HIF-1α is Overexpressed in Odontogenic Keratocyst Suggesting Activation of HIF-1α and NOTCH1 Signaling Pathways
Abstract
1. Introduction
2. Materials And Methods
2.1. Samples
2.2. Immunohistochemistry Assessment
2.3. Length, Inflammation, and Immunostaining Assessment
2.4. Statistical Analysis
3. Results
3.1. Basal Layer Length and Degree of Inflammation
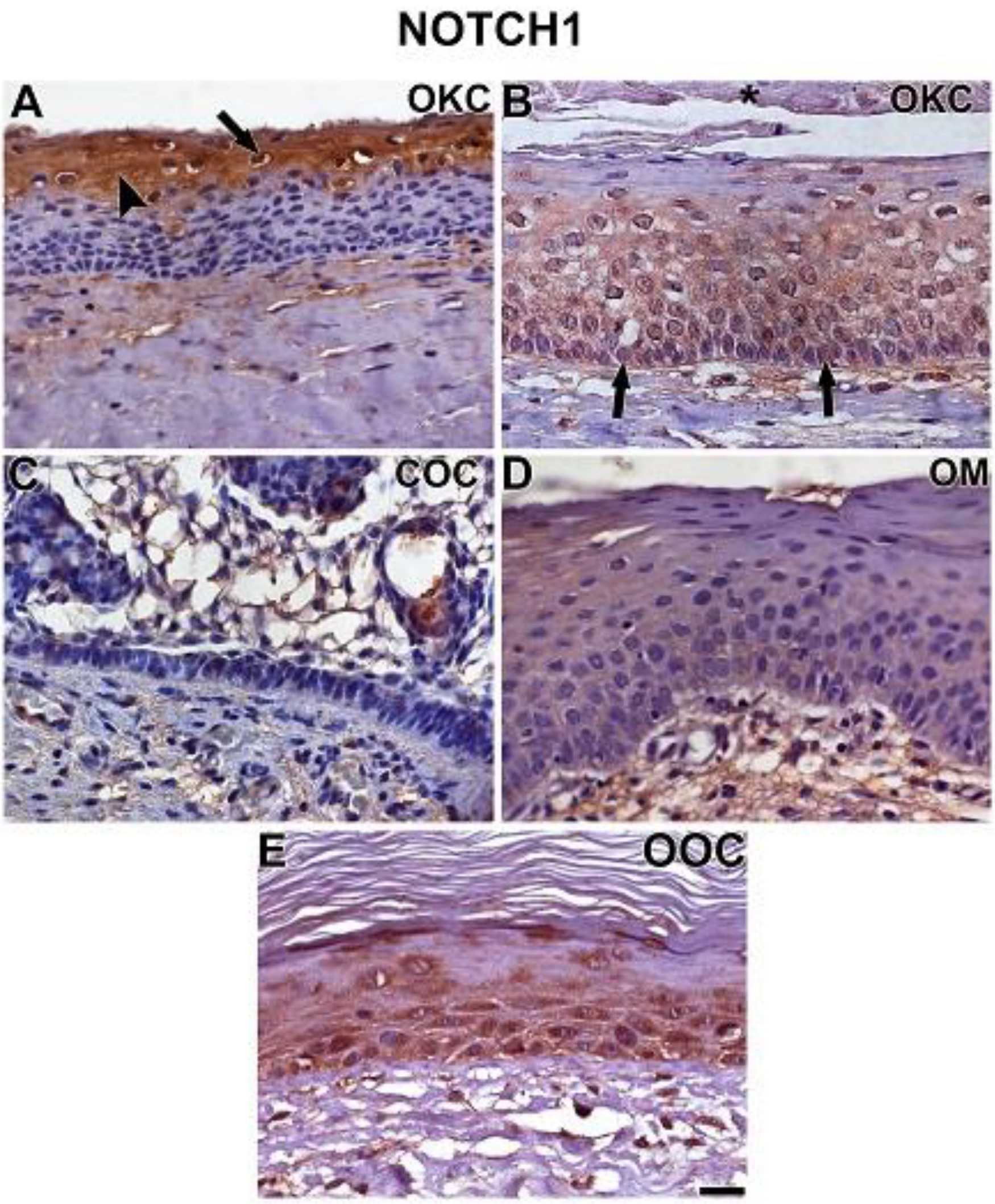
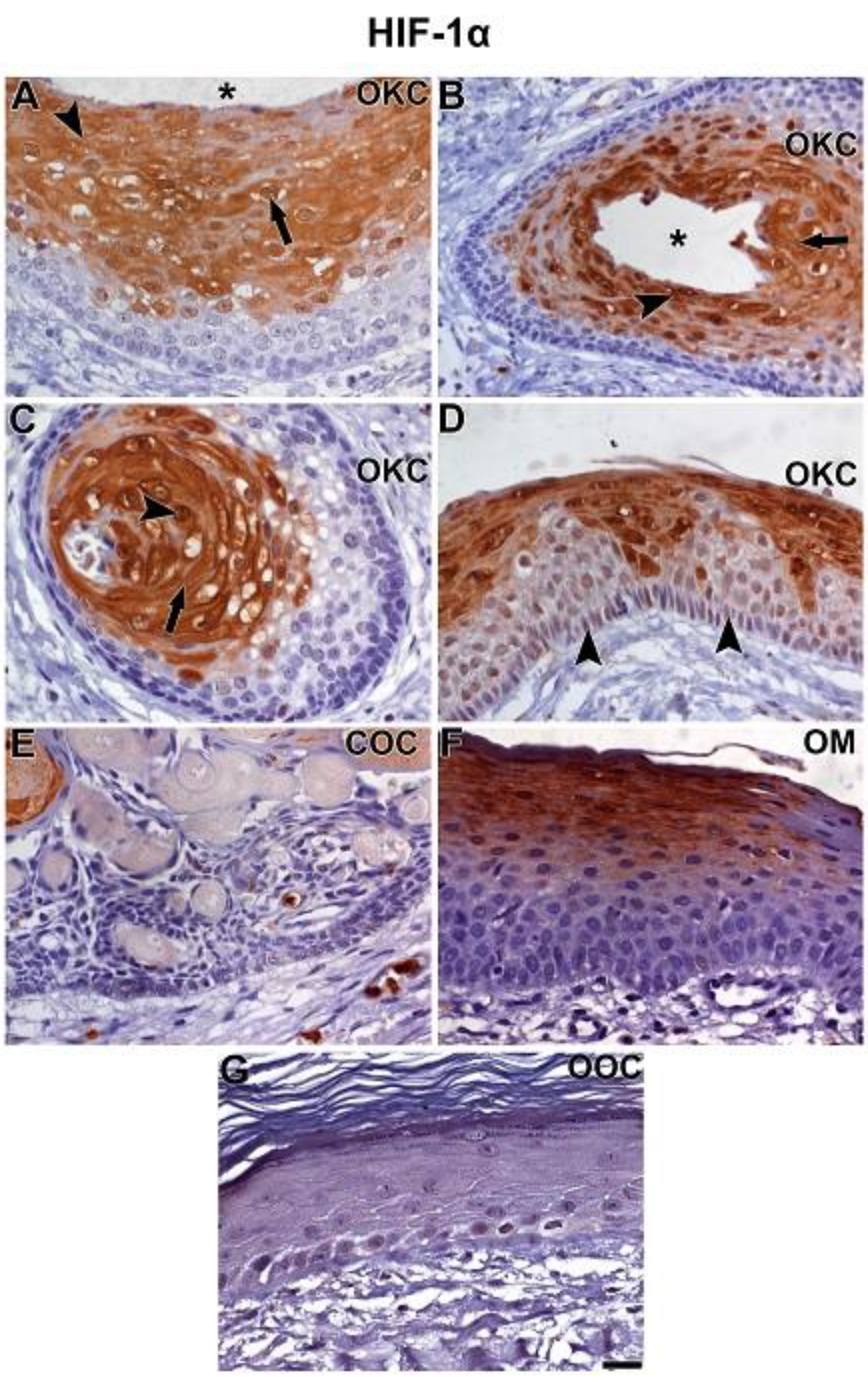
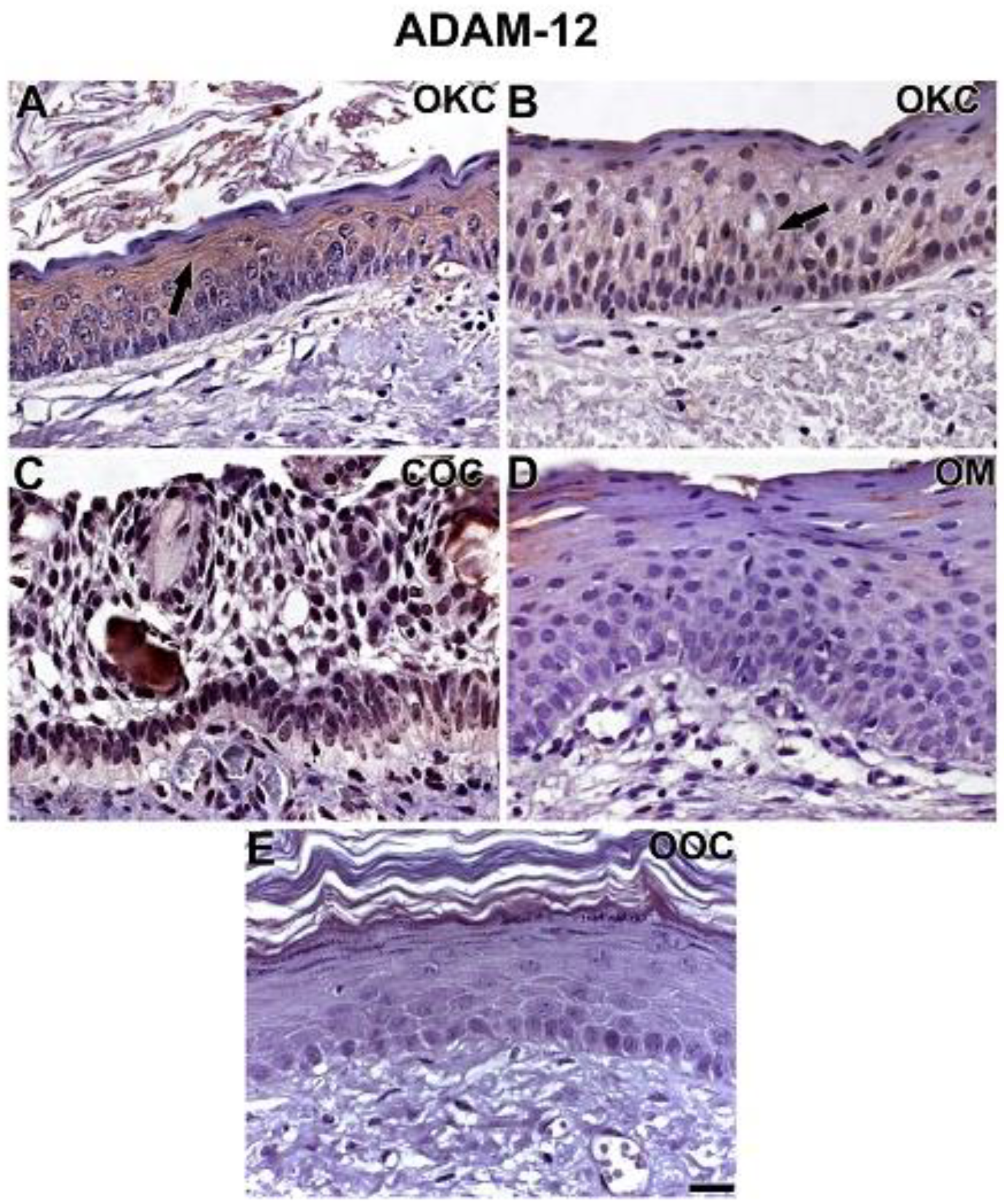
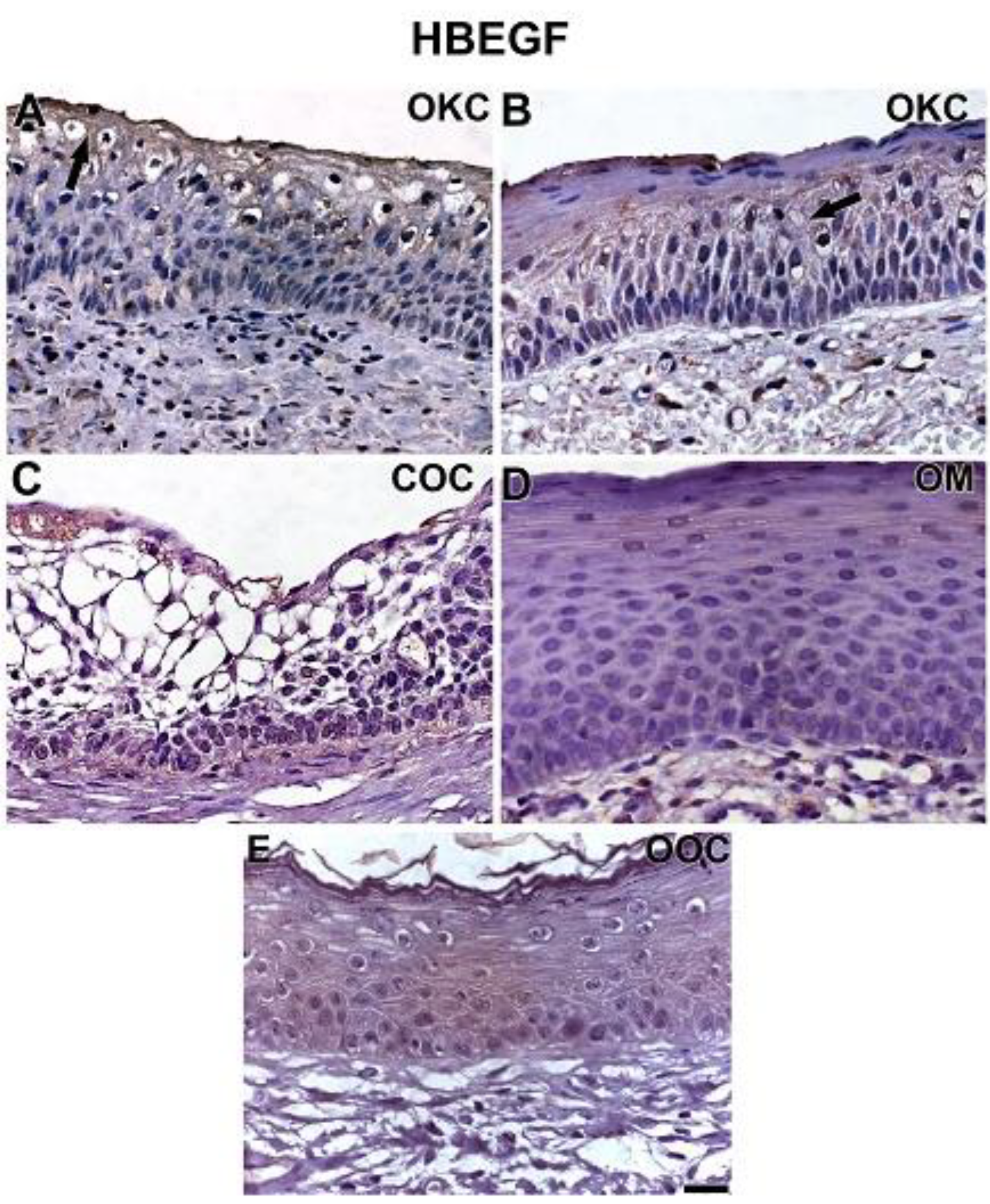
3.2. NOTCH1, HIF-1α, ADAM-12, and HBEGF were expressed in OKC, COC, OOC and OM
3.3. Expression of NOTCH1, HIF-1α, ADAM-12, and HBEGF is higher in OKC when compared to COC, OM, and OOC
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Head and Neck Tumours, 4th ed.; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; IARC Press: Lyon, France, 2017. [Google Scholar]
- World Health Organization Classification of Tumours Pathology and Genetics Head and Neck Tumours; Barnes, L., Eveson, J.W., Reichart, P., Sidransky, D., Eds.; IARC Press: Lyon, France, 2005. [Google Scholar]
- Shear, M. The aggressive nature of the odontogenic keratocyst: Is it a benign cystic neoplasm? Part 3. Immunocytochemistry of cytokeratin and other epithelial cell markers. Oral Oncol. 2002, 38, 407–415. [Google Scholar] [CrossRef]
