Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer
Abstract
:1. Introduction
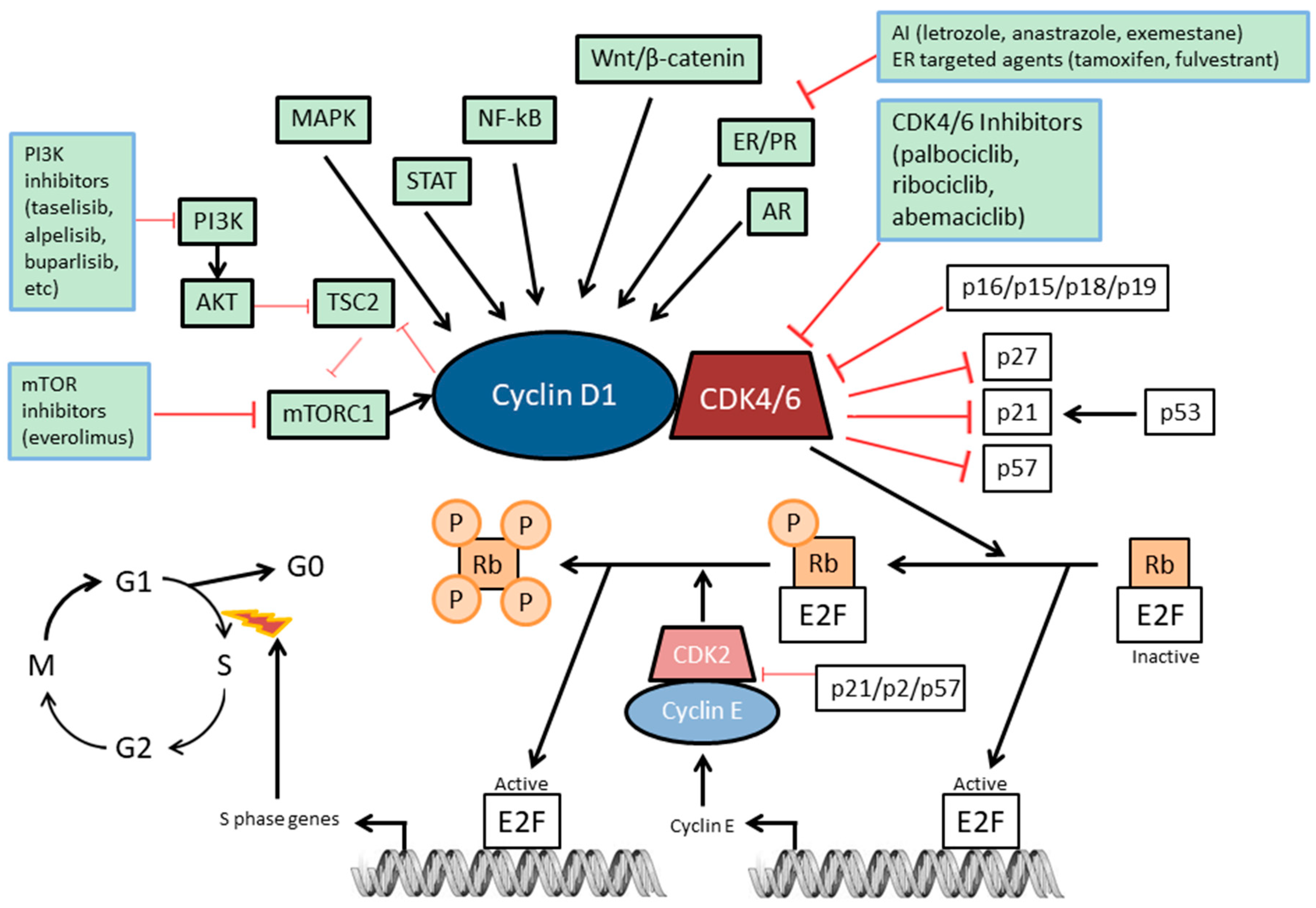
2. The Rb Pathway
3. Mechanism of Action of CDK4/6 Inhibitors
4. FDA-Approved CDK4/6 Inhibitors in Advanced or Metastatic ER-Positive Breast Cancer
5. CDK4/6 Inhibitors in HER2-Positive Breast Cancer
6. CDK4/6 Inhibitors in Triple-Negative Breast Cancer
7. CDK4/6 Inhibitor Combinations with Other Targeted and Immune Therapies
7.1. CDK4/6 and PI3K-mTOR Inhibitions
7.2. CDK4/6 and Immunotherapy Combinations
8. Biomarkers
8.1. Clinical Parameters
8.2. Molecular Biomarkers
8.2.1. Retinoblastoma Protein Expression
8.2.2. Cyclin D1 or p16Ink4a Alterations
8.2.3. Cyclin E1 Alterations
9. Conclusions
Funding
Conflicts of Interest
References
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. The Rb/E2F pathway: Expanding roles and emerging paradigms. Genes Dev. 2000, 14, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Classon, M.; Harlow, E. The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer 2002, 2, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Stevaux, O.; Dyson, N.J. A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol. 2002, 14, 684–691. [Google Scholar] [CrossRef]
- Lundberg, A.S.; Weinberg, R.A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 1998, 18, 753–761. [Google Scholar] [CrossRef]
- Harbour, J.W.; Luo, R.X.; Dei Santi, A.; Postigo, A.A.; Dean, D.C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 1999, 98, 859–869. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Cip/Kip proteins: More than just CDKs inhibitors. Genes Dev. 2004, 18, 851–855. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK Inhibitors: Cell Cycle Regulators and Beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, A.M.; Kaulich, M.; Shapiro, G.S.; Choi, Y.J.; Sicinski, P.; Dowdy, S.F. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Hannon, G.J.; Beach, D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.J.; Beach, D. pl5INK4B is a potentia| effector of TGF-β-induced cell cycle arrest. Nature 1994, 371, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.L.; Jenkins, C.W.; Li, Y.; Nichols, M.A.; Wu, X.; O’Keefe, C.L.; Matera, A.G.; Xiong, Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994, 8, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Roussel, M.F.; Kato, J.Y.; Ashmun, R.A.; Sherr, C.J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell. Biol. 1995, 15, 2672–2681. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Zhang, J.; Cheng, L.; Shapiro, D.N.; Winoto, A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol. Cell. Biol. 1995, 15, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.J.; Li, X.; Hydbring, P.; Sanda, T.; Stefano, J.; Christie, A.L.L.; Signoretti, S.; Look, A.T.T.; Kung, A.L.L.; von Boehmer, H.; et al. The Requirement for Cyclin D Function in Tumor Maintenance. Cancer Cell 2012, 22, 438–451. [Google Scholar] [CrossRef]
- el-Deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75, 817–825. [Google Scholar] [CrossRef]
- Gu, Y.; Turck, C.W.; Morgan, D.O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 1993, 366, 707–710. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Dulić, V.; Kaufmann, W.K.; Wilson, S.J.; Tlsty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Polyak, K.; Lee, M.H.; Erdjument-Bromage, H.; Koff, A.; Roberts, J.M.; Tempst, P.; Massagué, J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78, 59–66. [Google Scholar] [CrossRef]
