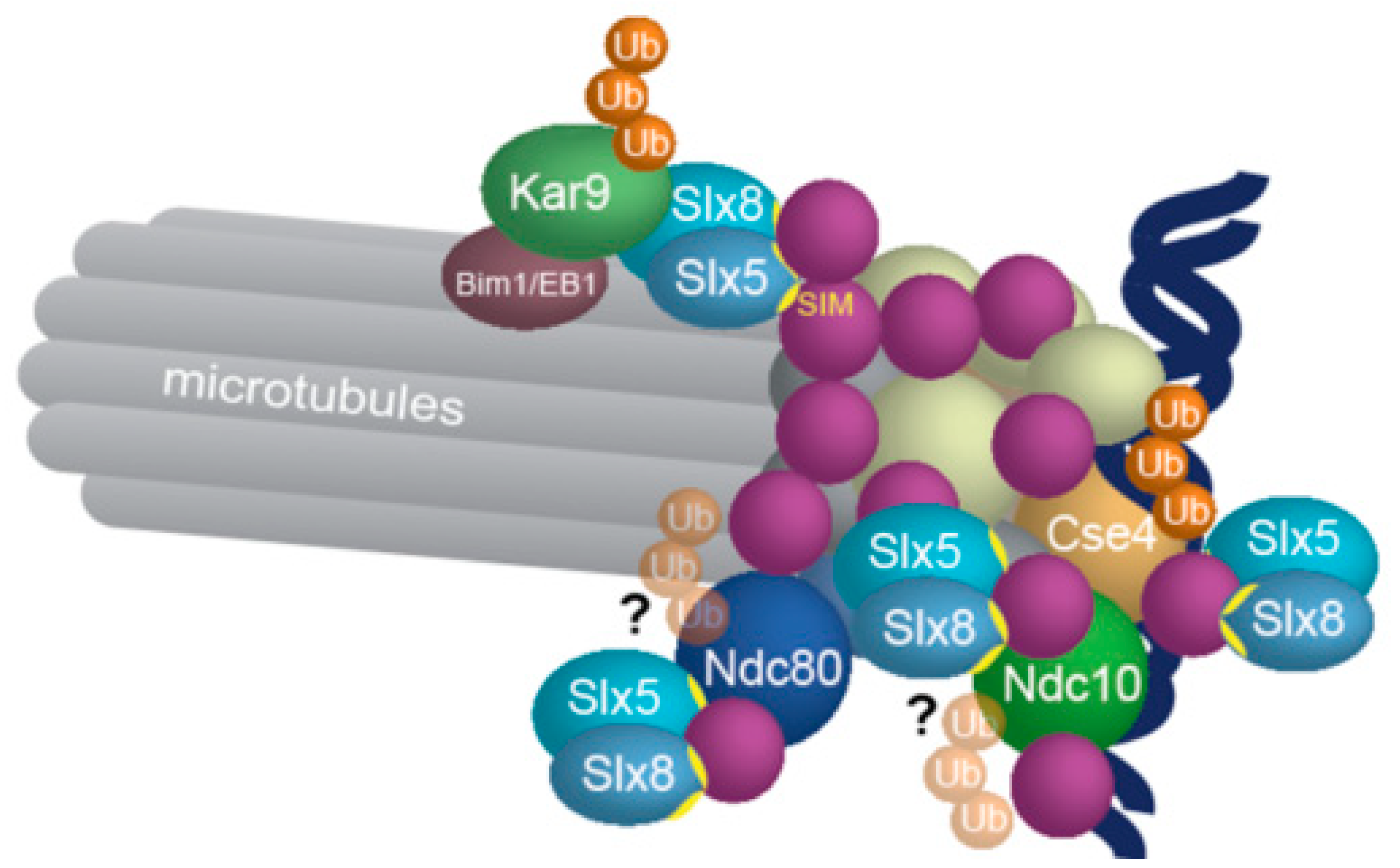
3.4. SUMO as a Signal for Degradation of Spindle Proteins: SUMO-targeted Ubiquitin Ligases
4. Many More Mitotic Proteins are SUMOylated–How to Study Them?
5. Spindle SUMOylation as Anticancer Target
6. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Prosser, S.L.; Pelletier, L. Mitotic spindle assembly in animal cells: A fine balancing act. Nat. Rev. Mol. Cell Biol. 2017, 18, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Petry, S.; Groen, A.C.; Ishihara, K.; Mitchison, T.J.; Vale, R.D. Branching microtubule nucleation in xenopus egg extracts mediated by augmin and TPX2. Cell 2013, 152, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Paz, J.; Lüders, J. Microtubule-Organizing Centers: Towards a Minimal Parts List. Trends Cell Biol. 2018, 28, 176–187. [Google Scholar] [CrossRef] [PubMed]
- David, A.F.; Roudot, P.; Legant, W.R.; Betzig, E.; Danuser, G.; Gerlich, D.W. Augmin accumulation on long-lived microtubules drives amplification and kinetochore-directed growth. J. Cell Biol. 2019, 218. [Google Scholar] [CrossRef]
- Alfaro-Aco, R.; Thawani, A.; Petry, S. Biochemical reconstitution of branching microtubule nucleation. bioRxiv 2019, 700047. [Google Scholar] [CrossRef]
- McIntosh, J.; Hays, T. A Brief History of Research on Mitotic Mechanisms. Biology (Basel) 2016, 5, 55. [Google Scholar] [CrossRef]
- Nixon, F.M.; Gutiérrez-Caballero, C.; Hood, F.E.; Booth, D.G.; Prior, I.A.; Royle, S.J. The mesh is a network of microtubule connectors that stabilizes individual kinetochore fibers of the mitotic spindle. Elife 2015, 4, e07635. [Google Scholar] [CrossRef]
- Dou, Z.; Prifti, D.; Gui, P.; Liu, X.; Elowe, S.; Yao, X. Recent Progress on the Localization of the Spindle Assembly Checkpoint Machinery to Kinetochores. Cells 2019, 8, 278. [Google Scholar] [CrossRef]
- Krenn, V.; Musacchio, A. The Aurora B Kinase in Chromosome Bi-Orientation and Spindle Checkpoint Signaling. Front. Oncol. 2015, 5, 1–18. [Google Scholar] [CrossRef]
- Scholey, J.; Civelekoglu-Scholey, G.; Brust-Mascher, I. Anaphase B. Biology (Basel) 2016, 5, 51. [Google Scholar] [CrossRef]
- Ubersax, J.A.; Woodbury, E.L.; Quang, P.N.; Paraz, M.; Blethrow, J.D.; Shah, K.; Shokat, K.M.; Morgan, D.O. Targets of the cyclin-dependent kinase Cdk1. Nature 2003, 425, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol. Cell 2002, 9, 931–943. [Google Scholar] [CrossRef]
- Matunis, M.J.; Rodriguez, M.S. Concepts and methodologies to study protein SUMOylation: An overview. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1475, pp. 57–88. ISBN 9781493963584. [Google Scholar]
- Mahajan, R.; Delphin, C.; Guan, T.; Gerace, L.; Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 1997, 88, 97–107. [Google Scholar] [CrossRef]
- Matunis, M.J.; Coutavas, E.; Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996, 135, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Schwienhorst, I.; Dohmen, R.J.; Blobel, G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 1997, 16, 5509–5519. [Google Scholar] [CrossRef] [PubMed]
- Meluh, P.B.; Koshland, D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol. Biol. Cell 1995, 6, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Garvin, A.J.; Morris, J.R. SUMO, a small, but powerful, regulator of double-strand break repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 372, 20160281. [Google Scholar] [CrossRef]
- Cappadocia, L.; Lima, C.D. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem. Rev. 2018, 118, 889–918. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Dasso, M. Modification in Reverse: The SUMO Proteases. Trends Biochem. Sci. 2007, 32, 286–295. [Google Scholar] [CrossRef]
- Desterro, J.M.P.; Rodriguez, M.S.; Kemp, G.D.; Ronald, T.H. Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. J. Biol. Chem. 1999, 274, 10618–10624. [Google Scholar] [CrossRef]
- Pichler, A.; Fatouros, C.; Lee, H.; Eisenhardt, N. SUMO conjugation—A mechanistic view. Biomol. Concepts 2017, 8, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Gupta, A.A. An E3-like Factor that Promotes SUMO Conjugation. Cell 2001, 106, 735–744. [Google Scholar] [CrossRef]
- Kahyo, T.; Nishida, T.; Yasuda, H. Involvement of PIAS1 in the Sumoylation of Tumor Suppressor p53. Mol. Cell 2001, 8, 713–718. [Google Scholar] [CrossRef]