- Tan, B.; Yan, T.S.; Shermin, L.; Teck, K.C.; Yoke, P.C.; Goh, C.; Balakrishnan, A. Malignant transformation of keratocystic odontogenic tumor: Two case reports. Am. J. Otolaryngol. Head Neck Med. Surg. 2013, 34, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Faurobert, E.; Bouin, A.P.; Albiges-Rizo, C. Microenvironment, tumor cell plasticity, and cancer. Curr. Opin. Oncol. 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Courtneidge, S.A. The “ins” and “outs” of podosomes and invadopodia: Characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Ribeiro, A.L.; da Costa, N.M.M.; de Siqueira, A.S.; Brasil da Silva, W.; da Silva Kataoka, M.S.; Jaeger, R.G.; de Melo Alves-Junior, S.; Smith, A.M.; de Jesus Viana Pinheiro, J. Keratocystic odontogenic tumor overexpresses invadopodia-related proteins, suggesting invadopodia formation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.S.; Pinto, M.P.; Cruz, M.C.; Smuczek, B.; Cruz, K.S.P.; Barbuto, J.A.M.; Hoshino, D.; Weaver, A.M.; Freitas, V.M.; Jaeger, R.G.; et al. Laminin-111 peptide C16 regulates invadopodia activity of malignant cells through β1 integrin, Src and ERK 1/2. Oncotarget 2016, 7, 47904–47917. [Google Scholar] [CrossRef] [PubMed]
- Buschman, M.D.; Bromann, P.A.; Cejudo-Martin, P.; Wen, F.; Pass, I.; Courtneidge, S.A. The Novel Adaptor Protein Tks4 (SH3PXD2B) Is Required for Functional Podosome Formation. Mol. Biol. Cell 2009, 20, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Amm, H.M.; Casimir, M.D.; Clark, D.B.; Sohn, P.; MacDougall, M. Matrix metalloproteinase expression in keratocystic odontogenic tumors and primary cells. Connect. Tissue Res. 