Zebrafish Carrying pycr1 Gene Deficiency Display Aging and Multiple Behavioral Abnormalities
Abstract
1. Introduction
1.1. Proline Functions in Stress Protection
1.2. Animal Model for Human Aging
2. Results
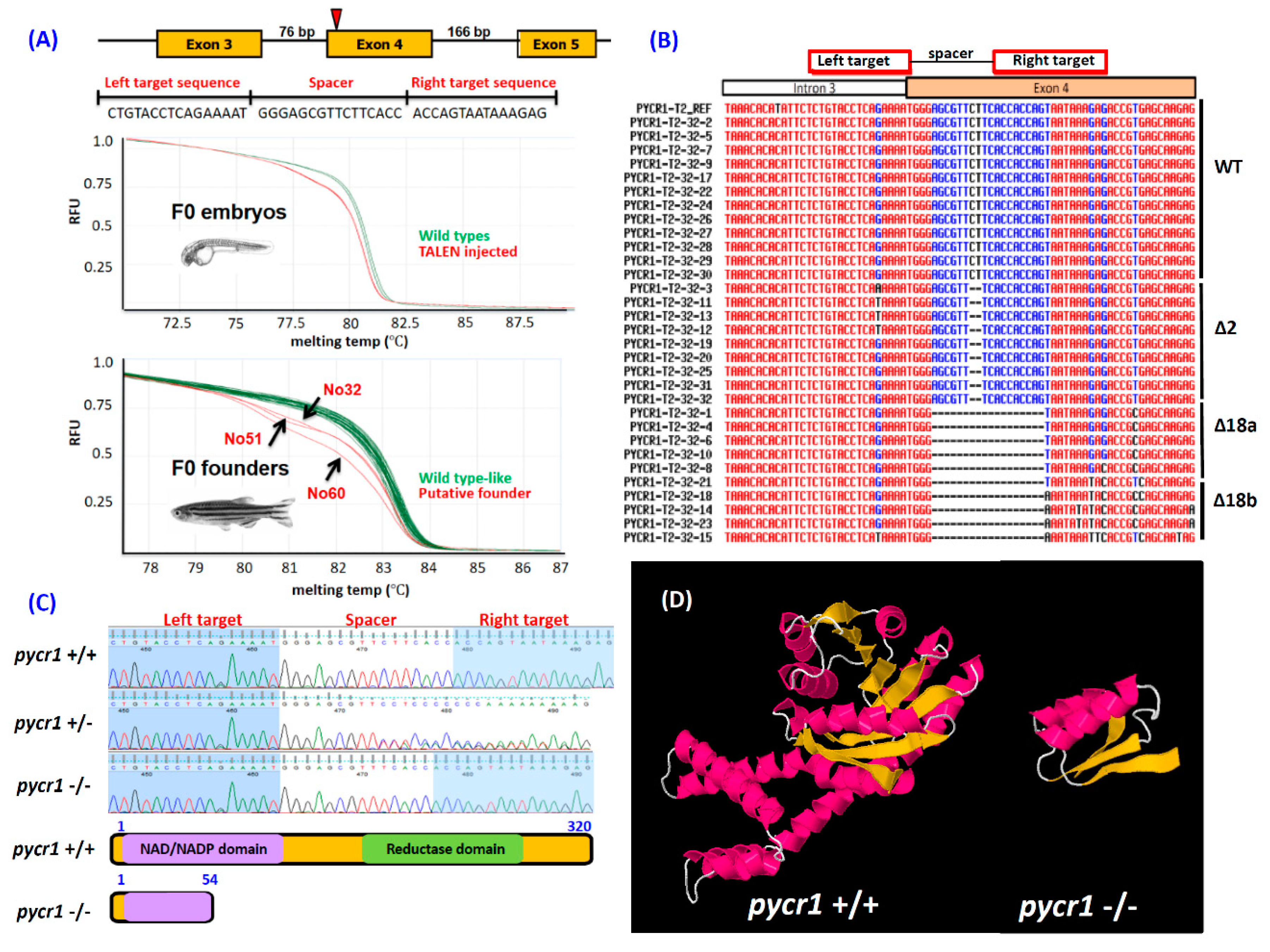
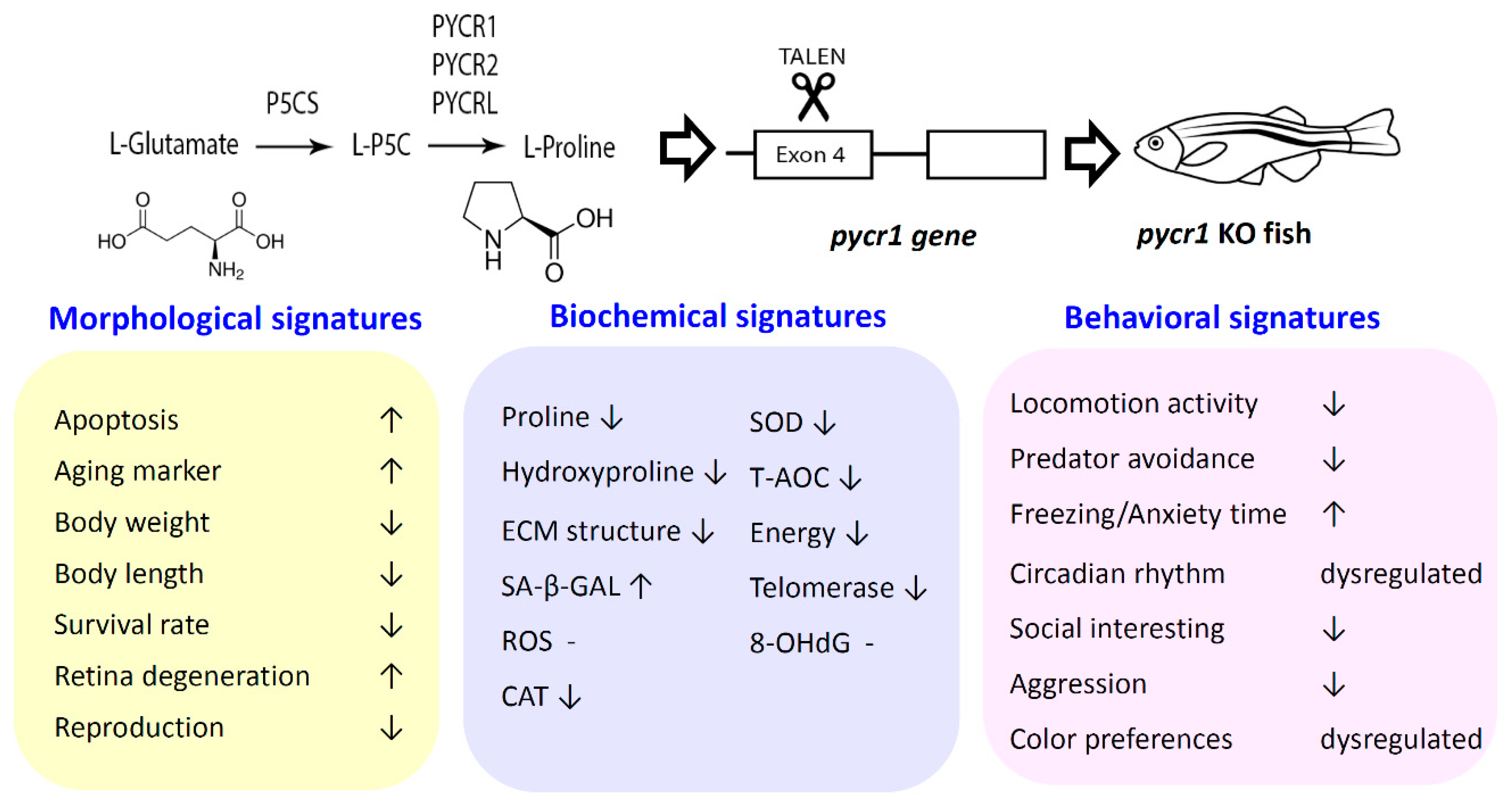
2.1. Ablation of pycr1 Gene in Zebrafish by TALEN
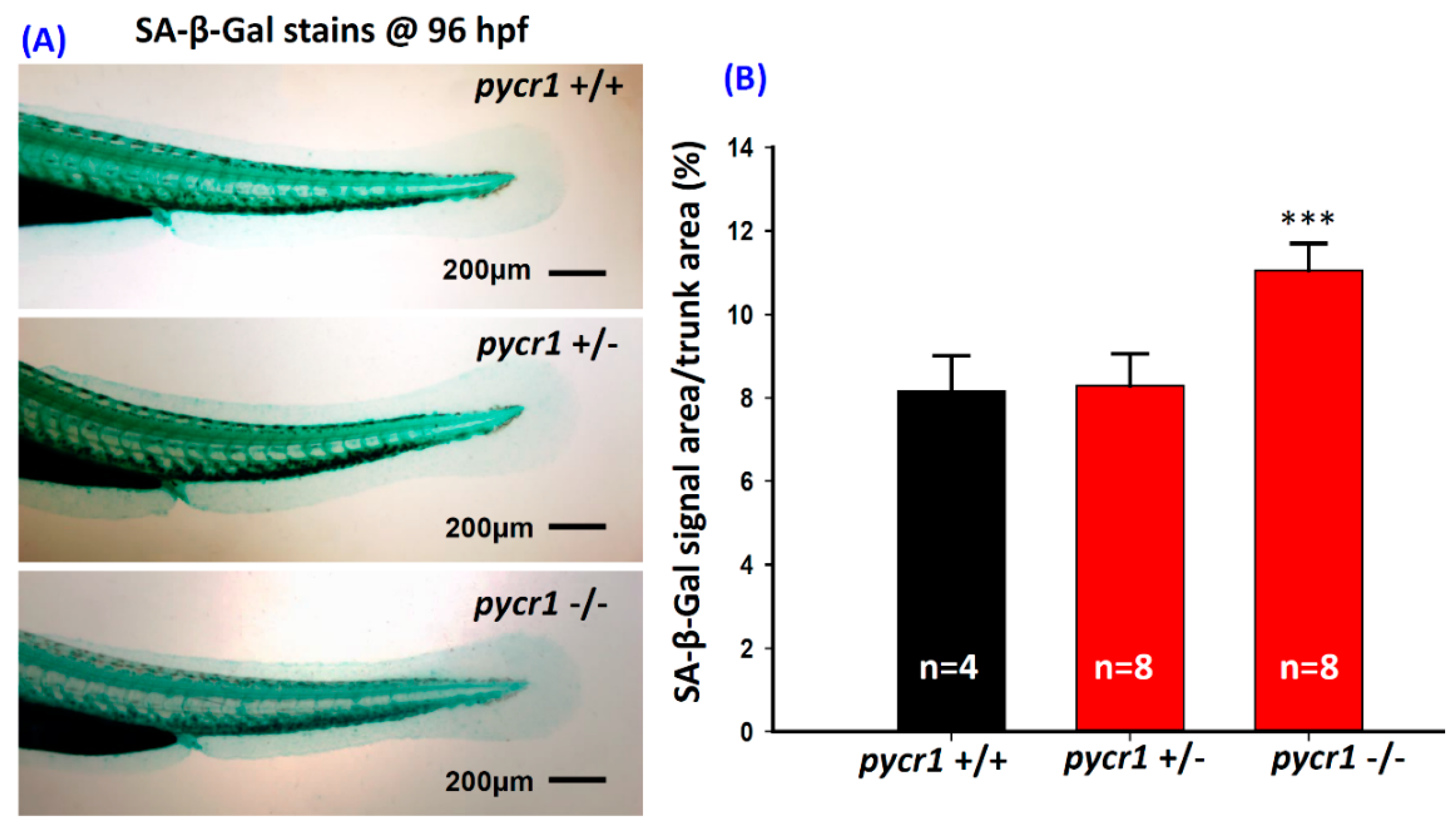
2.2. Ablation of pycr1 Gene Induced Senescence and Increased Intestinal Permeability
2.3. Knockout of pycr1 Gene Induces Dwarfism, Aging and Infertility
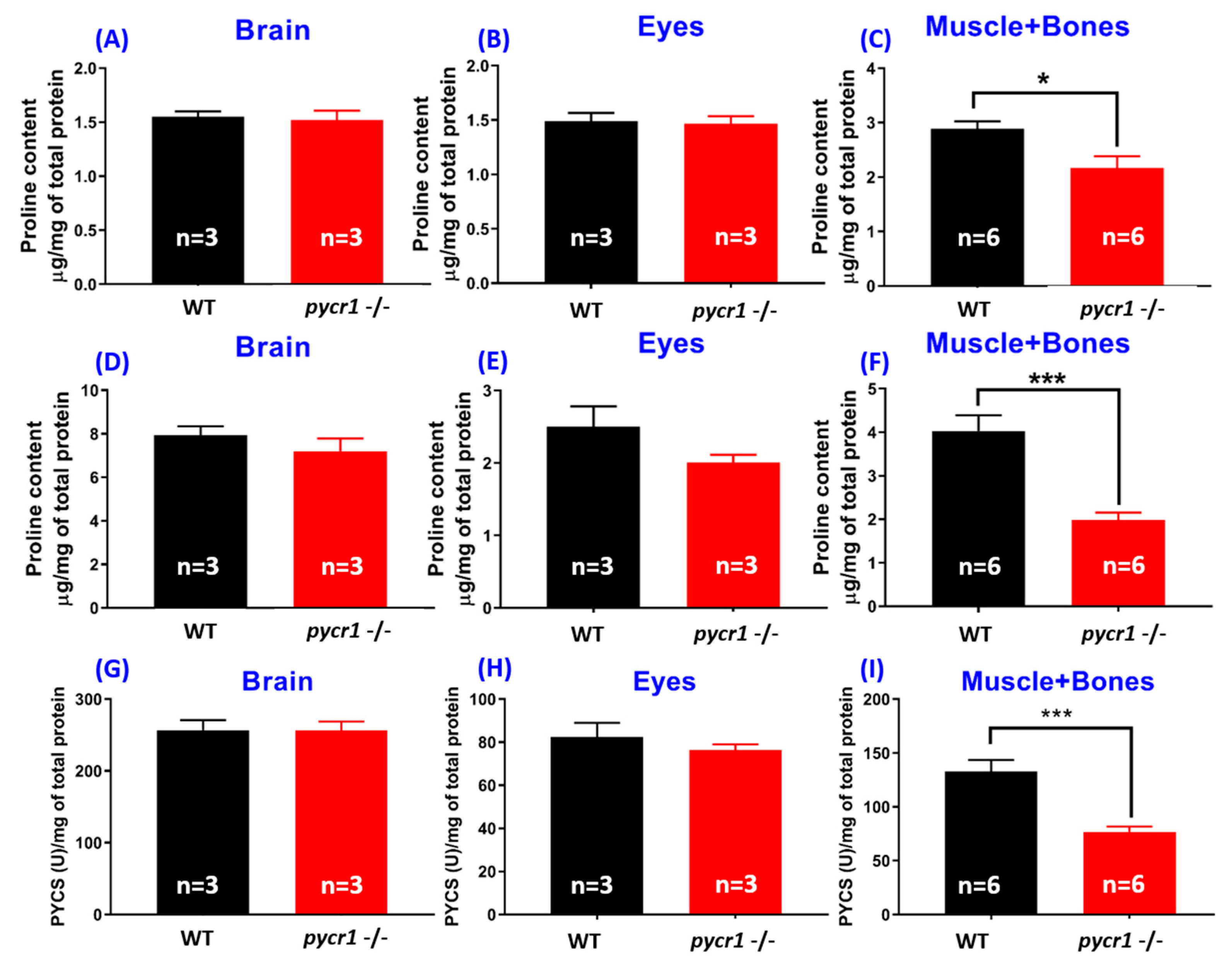
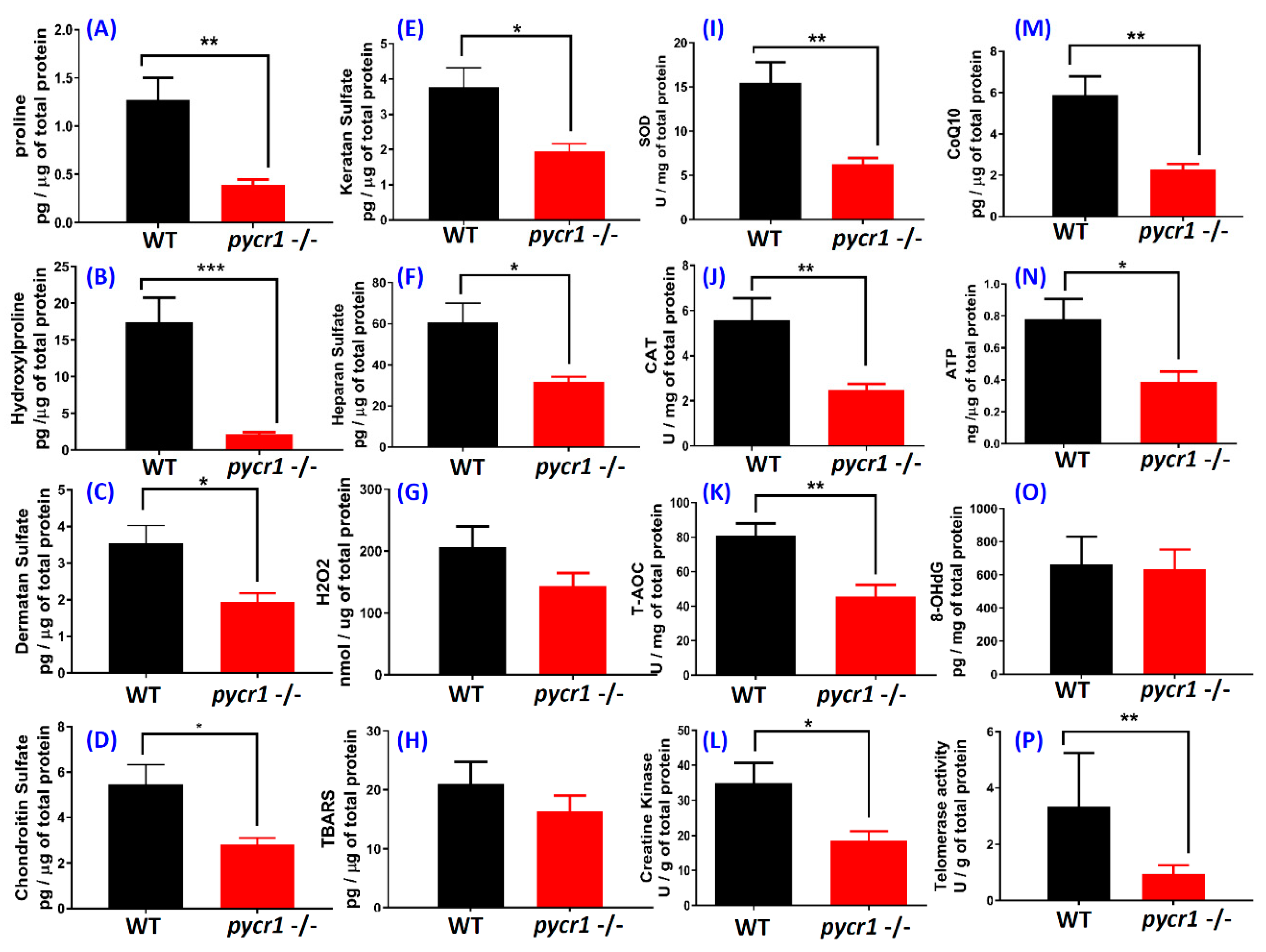
2.4. Biological Effects of Premature Aging in Adult pycr1 KO Fish
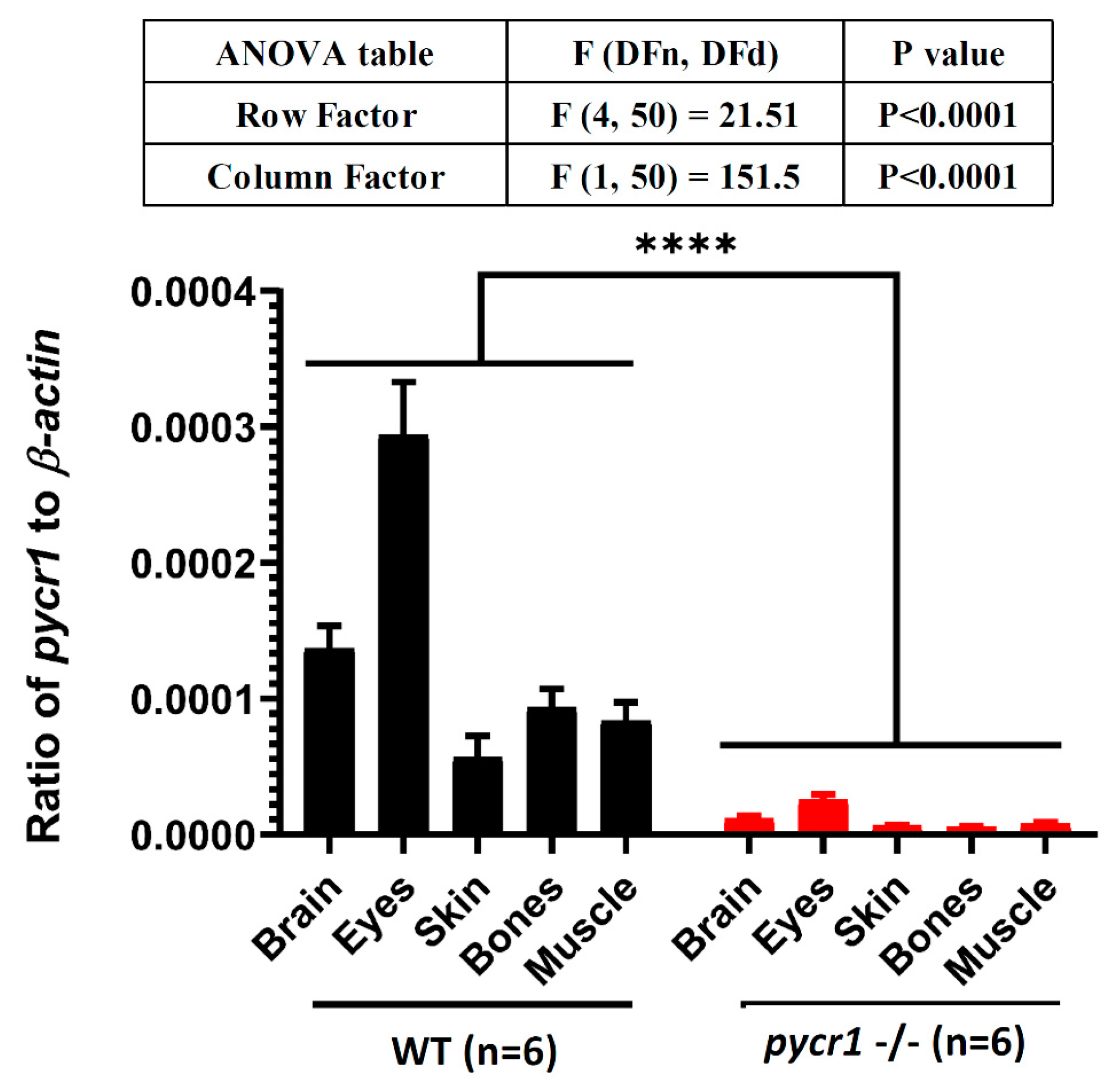
2.5. Knockout of pycr1 Gene Induces pycr1 Transcripts Degradation
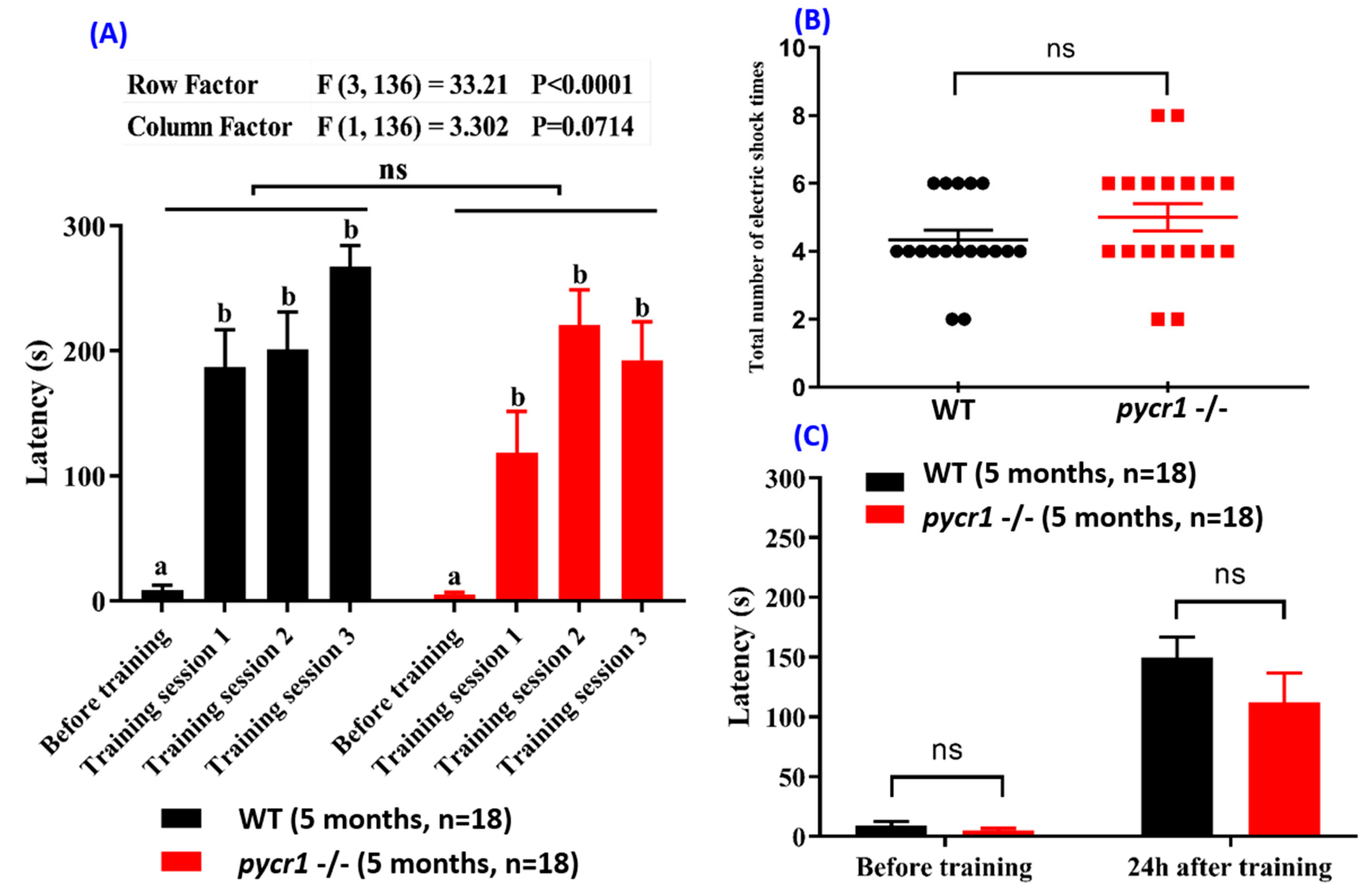
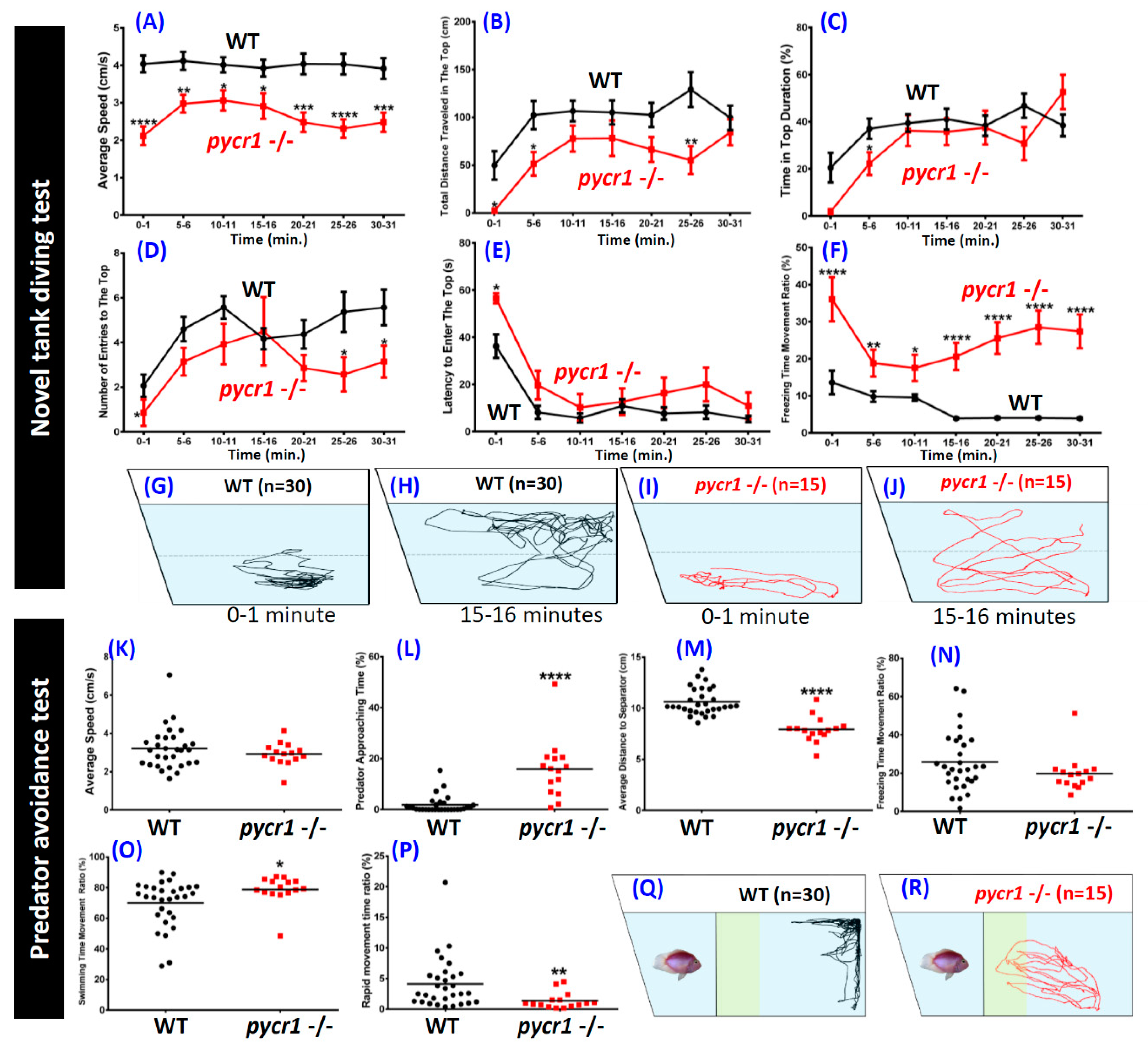
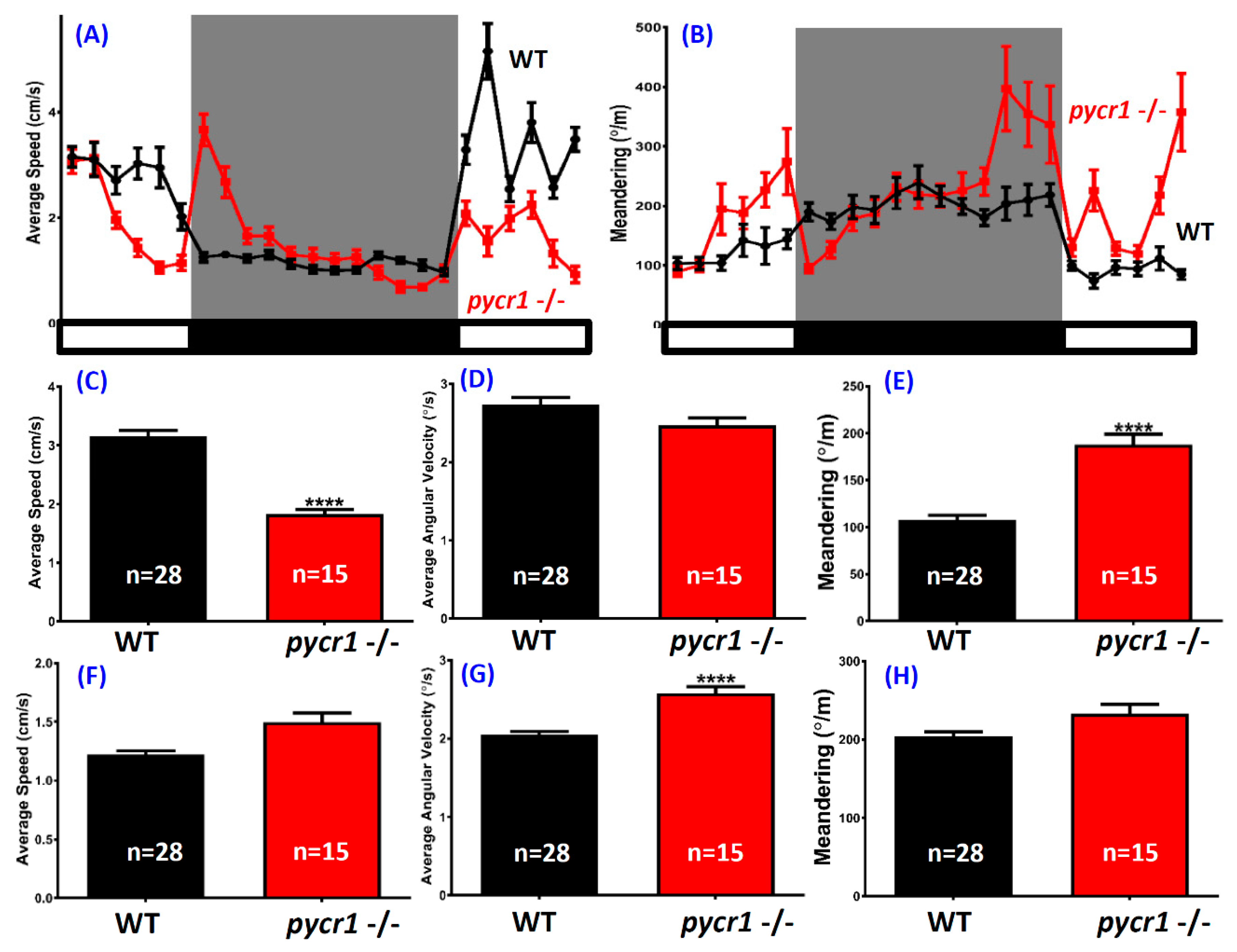
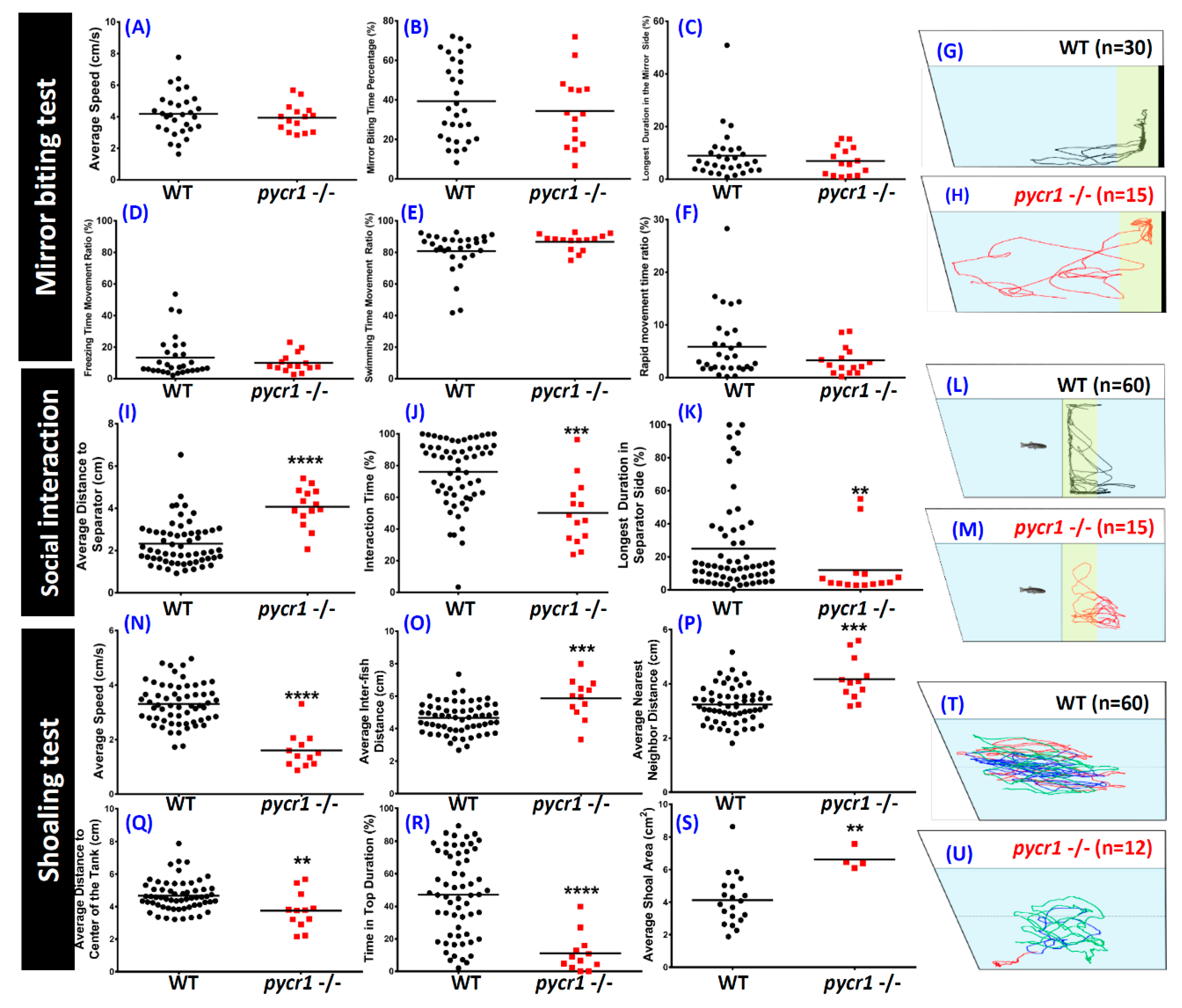
2.6. pycr1 Knockout Fish Showed Multiple Behavioral Alterations
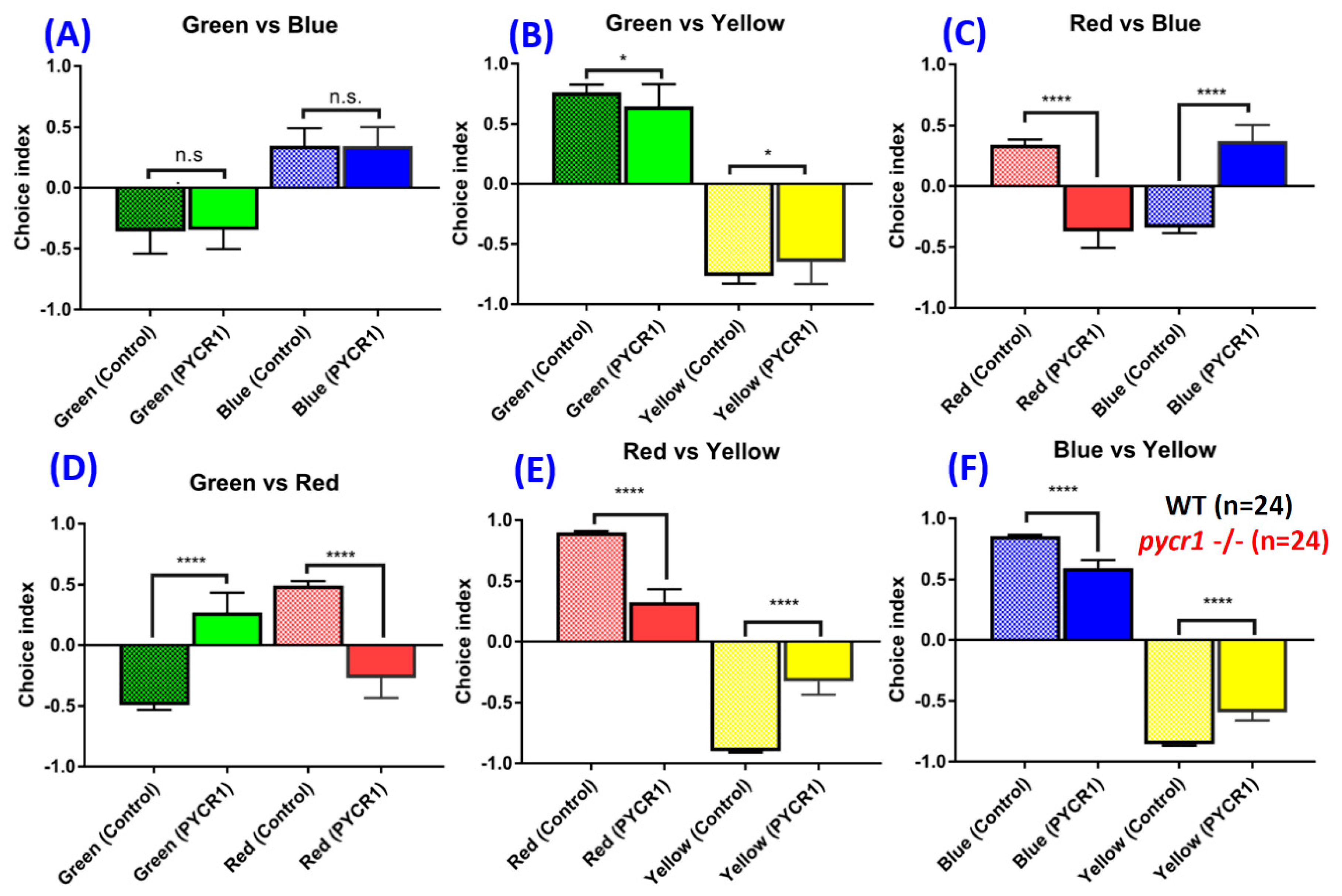
2.7. Pycr1 KO Fish Display Color Preference Abnormality
2.8. Pycr1 KO Fish Display Normal Short-Term Memory
2.9. Detection of Neurotransmitter Expression in pycr1 KO Fish Brain and Other Tissues
3. Discussion
3.1. Comparison of pycr1 Knockdown and Knockout Phenotypes
3.2. Comparison of pycr1 KO Fish with Other Aging Fish Models
3.3. Possible Mechanism for Behavioral Alteration in pycr1 KO Fish
4. Experimental Procedures
4.1. Animal Ethics and Rearing
4.2. Body Length, Body Weight and Survival Rate Measurement
4.3. Histology
4.4. Morphometric Analysis
4.5. Production of TALEN mRNA
4.6. Microinjection of Zebrafish Embryos
4.7. Identification of Indel and Targeted Mutations by HRMA
4.8. Production of pycr1 KO Fish
4.9. TUNEL Assay
4.10. SA-β-gal Assay
4.11. Smurf Dye Staining
4.12. Behavioral Assessment
4.13. Preparation of Brain and Body Extracts
4.14. Total Protein Determination
4.15. Determination of Biomarker Expression by ELISA
4.16. Real Time RT-PCR