- Wu, J.; Matunis, M.J.; Kraemer, D.; Blobel, G.; Coutavas, E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J. Biol. Chem. 1995, 270, 14209–14213. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Hayashi, N.; Seki, T.; Panté, N.; Ohba, T.; Nishii, K.; Kuma, K.; Hayashida, T.; Miyata, T.; Aebi, U.; et al. A giant nucleopore protein that binds Ran/TC4. Nature 1995, 376, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pichler, A.; Gast, A.; Seeler, J.S.; Dejean, A.; Melchior, F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 2002, 108, 109–120. [Google Scholar] [CrossRef]
- Reverter, D.; Lima, C.D. Insights into E3 ligase activity revealed by a SUMO–RanGAP1–Ubc9–Nup358 complex. Nature 2005, 435, 687–692. [Google Scholar] [CrossRef]
- Joseph, J.; Liu, S.; Jablonski, S.A.; Yen, T.J.; Dasso, M. The RanGAP1-RanBP2 Complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 2004, 14, 611–617. [Google Scholar] [CrossRef]
- Liang, Y.; Lee, C.; Yao, Y.; Lai, C.; Schmitz, M.L. SUMO5, a Novel Poly-SUMO Isoform, Regulates PML Nuclear Bodies. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Bohren, K.M.; Nadkarni, V.; Song, J.H.; Gabbay, K.H.; Owerbach, D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J. Biol. Chem. 2004, 279, 27233–27238. [Google Scholar] [CrossRef]
- Wang, L.; Wansleeben, C.; Zhao, S.; Miao, P.; Paschen, W.; Yang, W. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep. 2014, 15, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Nacerddine, K.; Lehembre, F.; Bhaumik, M.; Artus, J.; Cohen-Tannoudji, M.; Babinet, C.; Pandolfi, P.P.; Dejean, A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell 2005, 9, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.A.; Lyon, D.; Young, C.; Jensen, L.J.; Vertegaal, A.C.O.; Nielsen, M.L. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 2017, 24, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, B.; Hazbun, T.R.; Fields, S.; Hieter, P. Sumoylation of the budding yeast kinetochore protein Ndc10 is required for Ndc10 spindle localization and regulation of anaphase spindle elongation. J. Cell Biol. 2005, 174, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goeres, J.; Zhang, H.; Yen, T.J.; Porter, A.C.G.; Matunis, M.J. SUMO-2/3 Modification and Binding Regulate the Association of CENP-E with Kinetochores and Progression through Mitosis. Mol. Cell 2008, 29, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Goeres, J.; Sixt, K.M.; Békés, M.; Zhang, X.D.; Salvesen, G.S.; Matunis, M.J. Protection from Isopeptidase-Mediated Deconjugation Regulates Paralog-Selective Sumoylation of RanGAP1. Mol. Cell 2009, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, S.; Bonneil, E.; McManus, F.P.; Thibault, P. Gas-Phase Enrichment of Multiply Charged Peptide Ions by Differential Ion Mobility Extend the Comprehensiveness of SUMO Proteome Analyses. J. Am. Soc. Mass Spectrom. 2018, 29, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Coudreuse, D.; Nurse, P. Driving the cell cycle with a minimal CDK control network. Nature 2010, 468, 1074–1079. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Dasso, M. The fate of metaphase kinetochores is weighed in the balance of SUMOylation during S phase. Cell Cycle 2010, 9, 3194–3201. [Google Scholar] [CrossRef]
- Yong-Gonzales, V.; Hang, L.E.; Castellucci, F.; Branzei, D.; Zhao, X. The Smc5-Smc6 Complex Regulates Recombination at Centromeric Regions and Affects Kinetochore Protein Sumoylation during Normal Growth. PLoS ONE 2012, 7, e51540. [Google Scholar] [CrossRef]
- Sridharan, V.; Park, H.; Ryu, H. SUMOylation Regulates Polo-like Kinase 1-interacting Checkpoint Helicase (PICH) during Mitosis. J. Biol. Chem. 2015, 290, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Klein, U.R.; Haindl, M.; Nigg, E.A.; Muller, S. RanBP2 and SENP3 Function in a Mitotic SUMO2/3 Conjugation-Deconjugation Cycle on Borealin. Mol. Biol. Cell 2009, 20, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Miranda, G.; De Castro, I.P.; Carmena, M.; Aguirre-Portolés, C.; Ruchaud, S.; Fant, X.; Montoya, G.; Earnshaw, W.C.; Malumbres, M. SUMOylation modulates the function of Aurora-B kinase. J. Cell Sci. 2010, 1, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Ban, R.; Nishida, T.; Urano, T. Mitotic kinase Aurora-B is regulated by SUMO-2/3 conjugation/deconjugation during mitosis. Genes Cells 2011, 16, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Flotho, A.; Melchior, F. The RanBP2/RanGAP1*SUMO1/Ubc9 Complex is a Multisubunit SUMO E3 Ligase. Mol. Cell 2012, 46, 287–298. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Dai, W. Sumoylation of Kif18A plays a role in regulating mitotic progression. BMC Cancer 2015, 15, 4–13. [Google Scholar] [CrossRef]