2014, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.R.; Nobre, R.M.; Alves-Junior, S.M.; Kataoka, M.S.S.S.; Barroso, R.F.F.F.; Jaeger, R.G.; Pinheiro, J.J.V. V Matrix metalloproteinases, tissue inhibitors of metalloproteinases, and growth factors regulate the aggressiveness and proliferative activity of keratocystic odontogenic tumors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B.; Yuen, A.; Iizuka, S.; Higashiyama, S.; Courtneidge, S.A. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J. Cell Biol. 2013, 201, 279–292. [Google Scholar] [CrossRef]
- da Costa, N.M.M.; Fialho, A.D.V.; Proietti, C.C.; da Silva Kataoka, M.S.; Jaeger, R.G.; de Alves-Júnior, S.M.; de Jesus Viana Pinheiro, J. Role of hypoxia-related proteins in invasion of ameloblastoma cells: crosstalk between NOTCH1, hypoxia-inducible factor 1α, a disintegrin and metalloproteinase 12, and heparin-binding epidermal growth factor. Histopathology 2016, 69, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.R.; Trindade, A.; Fernandes, A.C.; Carvalho, C.; Gigante, J.; Tavares, A.T.; Diéguez-Hurtado, R.; Yagita, H.; Adams, R.H.; Duarte, A. Endothelial jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhu, Y.; Xu, L.; Chen, L.; Lin, Z.; Xu, J. Notch1 predicts recurrence and survival of patients with clear-cell renal cell carcinoma after surgical resection. Urology 2015, 85, 483.e9–483.e14. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Stegeman, I.; Willems, S.M.; Grolman, W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: A systematic review. Cancer Med. 2015, 4, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- da Costa, N.M.M.M.; de Siqueira, A.S.; Ribeiro, A.L.R.R.; da Silva Kataoka, M.S.; Jaeger, R.G.; de Alves-Júnior, S.M.; Smith, A.M.; de Jesus Viana Pinheiro, J. Role of HIF-1α and CASPASE-3 in cystogenesis of odontogenic cysts and tumors. Clin. Oral Investig. 2018, 22, 141–149. [Google Scholar] [CrossRef]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.; Saftig, P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin. Cell Dev. Biol. 2009, 20, 126–137. [Google Scholar] [CrossRef]
- Qi, Y.; Duhachek-Muggy, S.; Lino, H.; Zolkiewska, A. Phenotypic diversity of breast cancer-related mutations in metalloproteinase-disintegrin ADAM12. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Asakura, M.; Kitakaze, M.; Takashima, S.; Liao, Y.; Ishikura, F.; Yoshinaka, T.; Ohmoto, H.; Node, K.; Yoshino, K.; Ishiguro, H.; et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: Metalloproteinase inhibitors as a new therapy. Nat. Med. 2002, 8, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, S.; Abraham, J.; Miller, J.; Fiddes, J.; Klagsbrun, M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991, 251, 936–939. [Google Scholar] [CrossRef]
- Baselga, J.; Arteaga, C.L. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 2005, 23, 2445–2459. [Google Scholar] [CrossRef]