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Alia; Mohanty, P.; Matysik, J. Effect of proline on the production of singlet oxygen. Amino Acids 2001, 21, 195–200. [Google Scholar] [PubMed]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Guernsey, D.L.; Jiang, H.; Evans, S.C.; Ferguson, M.; Matsuoka, M.; Nightingale, M.; Rideout, A.L.; Provost, S.; Bedard, K.; Orr, A.; et al. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am. J. Hum. Genet. 2009, 85, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kretz, R.; Bozorgmehr, B.; Kariminejad, M.H.; Rohrbach, M.; Hausser, I.; Baumer, A.; Baumgartner, M.; Giunta, C.; Kariminejad, A.; Haberle, J. Defect in proline synthesis: Pyrroline-5-carboxylate reductase 1 deficiency leads to a complex clinical phenotype with collagen and elastin abnormalities. J. Inherit. Metab. Dis. 2011, 34, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Reversade, B.; Escande-Beillard, N.; Dimopoulou, A.; Fischer, B.; Chng, S.C.; Li, Y.; Shboul, M.; Tham, P.Y.; Kayserili, H.; Al-Gazali, L.; et al. Mutations in pycr1 cause cutis laxa with progeroid features. Nat. Genet. 2009, 41, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Chang, J.H.; Liu, H.L.; Wei, C.H.; Yeung, C.Y.; Ho, C.S.; Shu, C.H.; Chiang, M.F.; Chuang, C.K.; Huang, Y.W.; et al. Compound heterozygous mutations in pycr1 further expand the phenotypic spectrum of de barsy syndrome. Am. J. Hum. Genet. 2011, 155A, 3095–3099. [Google Scholar]
- Lin, D.S.; Yeung, C.Y.; Liu, H.L.; Ho, C.S.; Shu, C.H.; Chuang, C.K.; Huang, Y.W.; Wu, T.Y.; Huang, Z.D.; Jian, Y.R.; et al. A novel mutation in pycr1 causes an autosomal recessive cutis laxa with premature aging features in a family. Am. J. Hum. Genet. 2011, 155A, 1285–1289. [Google Scholar]
- Ni, Z.; Lee, S.S. Rnai screens to identify components of gene networks that modulate aging in caenorhabditis elegans. Brief. Funct. Genom. 2010, 9, 53–64. [Google Scholar] [CrossRef]
- Yang, Y.; Wilson, D.L. Characterization of a life-extending mutation in age-2, a new aging gene in caenorhabditis elegans. J. Gerontol. 1999, 54, B137–B142. [Google Scholar] [CrossRef]
- Brandt, A.; Vilcinskas, A. The fruit fly drosophila melanogaster as a model for aging research. Adv. Biochem. Eng./Biotechnol. 2013, 135, 63–77. [Google Scholar]
- Helfand, S.L.; Rogina, B. Genetics of aging in the fruit fly, drosophila melanogaster. Ann. Rev. Genet. 2003, 37, 329–348. [Google Scholar] [CrossRef]