- Polyak, K.; Kato, J.Y.; Solomon, M.J.; Sherr, C.J.; Massague, J.; Roberts, J.M.; Koff, A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994, 8, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Edwards, M.C.; Bai, C.; Parker, S.; Zhang, P.; Baldini, A.; Harper, J.W.; Elledge, S.J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995, 9, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Reynisdóttir, I.; Massagué, J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jackson, P.K.; Kirschner, M.W.; Dutta, A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 1995, 374, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Robetorye, R.S.; Adami, G.R.; Pereira-Smith, O.M.; Smith, J.R. Identification of the active region of the DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. EMBO J. 1995, 14, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Warbrick, E.; Lane, D.P.; Glover, D.M.; Cox, L.S. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr. Biol. 1995, 5, 275–282. [Google Scholar] [CrossRef]
- Lin, J.; Reichner, C.; Wu, X.; Levine, A.J. Analysis of wild-type and mutant p21WAF-1 gene activities. Mol. Cell. Biol. 1996, 16, 1786–1793. [Google Scholar] [CrossRef]
- Russo, A.A.; Jeffrey, P.D.; Patten, A.K.; Massagué, J.; Pavletich, N.P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inibitor bound to the cyclin A—Cdk2 complex. Nature 1996, 382, 325–331. [Google Scholar] [CrossRef]
- Yu, Q.; Sicinska, E.; Geng, Y.; Ahnström, M.; Zagozdzon, A.; Kong, Y.; Gardner, H.; Kiyokawa, H.; Harris, L.N.; Stål, O.; et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.G.; Anderson, J.J.; Milton, I.; Steward, M.; Parr, A.H.; Thomas, M.D.; Henry, J.A.; Angus, B.; Lennard, T.W.; Horne, C.H. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene 1995, 11, 885–891. [Google Scholar] [PubMed]
- Kenny, F.S.; Hui, R.; Musgrove, E.A.; Gee, J.M.; Blamey, R.W.; Nicholson, R.I.; Sutherland, R.L.; Robertson, J.F. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin. Cancer Res. 1999, 5, 2069–2076. [Google Scholar] [PubMed]
- Weinstat-Saslow, D.; Merino, M.J.; Manrow, R.E.; Lawrence, J.A.; Bluth, R.F.; Wittenbel, K.D.; Simpson, J.F.; Page, D.L.; Steeg, P.S. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat. Med. 1995, 1, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Gillett, C.; Fantl, V.; Smith, R.; Fisher, C.; Bartek, J.; Dickson, C.; Barnes, D.; Peters, G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994, 54, 1812–1817. [Google Scholar] [PubMed]
- Dean, J.L.; Thangavel, C.; McClendon, A.K.; Reed, C.A.; Knudsen, E.S. Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene 2010, 29, 4018–4032. [Google Scholar] [CrossRef]
- Malorni, L.; Curigliano, G.; Minisini, A.M.; Cinieri, S.; Tondini, C.; Arpino, G.; Pavesi, L.; Martignetti, A.; Criscitiello, C.; Puglisi, F.; et al. A phase II trial of the CDK4/6 inhibitor palbociclib (P) as single agent or in combination with the same endocrine therapy (ET) received prior to disease progression, in patients (pts) with hormone receptor positive (HR+) HER2 negative (HER2−) metastatic. J. Clin. Oncol. 2017, 35, 1002. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet. Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- DeMichele, A.; Clark, A.S.; Tan, K.S.; Heitjan, D.F.; Gramlich, K.; Gallagher, M.; Lal, P.; Feldman, M.; Zhang, P.; Colameco, C.; et al. CDK 4/6 Inhibitor Palbociclib (PD0332991) in Rb+ Advanced Breast Cancer: Phase II Activity, Safety, and Predictive Biomarker Assessment. Clin. Cancer Res. 2015, 21, 995–1001. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phas. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Infante, J.R.; Cassier, P.A.; Gerecitano, J.F.; Witteveen, P.O.; Chugh, R.; Ribrag, V.; Chakraborty, A.; Matano, A.; Dobson, J.R.; Crystal, A.S.; et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2016, 22, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Ellis, M.J.; Alley, H.M.; Eisele, K.; VanArsdale, T.; Dann, S.G.; Arndt, K.T.; Primeau, T.; Griffin, E.; Shao, J.; et al. Efficacy of SERD/SERM Hybrid-CDK4/6 Inhibitor Combinations in Models of Endocrine Therapy-Resistant Breast Cancer. Clin. Cancer Res. 2015, 21, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; LoRusso, P.M.; Dickson, M.A.; Randolph, S.S.; Shaik, M.N.; Wilner, K.D.; Courtney, R.; O’Dwyer, P.J. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br. J. Cancer 2011, 104, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Finn, R.S.; Martin, M.; Im, S.-A.; DeMichele, A.; Ettl, J.; Diéras, V.; Moulder, S.; Lipatov, O.; Colleoni, M.; et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann. Oncol. 2018, 29, 669–680. [Google Scholar] [CrossRef]
- Kim, S.; Loo, A.; Chopra, R.; Caponigro, G.; Huang, A.; Vora, S.; Parasuraman, S.; Howard, S.; Keen, N.; Sellers, W.; et al. Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6- Reactivating Rb in cancer. Mol. Cancer Ther. 2013, 12, PR02. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Raub, T.J.; Wishart, G.N.; Kulanthaivel, P.; Staton, B.A.; Ajamie, R.T.; Sawada, G.A.; Gelbert, L.M.; Shannon, H.E.; Sanchez-Martinez, C.; De Dios, A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. 2015, 43, 1360–1371. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Lin, N.U.; Thornton, D.; Klise, S.; Costigan, T.M.; Turner, P.K.; Anders, C.K. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer. J. Clin. Oncol. 2017, 35, 1019. [Google Scholar] [CrossRef]
- Tolaney, S.; Lam, A.; Mukundan, S.; Nanda, S.; Cox, J.; Barriga, S. Abstract P6-15-01: Analysis of renal function in MONARCH 1: A phase 2 study of abemaciclib, a CDK4 & 6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for metastatic breast cancer (MBC). Cancer Res. 2017, 77, P6-15-01. [Google Scholar] [CrossRef]
- Malorni, L.; Shetty, P.B.; De Angelis, C.; Hilsenbeck, S.; Rimawi, M.F.; Elledge, R.; Osborne, C.K.; De Placido, S.; Arpino, G. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 2012, 136, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Cox, D.; Knudsen, E.S. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 2014, 5, 261. [Google Scholar] [CrossRef]
- Landis, M.W.; Pawlyk, B.S.; Li, T.; Sicinski, P.; Hinds, P.W. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 2006, 9, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Geng, Y.; Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 2001, 411, 1017–1021. [Google Scholar] [CrossRef]
- O’Brien, N.; Conklin, D.; Beckmann, R.; Luo, T.; Chau, K.; Thomas, J.; Mc Nulty, A.; Marchal, C.; Kalous, O.; von Euw, E.; et al. Preclinical Activity of Abemaciclib Alone or in Combination with Antimitotic and Targeted Therapies in Breast Cancer. Mol. Cancer Ther. 2018, 17, 897–907. [Google Scholar] [CrossRef]
- Goel, S.; Wang, Q.; Watt, A.C.; Tolaney, S.M.; Dillon, D.A.; Li, W.; Ramm, S.; Palmer, A.C.; Yuzugullu, H.; Varadan, V.; et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016, 29, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Tamura, K.; Kondo, S.; Tanabe, Y.; Iwasa, S.; Shimomura, A.; Kitano, S.; Ogasawara, K.; Turner, P.K.; Mori, J.; et al. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother. Pharmacol. 2016, 78, 281–288. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef]