- Li, T.; Chen, L.; Cheng, J.; Dai, J.; Huang, Y.; Zhang, J.; Liu, Z.; Li, A.; Li, N. SUMOylated NKAP is essential for chromosome alignment by anchoring CENP-E to kinetochores. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Joseph, J.; Tan, S.; Karpova, T.S.; Mcnally, J.G.; Dasso, M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 2002, 156, 595–602. [Google Scholar] [CrossRef]
- Eifler, K.; Vertegaal, A.C.O. Sumoylation-Mediated Regulation of Cell Cycle Progression and Cancer. Trends Biochem. Sci. 2015, 40, 779–793. [Google Scholar] [CrossRef]
- Lee, C.C.; Li, B.; Yu, H.; Matunis, M.J. Sumoylation promotes optimal APC/C activation and timely anaphase. Elife 2018, 7, e29539. [Google Scholar] [CrossRef]
- Yang, F.; Hu, L.; Chen, C.; Yu, J.; Connell, C.B.O.; Khodjakov, A.; Pagano, M.; Dai, W. BubR1 Is Modified by Sumoylation during Mitotic. J. Biol. Chem. 2012, 287, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, A.; Yang, F.; Chen, C.; Lu, L.; Dai, W. Mps1 is SUMO-modified during the cell cycle. Oncotarget 2015, 7, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Al-Ani, G.; Deckert, K.; Kirkpatrick, D.; Gygi, S.P.; Dasso, M.; Azuma, Y. PIASy Mediates SUMO-2/3 Conjugation of Poly (ADP-ribose) Polymerase 1 (PARP1) on Mitotic Chromosomes. J. Biol. Chem. 2010, 285, 14415–14423. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Arnaoutov, A.; Dasso, M. SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 2003, 163, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Furuta, M.; Kirkpatrick, D.; Gygi, S.P.; Azuma, Y. PIASy-dependent SUMOylation regulates DNA topoisomerase IIalpha activity. J. Cell Biol. 2010, 191, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Bachant, J.; Alcasabas, A.; Blat, Y.; Kleckner, N.; Elledge, S.J. The SUMO-1 Isopeptidase Smt4 Is Linked to Centromeric Cohesion through SUMO-1 Modification of DNA Topoisomerase II. Mol. Cell 2002, 9, 1169–1182. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yong-Gonzalez, V.; Kikuchi, Y.; Strunnikov, A. SIZ1/SIZ2 Control of Chromosome Transmission Fidelity Is Mediated by the Sumoylation of Topoisomerase II. Genetics 2006, 172, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Huang, C.; Liu, B.; Wang, Y.; Xia, N.; Fan, Q.; Chen, G.-Q.; Cheng, J. Mitotic Phosphorylation of SENP3 Regulates De-SUMOylation of Chromosome-Associated Proteins and Chromosome Stability. Cancer Res. 2018, 78, 2171–2178. [Google Scholar] [CrossRef]
- Eifler, K.; Cuijpers, S.A.G.; Willemstein, E.; Raaijmakers, J.A.; El Atmioui, D.; Ovaa, H.; Medema, R.H.; Vertegaal, A.C.O. SUMO targets the APC/C to regulate transition from metaphase to anaphase. Nat. Commun. 2018, 9, 1119. [Google Scholar] [CrossRef]
- Pelisch, F.; Sonneville, R.; Pourkarimi, E.; Agostinho, A.; Blow, J.J.; Gartner, A.; Hay, R.T. Dynamic SUMO modification regulates mitotic chromosome assembly and cell cycle progression in Caenorhabditis elegans. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Davis-Roca, A.C.; Divekar, N.S.; Ng, R.K.; Wignall, S.M. Dynamic SUMO remodeling drives a series of critical events during the meiotic divisions in C. elegans. PLoS Genet. 2018, 14, e1007626. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Arnaoutov, A.; Dasso, M. The SUMO protease SENP6 is essential for inner kinetochore assembly. J. Cell Biol. 2010, 188, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Ohkuni, K.; Takahashi, Y.; Fulp, A.; Lawrimore, J.; Au, W.-C.; Pasupala, N.; Levy-Myers, R.; Warren, J.; Strunnikov, A.; Baker, R.E.; et al. SUMO-targeted ubiquitin ligase (STUbL) Slx5 regulates proteolysis of centromeric histone H3 variant Cse4 and prevents its mislocalization to euchromatin. Mol. Biol. Cell 2016, 27, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Bao, X.; Gan, X.; Luo, S.; Rao, H. Multiple E3s promote the degradation of histone H3 variant Cse4. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, J.; Stevermann, L.; Panigada, D.; Kammerer, D.; Liakopoulos, D. Regulation of a Spindle Positioning Factor at Kinetochores by SUMO-Targeted Ubiquitin Ligases. Dev. Cell 2016, 36, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Meednu, N.; Hoops, H.; D’Silva, S.; Pogorzala, L.; Wood, S.; Farkas, D.; Sorrentino, M.; Sia, E.; Meluh, P.; Miller, R.K. The spindle positioning protein Kar9p interacts with the SUMOylation machinery in Saccharomyces cerevisiae. Genetics 2008, 180, 2033–2055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pelisch, F.; Tammsalu, T.; Wang, B.; Jaffray, E.G.; Gartner, A.; Hay, R.T.; Pelisch, F.; Tammsalu, T.; Wang, B.; Jaffray, E.G.; et al. Article A SUMO-Dependent Protein Network Regulates Chromosome Congression during Oocyte Meiosis. Mol. Cell 2016, 65, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, S.A.G.; Willemstein, E.; Vertegaal, A.C.O. Converging Small Ubiquitin-like Modifier (SUMO) and Ubiquitin Signaling: Improved Methodology Identifies Co-modified Target Proteins. Mol. Cell. Proteomics 2017, 16, 2281–2295. [Google Scholar] [CrossRef]
- Fu, H.; Liu, N.; Dong, Q.; Ma, C.; Yang, J.; Xiong, J.; Zhang, Z.; Qi, X.; Huang, C.; Zhu, B. SENP6-mediated M18BP1 deSUMOylation regulates CENP-A centromeric localization. Cell Res. 2019, 29, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Kim, H.N.; Kim, S.; Bang, J.; Kim, E.; Sung, K.S.; Yoon, H.; Yoo, H.Y.; Choi, C.Y. Cell cycle-dependent SUMO-1 conjugation to nuclear mitotic apparatus protein (NuMA). Biochem. Biophys. Res. Commun. 2014, 443, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Xie, Y.; Loo, J.A.; Courey, A.J. Genetic and Proteomic Evidence for Roles of Drosophila SUMO in Cell Cycle Control, Ras Signaling, and Early Pattern Formation. PLoS ONE 2009, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, W.B.; Hwang, K.; Morris, P.L. Temporal and SUMO-specific SUMOylation contribute to the dynamics of Polo-like kinase 1 (PLK1) and spindle integrity during mouse oocyte meiosis. Dev. Biol. 2018, 434, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Sparrow, D.B.; Shiomi, T.; Pu, R.T.; Nishimoto, T.; Mohun, T.J.; Dasso, M. Ubc9p and the conjugation of SUMO-1 to RanGAP1 and RanBP2. Curr. Biol. 1998, 8, 121–124. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, S.; Li, M.; Ouyang, Y.; Hou, Y.; Schatten, H.; Sun, Q. Septin2 is modified by SUMOylation and required for chromosome congression in mouse oocytes. Cell Cycle 2010, 9, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Ribet, D.; Boscaini, S.; Cauvin, C.; Siguier, M.; Mostowy, S.; Echard, A.; Cossart, P. SUMOylation of human septins is critical for septin filament bundling and cytokinesis. J. Cell Biol. 2017, 216, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, M.; Alonso, A.; Rahman, M.; Meednu, N.; Davis, K.; Tabb, V.; Cook, R.; Miller, R.K. The TOG protein Stu2 / XMAP215 interacts covalently and noncovalently with SUMO. Cytoskeleton 2018, 75, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Dawlaty, M.M.; Malureanu, L.; Jeganathan, K.B.; Kao, E.; Sustmann, C.; Tahk, S.; Shuai, K.; Grosschedl, R.; van Deursen, J.M. Resolution of Sister Centromeres Requires RanBP2-Mediated SUMOylation of Topoisomerase IIα. Cell 2008, 133, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Wu, J.; Wang, L.; Fu, Z. SUMOylation Promotes Nuclear Import and Stabilization of Polo-like Kinase 1 to Support Its Mitotic Function. Cell Rep. 2017, 21, 2147–2159. [Google Scholar] [CrossRef]
- Leisner, C.; Kammerer, D.; Denoth, A.; Britschi, M.; Barral, Y.; Liakopoulos, D. Regulation of Mitotic Spindle Asymmetry by SUMO and the Spindle-Assembly Checkpoint in Yeast. Curr. Biol. 2008, 18, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Blobel, G. Cell Cycle—Regulated Attachment of the Ubiquitin-Related Protein SUMO to the Yeast Septins. J. Cell Biol. 1999, 147, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Makhnevych, T.; Ptak, C.; Lusk, C.P.; Aitchison, J.D.; Wozniak, R.W. The role of karyopherins in the regulated SUMOylation of septins. J. Cell Biol. 2007, 177, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Durrin, L.K.; Wilkinson, T.A.; Krontiris, T.G.; Chen, Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 14373–14378. [Google Scholar] [CrossRef] [PubMed]