- Helfand, S.L.; Rogina, B. Molecular genetics of aging in the fly: Is this the end of the beginning? BioEssays 2003, 25, 134–141. [Google Scholar] [CrossRef]
- Terzibasi, E.; Valenzano, D.R.; Cellerino, A. The short-lived fish nothobranchius furzeri as a new model system for aging studies. Exp. Gerontol. 2007, 42, 81–89. [Google Scholar] [CrossRef]
- Herrera, M.; Jagadeeswaran, P. Annual fish as a genetic model for aging. J. Gerontol. 2004, 59, 101–107. [Google Scholar] [CrossRef]
- Peinado, J.R.; Quiros, P.M.; Pulido, M.R.; Marino, G.; Martinez-Chantar, M.L.; Vazquez-Martinez, R.; Freije, J.M.; Lopez-Otin, C.; Malagon, M.M. Proteomic profiling of adipose tissue from zmpste24−/− mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Mol. Cell. Proteom. 2011, 10, M111.008094. [Google Scholar] [CrossRef]
- De Boer, J.; Andressoo, J.O.; de Wit, J.; Huijmans, J.; Beems, R.B.; van Steeg, H.; Weeda, G.; van der Horst, G.T.; van Leeuwen, W.; Themmen, A.P.; et al. Premature aging in mice deficient in DNA repair and transcription. Science 2002, 296, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Kao, C.H.; Chen, Y.T.; Wang, C.H.; Wu, C.Y.; Tsai, C.Y.; Liu, F.C.; Yang, C.W.; Wei, Y.H.; Hsu, M.T.; et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009, 23, 1183–1194. [Google Scholar] [CrossRef]
- Lanske, B.; Razzaque, M.S. Premature aging in klotho mutant mice: Cause or consequence? Ageing Res. Rev. 2007, 6, 73–79. [Google Scholar] [CrossRef]
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 2008, 4, e1000152. [Google Scholar] [CrossRef]
- Kishi, S. Functional aging and gradual senescence in zebrafish. Ann. N. Y. Acad. Sci. 2004, 1019, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Uchiyama, J.; Baughman, A.M.; Goto, T.; Lin, M.C.; Tsai, S.B. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp. Gerontol. 2003, 38, 777–786. [Google Scholar] [CrossRef]
- Martins, R.R.; McCracken, A.W.; Simons, M.J.; Henriques, C.M.; Rera, M. How to catch a smurf?–ageing and beyond. in vivo assessment of intestinal permeability in multiple model organisms. Bio-protocol 2018, 8. [Google Scholar] [CrossRef]
- Gilbert, M.J.; Zerulla, T.C.; Tierney, K.B. Zebrafish (danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp. Gerontol. 2014, 50, 10–113. [Google Scholar] [CrossRef]