- Takebe, N.; Nguyen, D.; Yang, S.X. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol. Ther. 2014, 141, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Hägg, M.; Wennström, S. Activation of hypoxia-induced transcription in normoxia. Exp. Cell Res. 2005, 306, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Zhang, X.P.; Liu, F.; Wang, W. Modeling the interplay between the HIF-1 and p53 pathways in hypoxia. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simón, R.; Aparicio, R.; Housden, B.E.; Bray, S.; Busturia, A. Drosophila p53 controls Notch expression and balances apoptosis and proliferation. Apoptosis 2014, 19, 1430–1443. [Google Scholar] [CrossRef]
- Miller, M.A.; Moss, M.L.; Powell, G.; Petrovich, R.; Edwards, L.; Meyer, A.S.; Griffith, L.G.; Lauffenburger, D.A. Targeting autocrine HB-EGF signaling with specific ADAM12 inhibition using recombinant ADAM12 prodomain. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, Y.; Zolkiewska, A. Direct Interaction between the Cytoplasmic Tail of ADAM 12 and the Src Homology 3 Domain of p85α Activates Phosphatidylinositol 3-Kinase in C2C12 Cells. J. Biol. Chem. 2001, 276, 24466–24472. [Google Scholar] [CrossRef]

| Length of the Basement Membrane (mm) | Inflammation Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SD | 0 | 1 | 2 | 3 | A × B | |
| OKC | 8.8 | 5.9 | 15.7 | 1.8 | - | 4 | 12 | 4 | ns |
| COC | 8.9 | 6.3 | 13.9 | 1.7 | - | - | 7 | 1 | na |
| OOC | 8.4 | 5.2 | 17.4 | 2.1 | - | 5 | 1 | 2 | na |