- Cappadocia, L.; Mascle, X.H.; Bourdeau, V.; Tremblay-Belzile, S.; Chaker-Margot, M.; Lussier-Price, M.; Wada, J.; Sakaguchi, K.; Aubry, M.; Ferbeyre, G.; et al. Article Structural and Functional Characterization of the Phosphorylation-Dependent Interaction between PML and SUMO1. Structure 2015, 23, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Praefcke, G.J.K.; Hofmann, K.; Dohmen, R.J. SUMO playing tag with ubiquitin. Trends Biochem. Sci. 2012, 37, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Knipscheer, P.; Van Dijk, W.J.; Olsen, J.V.; Mann, M.; Sixma, T.K. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007, 26, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, J.R.; Povlsen, L.K.; Villumsen, B.H.; Streicher, W.; Nilsson, J.; Wikström, M.; Bekker-jensen, S.; Mailand, N. DNA damage–inducible SUMOylation of HERC2 promotes RNF8 binding via a novel SUMO-binding Zinc finger. J. Cell Biol. 2012, 197, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, S.; Psakhye, I. Control of Nuclear Activities by Substrate-Selective and Protein-group Sumoylation. Annu. Rev. Genet. 2013, 47, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Rice, A.M.; Peeples, W.B.; Lin, Y.; Jain, S.; Parker, R.; Rosen, M.K. Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Pelisch, F.; Bel Borja, L.; Jaffray, E.G.; Hay, R.T. Sumoylation regulates protein dynamics during meiotic chromosome segregation in C. elegans oocytes. J. Cell Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Psakhye, I.; Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012, 151, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Wright, W.E.; Shay, J.W. Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes Dev. 2019, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hecker, C.M.; Rabiller, M.; Haglund, K.; Bayer, P.; Dikic, I. Specification of SUMO1-and SUMO2-interacting motifs. J. Biol. Chem. 2006, 281, 16117–16127. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, R.; Chien, C.D.; Avantaggiati, M.L.; Muller, S. An Acetylation Switch Regulates SUMO-Dependent Protein Interaction Networks. Mol. Cell 2012, 46, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Sriramachandran, A.M.; Dohmen, R.J. SUMO-targeted ubiquitin ligases. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Keusekotten, K.; Bade, V.N.; Meyer-Teschendorf, K.; Sriramachandran, A.M.; Fischer-Schrader, K.; Krause, A.; Horst, C.; Hofmann, K. Multivalent interactions of the SUMO-interaction motifs in RING finger protein 4 determine the specificity for chains of the SUMO. Biochem. J. 2014, 457, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, K.; Göttsche, K.; Miteva, M.; Weisshaar, S.R.; Glanemann, C.; Schnellhardt, M.; Niessen, M.; Scheel, H.; Hofmann, K.; Johnson, E.S.; et al. Ubiquitin-dependent proteolytic control of SUMO conjugates. J. Biol. Chem. 2007, 282, 34167–34175. [Google Scholar] [CrossRef] [PubMed]
- Tatham, M.H.; Geoffroy, M.C.; Shen, L.; Plechanovova, A.; Hattersley, N.; Jaffray, E.G.; Palvimo, J.J.; Hay, R.T. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 2008, 10, 538–546. [Google Scholar] [CrossRef]
- Weisshaar, S.R.; Keusekotten, K.; Krause, A.; Horst, C.; Springer, H.M.; Göttsche, K.; Dohmen, R.J.; Praefcke, G.J.K. Arsenic trioxide stimulates SUMO-2/3 modification leading to RNF4-dependent proteolytic targeting of PML. FEBS Lett. 2008, 582, 3174–3178. [Google Scholar] [CrossRef]
- Yin, Y.; Seifert, A.; Chua, J.S.; Golebiowski, F.; Hay, R.T. SUMO-targeted ubiquitin E3 ligase RNF4 is required for the response of human cells to DNA damage. Genes Dev. 2012, 26, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; González-Prieto, R.; Xiao, Z.; Verlaan-de Vries, M.; Vertegaal, A.C.O. The STUbL RNF4 regulates protein group SUMOylation by targeting the SUMO conjugation machinery. Nat. Commun. 2017, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Psakhye, I.; Jentsch, S. Identification of substrates of protein-group SUMOylation. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2016; Volume 1475, pp. 219–231. ISBN 9781493963584. [Google Scholar]