- Keller, E.T.; Murtha, J.M. The use of mature zebrafish (danio rerio) as a model for human aging and disease. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 335–341. [Google Scholar] [CrossRef]
- Mitani, H.; Kamei, Y.; Fukamachi, S.; Oda, S.; Sasaki, T.; Asakawa, S.; Todo, T.; Shimizu, N. The medaka genome: Why we need multiple fish models in vertebrate functional genomics. Genom. Dyn. 2006, 2, 165–182. [Google Scholar]
- Takeda, H. Draft genome of the medaka fish: A comprehensive resource for medaka developmental genetics and vertebrate evolutionary biology. Dev. Growth Differ. 2008, 50, S157–S166. [Google Scholar] [CrossRef]
- Taylor, J.S.; Van de Peer, Y.; Braasch, I.; Meyer, A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos. Trans. Royal Soc. Lond. 2001, 356, 1661–1679. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Del Bene, F. Crispr/cas9 and talen-mediated knock-in approaches in zebrafish. Methods 2014. [Google Scholar] [CrossRef]
- Zu, Y.; Tong, X.; Wang, Z.; Liu, D.; Pan, R.; Li, Z.; Hu, Y.; Luo, Z.; Huang, P.; Wu, Q.; et al. Talen-mediated precise genome modification by homologous recombination in zebrafish. Nat. Methods 2013, 10, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Wang, Y.; Campbell, J.M.; Poshusta, T.L.; Starker, C.G.; Krug, R.G., 2nd; Tan, W.; Penheiter, S.G.; Ma, A.C.; Leung, A.Y.; et al. In vivo genome editing using a high-efficiency talen system. Nature 2012, 491, 114–118. [Google Scholar] [CrossRef]
- Huang, P.; Xu, L.; Liang, W.; Tam, C.I.; Zhang, Y.; Qi, F.; Zhu, Z.; Lin, S.; Zhang, B. Genomic deletion induced by tol2 transposon excision in zebrafish. Nucleic Acids Res. 2013, 41, e36. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Tol2: A versatile gene transfer vector in vertebrates. Genome Biol. 2007, 8, S7. [Google Scholar] [CrossRef]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Parant, J.M.; Yost, H.J.; Kanki, J.P.; Chien, C.B. The tol2kit: A multisite gateway-based construction kit for tol2 transposon transgenesis constructs. Dev. Dyn. 2007, 236, 3088–3099. [Google Scholar] [CrossRef]