| OM | 8.9 | 3.7 | 18.3 | 2.9 | - | 6 | 2 | - | na |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda da Costa, N.M.; Saab Abe, C.T.; Mitre, G.P.; Mesquita, R.A.; Silva Kataoka, M.S.d.; Ribeiro Ribeiro, A.L.; Jaeger, R.G.; Alves-Júnior, S.d.M.; Smith, A.M.; Pinheiro, J.d.J.V. HIF-1α is Overexpressed in Odontogenic Keratocyst Suggesting Activation of HIF-1α and NOTCH1 Signaling Pathways. Cells 2019, 8, 731. https://doi.org/10.3390/cells8070731
Miranda da Costa NM, Saab Abe CT, Mitre GP, Mesquita RA, Silva Kataoka MSd, Ribeiro Ribeiro AL, Jaeger RG, Alves-Júnior SdM, Smith AM, Pinheiro JdJV. HIF-1α is Overexpressed in Odontogenic Keratocyst Suggesting Activation of HIF-1α and NOTCH1 Signaling Pathways. Cells. 2019; 8(7):731. https://doi.org/10.3390/cells8070731
Chicago/Turabian StyleMiranda da Costa, Natacha Malu, Caio Tadashi Saab Abe, Geovanni Pereira Mitre, Ricardo Alves Mesquita, Maria Sueli da Silva Kataoka, André Luis Ribeiro Ribeiro, Ruy Gastaldoni Jaeger, Sérgio de Melo Alves-Júnior, Andrew Mark Smith, and João de Jesus Viana Pinheiro. 2019. "HIF-1α is Overexpressed in Odontogenic Keratocyst Suggesting Activation of HIF-1α and NOTCH1 Signaling Pathways" Cells 8, no. 7: 731. https://doi.org/10.3390/cells8070731
APA StyleMiranda da Costa, N. M., Saab Abe, C. T., Mitre, G. P., Mesquita, R. A., Silva Kataoka, M. S. d., Ribeiro Ribeiro, A. L., Jaeger, R. G., Alves-Júnior, S. d. M., Smith, A. M., & Pinheiro, J. d. J. V. (2019). HIF-1α is Overexpressed in Odontogenic Keratocyst Suggesting Activation of HIF-1α and NOTCH1 Signaling Pathways. Cells, 8(7), 731. https://doi.org/10.3390/cells8070731