- Van De Pasch, L.A.L.; Miles, A.J.; Nijenhuis, W.; Brabers, N.A.C.H.; Van Leenen, D.; Lijnzaad, P.; Brown, M.K.; Ouellet, J.; Barral, Y.; Kops, G.J.P.L.; et al. Centromere Binding and a Conserved Role in Chromosome Stability for SUMO-Dependent Ubiquitin Ligases. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Tsuda, M.; Murai, J.; Takagi, T.; Keka, I.S.; Narita, T.; Fujita, M.; Sasanuma, H.; Kobayashi, J.; Takeda, S. SUMO-targeted ubiquitin ligase RNF4 plays a critical role in preventing chromosome loss. Genes Cells 2014, 19, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Thu, Y.M.; Van Riper, S.K.; Higgins, L.A.; Zhang, T.; Becker, J.R.; Markowski, T.W.; Nguyen, H.D.; Griffin, T.J.; Bielinsky, A.K. Slx5/Slx8 Promotes Replication Stress Tolerance by Facilitating Mitotic Progression. Cell Rep. 2016, 15, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Ranjitkar, P.; Press, M.O.; Yi, X.; Baker, R.; MacCoss, M.J.; Biggins, S. An E3 Ubiquitin Ligase Prevents Ectopic Localization of the Centromeric Histone H3 Variant via the Centromere Targeting Domain. Mol. Cell 2010, 40, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Hewawasam, G.; Shivaraju, M.; Mattingly, M.; Venkatesh, S.; Martin-Brown, S.; Florens, L.; Workman, J.L.; Gerton, J.L. Psh1 Is an E3 Ubiquitin Ligase that Targets the Centromeric Histone Variant Cse4. Mol. Cell 2010, 40, 444–454. [Google Scholar] [CrossRef]
- Caldas, G.V.; DeLuca, J.G. KNL1: Bringing order to the kinetochore. Chromosoma 2014, 123, 169–181. [Google Scholar] [CrossRef][Green Version]
- Weir, J.R.; Faesen, A.C.; Klare, K.; Petrovic, A.; Basilico, F.; Fischböck, J.; Pentakota, S.; Keller, J.; Pesenti, M.E.; Pan, D.; et al. Insights from biochemical reconstitution into the architecture of human kinetochores. Nature 2016, 537, 249–253. [Google Scholar] [CrossRef]
- Meulmeester, E.; Kunze, M.; Hsiao, H.H.; Urlaub, H.; Melchior, F. Mechanism and Consequences for Paralog-Specific Sumoylation of Ubiquitin-Specific Protease 25. Mol. Cell 2008, 30, 610–619. [Google Scholar] [CrossRef]
- Nathan, D.; Ingvarsdottir, K.; Sterner, D.E.; Bylebyl, G.R.; Dokmanovic, M.; Dorsey, J.A.; Whelan, K.A.; Krsmanovic, M.; Lane, W.S.; Meluh, P.B.; et al. Histone SUMOylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 2006, 20, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Strunnikov, A. In vivo modeling of polysumoylation uncovers targeting of Topoisomerase II to the nucleolus via optimal level of SUMO modification. Chromosoma 2008, 117, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Chang, Y.C.; Lee, D.S.W.; Berry, J.; Sanders, D.W.; Ronceray, P.; Wingreen, N.S.; Haataja, M.; Brangwynne, C.P. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 2018, 175, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Seeler, J.S.; Dejean, A. SUMO and the robustness of cancer. Nat. Rev. Cancer 2017, 17, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, Y.; Wang, X.; Liang, Z.; He, G.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Protein SUMOylation modification and its associations with disease. Open Biol. 2017, 7. [Google Scholar] [CrossRef]
- Poruchynsky, M.S.; Komlodi-Pasztor, E.; Trostel, S.; Wilkerson, J.; Regairaz, M.; Pommier, Y.; Zhang, X.; Kumar Maity, T.; Robey, R.; Burotto, M.; et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Senese, S.; France, B.; Gholkar, A.A.; Damoiseaux, R.; Torres, J.Z. Computational Cell Cycle Profiling of Cancer Cells for Prioritizing FDA-Approved Drugs with Repurposing Potential. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Yang, W.; Ye, D.G.; Shen, Y.; Pluchino, S.; Lee, Y.J.; Hallenbeck, J.M.; Paschen, W. SUMOylation in brain ischemia: Patterns, targets, and translational implications. J. Cereb. Blood Flow Metab. 2018, 38, 5–16. [Google Scholar] [CrossRef]
- Fukuda, I.; Ito, A.; Hirai, G.; Nishimura, S.; Kawasaki, H.; Saitoh, H.; Kimura, K.; Sodeoka, M.; Yoshida, M. Ginkgolic Acid Inhibits Protein SUMOylation by Blocking Formation of the E1-SUMO Intermediate. Chem. Biol. 2009, 16, 133–140. [Google Scholar] [CrossRef]