- Kawakami, K.; Koga, A.; Hori, H.; Shima, A. Excision of the tol2 transposable element of the medaka fish, oryzias latipes, in zebrafish, danio rerio. Gene 1998, 225, 17–22. [Google Scholar] [CrossRef]
- Koshimizu, E.; Imamura, S.; Qi, J.; Toure, J.; Valdez, D.M., Jr.; Carr, C.E.; Hanai, J.; Kishi, S. Embryonic senescence and laminopathies in a progeroid zebrafish model. PLoS ONE 2011, 6, e17688. [Google Scholar] [CrossRef] [PubMed]
- Anchelin, M.; Alcaraz-Perez, F.; Martinez, C.M.; Bernabe-Garcia, M.; Mulero, V.; Cayuela, M.L. Premature aging in telomerase-deficient zebrafish. Dis. Models Mech. 2013, 6, 1101–1112. [Google Scholar] [CrossRef]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the drosophila pgc-1 homolog. Cell. Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Dambroise, E.; Monnier, L.; Ruisheng, L.; Aguilaniu, H.; Joly, J.-S.; Tricoire, H.; Rera, M. Two phases of aging separated by the smurf transition as a public path to death. Sci. Rep. 2016, 6, 23523. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imagej. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Schipper, H.; Brawer, J.R.; Nelson, J.F.; Felicio, L.S.; Finch, C.E. Role of the gonads in the histologic aging of the hypothalamic arcuate nucleus. Biol. Reprod. 1981, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.M.; Akhtar, S.; Currie, Z. Ageing changes in the eye. Postgrad. Med. J. 2006, 82, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Bonnel, S.; Mohand-Said, S.; Sahel, J.A. The aging of the retina. Exp. Gerontol. 2003, 38, 825–831. [Google Scholar] [CrossRef]
- Marshall, J. The ageing retina: Physiology or pathology. Eye 1987, 1, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C.; Medeiros, N.E.; Curcio, C.A. Phenotypic variation of retinal pigment epithelium in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2002, 43, 267–273. [Google Scholar]
- Kuo, M.-L.; Lee, M.B.-E.; Tang, M.; den Besten, W.; Hu, S.; Sweredoski, M.J.; Hess, S.; Chou, C.-M.; Changou, C.A.; Su, M.; et al. Pycr1 and pycr2 interact and collaborate with rrm2b to protect cells from overt oxidative stress. Sci. Rep. 2016, 6, 18846. [Google Scholar] [CrossRef]
- Wittkopp, N.; Huntzinger, E.; Weiler, C.; Saulière, J.; Schmidt, S.; Sonawane, M.; Izaurralde, E. Nonsense-mediated mrna decay effectors are essential for zebrafish embryonic development and survival. Mol. Cell. Biol. 2009, 29, 3517–3528. [Google Scholar] [CrossRef]
- Hwang, J.; Maquat, L.E. Nonsense-mediated mrna decay (nmd) in animal embryogenesis: To die or not to die, that is the question. Curr. Opin. Genet. Dev. 2011, 21, 422–430. [Google Scholar] [CrossRef]
- Gardeitchik, T.; Mohamed, M.; Fischer, B.; Lammens, M.; Lefeber, D.; Lace, B.; Parker, M.; Kim, K.-J.; Lim, B.C.; Häberle, J. Clinical and biochemical features guiding the diagnostics in neurometabolic cutis laxa. Eur. J. Hum. Genet. 2014, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef]
- Blaser, R.; Chadwick, L.; McGinnis, G. Behavioral measures of anxiety in zebrafish (danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tucci, V.; Kishi, S.; Zhdanova, I.V. Cognitive aging in zebrafish. PLoS ONE 2006, 1, e14. [Google Scholar] [CrossRef]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Humana Press: Totowa, NJ, USA, 2012; pp. 231–246. [Google Scholar]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arellano, I.; Carmona-Álvarez, F.; Martínez, A.I.; Rodríguez-Díaz, J.; Cervera, J. Pyrroline-5-carboxylate synthase and proline biosynthesis: From osmotolerance to rare metabolic disease. Protein Sci. 2010, 19, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Chiang, M.-F.; Ho, C.-S.; Hung, P.-L.; Hsu, M.-H.; Lee, T.-H.; Chu, L.J.; Liu, H.; Tang, P.; Ng, W.V.; et al. A transcriptome study of progeroid neurocutaneous syndrome reveals postn as a new element in proline metabolic disorder. Aging 2018, 9, 1043. [Google Scholar]
- Henriques, C.M.; Carneiro, M.C.; Tenente, I.M.; Jacinto, A.; Ferreira, M.G. Telomerase is required for zebrafish lifespan. PLoS Genet. 2013, 9, e1003214. [Google Scholar] [CrossRef] [PubMed]
- Anchelin, M.; Alcaraz-Pérez, F.; Martínez, C.M.; Bernabé-García, M.; Mulero, V.; Cayuela, M.L. mechanisms. Premature aging in telomerase-deficient zebrafish. Dis. Models Mech. 2013, 6, 1101–1112. [Google Scholar] [CrossRef]