- Fukuda, I.; Ito, A.; Uramoto, M.; Saitoh, H.; Kawasaki, H.; Osada, H.; Yoshida, M. Kerriamycin B inhibits protein SUMOylation. J. Antibiot. 2014, 67, 335–338. [Google Scholar] [CrossRef]
- Takemoto, M.; Kawamura, Y.; Hirohama, M.; Yamaguchi, Y.; Handa, H.; Saitoh, H.; Nakao, Y.; Kawada, M.; Khalid, K.; Koshino, H.; et al. Inhibition of protein SUMOylation by davidiin, an ellagitannin from Davidia involucrata. J. Antibiot. 2014, 67, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yuan, L.; Atkison, J.H.; Williams, K.M.; Vega, R.; Sessions, E.H.; Divlianska, D.B.; Davies, C.; Chen, Y.; Olsen, S.K. Molecular mechanism of a covalent allosteric inhibitor of SUMO E1 activating enzyme. Nat. Commun. 2018, 9, 5145. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Nagy, K.; Keyser, S.; Schneekloth, J.S. An electrophoretic mobility shift assay identifies a mechanistically unique inhibitor of protein SUMOylation. Chem. Biol. 2013, 20, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Hirohama, M.; Kumar, A.; Fukuda, I.; Matsuoka, S.; Igarashi, Y.; Saitoh, H.; Takagi, M.; Shin-Ya, K.; Honda, K.; Kondoh, Y.; et al. Spectomycin B1 as a novel SUMOylation inhibitor that directly binds to SUMO E2. ACS Chem. Biol. 2013, 8, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Bossis, G.; Sarry, J.E.; Kifagi, C.; Ristic, M.; Saland, E.; Vergez, F.; Salem, T.; Boutzen, H.; Baik, H.; Brockly, F.; et al. The ROS/SUMO Axis Contributes to the Response of Acute Myeloid Leukemia Cells to Chemotherapeutic Drugs. Cell Rep. 2014, 7, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Bogachek, M.V.; Chen, Y.; Kulak, M.V.; Woodfield, G.W.; Cyr, A.R.; Park, J.M.; Spanheimer, P.M.; Li, Y.; Li, T.; Weigel, R.J. Sumoylation pathway is required to maintain the basal breast cancer subtype. Cancer Cell 2014, 25, 748–761. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Riceberg, J.; Soucy, T.; Koenig, E.; Minissale, J.; Gallery, M.; Bernard, H.; Yang, X.; Liao, H.; Rabino, C.; et al. Probing the roles of SUMOylation in cancer cell biology by using a selective SAE inhibitor. Nat. Chem. Biol. 2017, 13, 1164–1171. [Google Scholar] [CrossRef]
- De Thé, H.; Pandolfi, P.P.; Chen, Z. Acute Promyelocytic Leukemia: A Paradigm for Oncoprotein-Targeted Cure. Cancer Cell 2017, 32, 552–560. [Google Scholar] [CrossRef]
| SUMOylated Protein Studied | Localization in Mitosis | SUMO Regulates Localization? | SUMO Regulates Enzymatic Activity? | SUMO Pathway Components Involved | Model System | Reference |
|---|---|---|---|---|---|---|
| ANAPC4 | kinetochore | no | N.A. | SUMO2 | Mammalian cell culture | [51,60] |
| Aurora B/AURKB (AIR-2, CPC) | chromosomes, midzone, Ring complexes | yes | N.D. | SUMO2/3, PIAS2/3/4, ULP-1 (SENP2/3/5, SENP6/7 | C. elegans mitosis, meiosis | [61,62] |
| Aurora B/AURKB (CPC) | centromere | yes | no/yes | SUMO2/3, PIAS3, SENP2 | Mammalian cell culture | [44,45] |
| BIRC5 (Survivin, Bir1, CPC) | centromere | yes | N.A. | N.D. | S. cerevisiae | [35] |
| BUB1B (BubR1) | kinetochore | no | N.D. | SUMO1,2/3, SENP2 | Mammalian cell culture | [36,52] |
| CDCA8 (Borealin) | Centromere, central spindle | no | N.A. | SUMO2/3, RanBP2, RRSU, SENP3 | Mammalian cell culture | [43,46] |
| CENPI | centromere/kinetochore | yes | N.A. | SENP6 | Mammalian cell culture | [40,63] |
| Cep3 | centromere | yes | N.A. | Siz1, Siz2 | S. cerevisiae | [35] |
| Cse4/CENPA | centromere | yes | N.A. | Siz1, Siz2, Slx5/8 | S. cerevisiae | [64,65] |
| Kar9 | astral microtubules | yes | N.A. | Siz1, Siz2, Slx5/8 | S. cerevisiae | [66,67] |
| KIF10 (CENP-E, kinesin-7) | kinetochore | no | N.D. | SUMO2/3, RNF4 | Mammalian cell culture | [36] |
| KIF18A (kinesin 8) | kinetochore | no | N.D. | SUMO2 | Mammalian cell culture | [47] |
| KLP-19 (kinesin 4) | Ring complexes | yes | N.A. | PIAS2/3/4 (GEI-17) | C. elegans meiosis | [68] |
| KIF23/MKLP1 | midzone | N.D. | N.D. | SUMO2, RNF4 | Mammalian cell culture | [69] |
| MIS18BP1 | kinetochore | N.D. | N.D. | SUMO2, SENP6, RNF4 | Mammalian cell culture | [69,70] |
| Ndc10 | centromere | yes | N.A. | Siz1, Siz2 | S. cerevisiae | [35] |
| Ndc80 | kinetochore | no | N.A. | N.D. | S. cerevisiae | [35] |
| NKAP | kinetochore | no | N.A. | SUMO1, SUMO2 | Mammalian cell culture | [48] |