- Anchelin, M.; Murcia, L.; Alcaraz-Pérez, F.; García-Navarro, E.M.; Cayuela, M.L. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS ONE 2011, 6, e16955. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; DePinho, R.A. Axis of ageing: Telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012, 13, 397. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, J.; Liu, L.; Chen, J. Proline enhances torulopsis glabrata growth during hyperosmotic stress. Biotechnol. Bioprocess Eng. 2010, 15, 285–292. [Google Scholar] [CrossRef]
- Chen, C.F.; Chu, C.Y.; Chen, T.H.; Lee, S.J.; Shen, C.N.; Hsiao, C.D. Establishment of a transgenic zebrafish line for superficial skin ablation and functional validation of apoptosis modulators in vivo. PLoS ONE 2011, 6, e20654. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc chloride exposure inhibits brain acetylcholine levels, produces neurotoxic signatures, and diminishes memory and motor activities in adult zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.; Lai, Y.-H.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Evaluation of the effects of carbon 60 nanoparticle exposure to adult zebrafish: A behavioral and biochemical approach to elucidate the mechanism of toxicity. Int. J. Mol. Sci. 2018, 19, 3853. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ (–delta delta ct) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath 2013, 3, 71. [Google Scholar]

| Biomarker | WT | pycr1 KO | Unit | Significance | p Value |
|---|---|---|---|---|---|
| Brain | |||||
| Dopamine | 0.282 ± 0.027 | 0.388 ± 0.086 | pg/μg total protein | NO | 0.3034 |
| GABA | 171.000 ± 13.930 | 235.700 ± 48.200 | pg/μg total protein | NO | 0.2671 |
| 5-HT | 3.862 ± 0.432 | 5.553 ± 1.264 | pg/μg total protein | NO | 0.2742 |
| Melatonin | 13.170 ± 0.232 | 20.220 ± 4.245 | pg/mg total protein | NO | 0.1727 |
| Norepinephrine | 1.564 ± 0.071 | 2.281 ± 0.492 | pg/μg total protein | NO | 0.2224 |
| Epinephrine | 3.274 ± 0.166 | 5.178 ± 1.145 | pg/μg total protein | NO | 0.1751 |
| Cortisol | 1.253 ± 0.144 | 1.944 ± 0.613 | pg/μg total protein | NO | 0.3339 |
| Acetylcholine | 473.700 ± 23.370 | 678.800 ± 133.400 | pg/μg total protein | NO | 0.2044 |
| Acetylcholinesterase | 21.470 ± 0.947 | 30.530 ± 4.843 | pg/μg total protein | NO | 0.1402 |
| Glutamate | 31.870 ± 2.765 | 47.340 ± 7.596 | pg/μg total protein | NO | 0.1283 |
| Glycine | 27.820 ± 1.232 | 37.770 ± 8.194 | μg/μg total protein | NO | 0.2962 |
| Histamine | 8.295 ± 0.360 | 11.970 ± 2.025 | pg/μg total protein | NO | 0.1484 |
| Body | |||||
| Dopamine | 128.800 ± 19.520 | 65.790 ± 9.745 | pg/μg total protein | YES | 0.0136 |
| GABA | 77.630 ± 12.480 | 37.780 ± 5.180 | pg/μg total protein | YES | 0.0122 |
| 5-HT | 1.854 ± 0.306 | 0.977 ± 0.114 | pg/μg total protein | YES | 0.0200 |
| Melatonin | 23.200 ± 4.166 | 12.870 ± 1.486 | pg/mg total protein | YES | 0.0376 |
| Norepinephrine | 0.653 ± 0.120 | 0.316 ± 0.030 | pg/μg total protein | YES | 0.0184 |
| Epinephrine | 2.122 ± 0.319 | 0.807 ± 0.115 | pg/μg total protein | YES | 0.0022 |
| Cortisol | 0.672 ± 0.096 | 0.299 ± 0.0419 | pg/μg total protein | YES | 0.0038 |
| Acetylcholine | 84.260 ± 11.860 | 48.320 ± 6.940 | pg/μg total protein | YES | 0.0225 |
| Acetylcholinesterase | 8.596 ± 1.636 | 4.097 ± 0.498 | pg/μg total protein | YES | 0.0220 |
| Glutamate | 4.121 ± 0.764 | 1.898 ± 0.267 | pg/μg total protein | YES | 0.0177 |
| Glycine | 32.620 ± 4.556 | 13.820 ± 1.194 | μg/μg total protein | YES | 0.0018 |
| Histamine | 1.466 ± 0.263 | 0.706 ± 0.089 | pg/μg total protein | YES | 0.0181 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.-T.; Audira, G.; Juniardi, S.; Chen, J.-R.; Lai, Y.-H.; Du, Z.-C.; Lin, D.-S.; Hsiao, C.-D. Zebrafish Carrying pycr1 Gene Deficiency Display Aging and Multiple Behavioral Abnormalities. Cells 2019, 8, 453. https://doi.org/10.3390/cells8050453
Liang S-T, Audira G, Juniardi S, Chen J-R, Lai Y-H, Du Z-C, Lin D-S, Hsiao C-D. Zebrafish Carrying pycr1 Gene Deficiency Display Aging and Multiple Behavioral Abnormalities. Cells. 2019; 8(5):453. https://doi.org/10.3390/cells8050453
Chicago/Turabian StyleLiang, Sung-Tzu, Gilbert Audira, Stevhen Juniardi, Jung-Ren Chen, Yu-Heng Lai, Zheng-Cai Du, Dar-Shong Lin, and Chung-Der Hsiao. 2019. "Zebrafish Carrying pycr1 Gene Deficiency Display Aging and Multiple Behavioral Abnormalities" Cells 8, no. 5: 453. https://doi.org/10.3390/cells8050453
APA StyleLiang, S.-T., Audira, G., Juniardi, S., Chen, J.-R., Lai, Y.-H., Du, Z.-C., Lin, D.-S., & Hsiao, C.-D. (2019). Zebrafish Carrying pycr1 Gene Deficiency Display Aging and Multiple Behavioral Abnormalities. Cells, 8(5), 453. https://doi.org/10.3390/cells8050453