| NUF2 | kinetochore | N.D. | N.A. | SUMO2/3, SENP2 | Mammalian cell culture | [36] |
| NuMA | spindle pole | not clear | N.A. | SUMO1 | Mammalian cell culture | [71] |
| PARP1 | chromosomes | no | no | SUMO2/3, PIAS4 | Xenopus egg extracts | [42,54] |
| PICH | centromere | no | likely | SUMO2/3, PIAS4 | Xenopus egg extracts | [42] |
| PLK1 | kinetochore, midbody | N.D. | N.D. | N.D. | Drosophila | [72] |
| PLK1 | kinetochore | N.D. | N.D. | SUMO2/3 | Mouse oocyte (meiosis) | [73] |
| PLK1 | spindle pole | N.D. | N.D. | SUMO1 | Mouse oocyte (meiosis) | [73] |
| RANGAP1 | kinetochore, microtubules | yes | N.A. | SUMO1 | Mammalian cell culture | [29,49,74] |
| SEPT2 | spindle, midbody | N.D. | N.A. | SUMO1 | Mouse oocyte (meiosis) | [75] |
| SEPT3, SEPT6, SEPT7, SEPT9 (Septins) | cleavage furrow, actin | yes | N.A. | SUMO1, SUMO2 | Mammalian cell culture | [76] |
| Stu2/XMAP215, TOG) | kinetochore, microtubules | N.D. | N.A. | Smt3, Siz1, Siz2 | S. cerevisiae | [77] |
| Topoisom. II/TOP2 | chromosomes | N.D. | yes | SUMO2/3, PIAS4 | Xenopus egg extracts | [55,56] |
| Topoisom. II/TOP2 | chromosomes | N.D. | N.D. | RanBP2, SENP3 | Mouse Embryonic Fibroblasts, Mammalian cell culture | [59,78] |
| Topoisom. II/Top2 | chromosomes | N.D. | N.D. | Siz1, Siz2 | S. cerevisiae | [57,58] |
| TTK (Mps1) | kinetochore | no | N.D. | SUMO1, SUMO2 | Mammalian cell culture | [53] |
| N.A. | Not Applicable | |||||
| N.D. | Not determined |
| SUMOylated Protein | Nr of SUMO Sites | Localization in Mitosis | Studied (Table 1) |
|---|---|---|---|
| ANAPC4 | 2 | kinetochore | yes |
| AURKA (Aurora A) | 9 | spindle pole | |
| AURKB (Aurora B) | 13 | centromere | yes |
| BIRC5 (Survivin) | 7 | centromere | yes |
| BUB1 | 9 | kinetochore | |
| BUB1B | 1 | kinetochore | yes |
| BUB3 | 12 | kinetochore | |
| CASC5 (KNL1) | 87 | kinetochore | |
| CDCA8 (Borealin) | 7 | centromere | yes |
| CENPB | 5 | centromere | |
| CENPC (Mif2) | 28 | centromere/kinetochore | |
| CENPH | 7 | centromere/kinetochore | |
| CENPI | 1 | centromere/kinetochore | yes |
| CENPK | 2 | centromere/kinetochore | |
| CENPN | 6 | centromere/kinetochore | |
| CENPO | 7 | centromere/kinetochore | |
| CENPQ | 8 | centromere/kinetochore | |
| CENPT | 2 | centromere/kinetochore | |
| CENPU | 11 | centromere/kinetochore | |
| CENPV | 3 | centromere/kinetochore | |
| CENPW | 1 | centromere/kinetochore | |
| INCENP | 10 | centromere | |
| KIF11 (Cin8, Eg5) | 2 | microtubule | |
| KIF13A | 1 | microtubule | |
| KIF14 | 1 | spindle pole, microtubule | |
| KIF15 | 2 | microtubule | |
| KIF18A | 32 | kinetochore | yes |
| KIF18B | 7 | microtubule | |
| KIF22 | 24 | chromosome | |
| KIF23 | 1/31 | spindle midzone (from anaphase) | yes |
| KIF2A | 1/5 | centromere | |
| KIF2C | 1/16 | centromere | |
| KIF4A | 22 | chromosome | yes |
| KIFC1 (kin-14) | 6 | microtubule | |
| KIFC3 | 1 | microtubule | |
| MAP9 | 1 | microtubule | |
| MIS18A | 5 | centromere | |
| MIS18BP1 (KNL2) | 80 | centromere | yes |
| NDC80 | 17 | kinetochore | yes |
| NKAP | 5 | kinetochore | yes |
| NUF2 | 6 | kinetochore | yes |
| NuMA1 | 10 | spindle pole | yes |
| PARP1 | 48 | chromosomes | yes |
| PLK1 | 5 | centromere/kinetochore, spindle pole | yes |
| RANBP1 | 2 | kinetochore | |
| RANBP2 | 48 | kinetochore | |
| RANGAP1 | 9 | kinetochore, microtubule | yes |
| RCC1 | 8 | chromosome | |
| RCC2 (TD-60) | 12 | centromere/kinetochore | |
| SPC25 | 5 | kinetochore | |
| TOP1 | 25 | chromosome | |
| TOP2A | 85 | chromosome | yes |
| TOP2B | 2/75 | chromosome | yes |
| TPX2 | 39 | spindle pole, microtubule | |
| TTK (Mps1) | 16 | kinetochore | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrieu, A.; Liakopoulos, D. How Does SUMO Participate in Spindle Organization? Cells 2019, 8, 801. https://doi.org/10.3390/cells8080801
Abrieu A, Liakopoulos D. How Does SUMO Participate in Spindle Organization? Cells. 2019; 8(8):801. https://doi.org/10.3390/cells8080801
Chicago/Turabian StyleAbrieu, Ariane, and Dimitris Liakopoulos. 2019. "How Does SUMO Participate in Spindle Organization?" Cells 8, no. 8: 801. https://doi.org/10.3390/cells8080801
APA StyleAbrieu, A., & Liakopoulos, D. (2019). How Does SUMO Participate in Spindle Organization? Cells, 8(8), 801. https://doi.org/10.3390/cells8080801