- Gianni, L.; Bisagni, G.; Colleoni, M.; Del Mastro, L.; Zamagni, C.; Mansutti, M.; Zambetti, M.; Frassoldati, A.; De Fato, R.; Valagussa, P.; et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): An exploratory, open-label, phase 2 study. Lancet Oncol. 2018, 19, 249–256. [Google Scholar] [CrossRef]
- Raspé, E.; Coulonval, K.; Pita, J.M.; Paternot, S.; Rothé, F.; Twyffels, L.; Brohée, S.; Craciun, L.; Larsimont, D.; Kruys, V.; et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol. Med. 2017, 9, 1052–1066. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.U.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular Characterization of Basal-Like and Non-Basal-Like Triple-Negative Breast Cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.S.; Barr, A.R.; Cutts, R.; Beaney, M.; Babina, I.; Sampath, D.; Giltnane, J.; Lacap, J.A.; Crocker, L.; Young, A.; et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin. Cancer Res. 2017, 23, 5561–5572. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, J.; Deng, M.; Yin, Y.; Zhang, H.; Luo, K.; Qin, B.; Li, Y.; Wu, C.; Ren, T.; et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 2017, 8, 13923. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Versaci, S.; Dushyanthen, S.; Caramia, F.; Savas, P.; Mintoff, C.P.; Zethoven, M.; Virassamy, B.; Luen, S.J.; McArthur, G.A.; et al. Combined CDK4/6 and PI3Kα Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 6340–6352. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kanaya, N.; Somlo, G.; Chen, S. Synergistic anti-cancer activity of CDK4/6 inhibitor palbociclib and dual mTOR kinase inhibitor MLN0128 in pRb-expressing ER-negative breast cancer. Breast Cancer Res. Treat. 2019. [Google Scholar] [CrossRef] [PubMed]
- Foidart, P.; Yip, C.; Radermacher, J.; Blacher, S.; Lienard, M.; Montero-Ruiz, L.; Maquoi, E.; Montaudon, E.; Château-Joubert, S.; Collignon, J.; et al. Expression of MT4-MMP, EGFR, and RB in Triple-Negative Breast Cancer Strongly Sensitizes Tumors to Erlotinib and Palbociclib Combination Therapy. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Lau, K.-Y.; Hsu, C.-C.; Chen, J.-L.; Lee, C.-H.; Huang, T.-T.; Chen, Y.-T.; Huang, C.-T.; Lin, P.-H.; Tseng, L.-M. Combination of palbociclib with enzalutamide shows in vitro activity in RB proficient and androgen receptor positive triple negative breast cancer cells. PLoS ONE 2017, 12, e0189007. [Google Scholar] [CrossRef]
- Martin, M.; Hurvitz, S.; Chan, D.; Fernandez-Abad, M.; Petru, E.; Rostorfer, R.; Guarneri, V.; Huang, C.-S.; Press, M.; Costigan, T.; et al. Abstract PD5-01: Final results of NeoMONARCH: A phase 2 neoadjuvant study of abemaciclib in postmenopausal women with hormone receptor positive (HR+), HER2 negative breast cancer (BC). Cancer Res. 2018, 78, PD5-01. [Google Scholar] [CrossRef]
- Vora, S.R.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.L.L.; Pollack, S.F.F.; Liu, M.; Li, X.; et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef]
- Herrera-Abreu, M.T.; Palafox, M.; Asghar, U.; Rivas, M.A.; Cutts, R.J.; Garcia-Murillas, I.; Pearson, A.; Guzman, M.; Rodriguez, O.; Grueso, J.; et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016, 76, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Kabos, P.; Dickler, M.; John, W.; Smith, I.; Lu, Y.; Young, S.; Tolaney, S. Abstract P1-09-01: A phase 1b study of abemaciclib plus pembrolizumab for patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (MBC). Cancer Res. 2018, 78, P1-09-01. [Google Scholar] [CrossRef]
- Goetz, M.; O’Shaughnessy, J.; Sledge, G.; Martin, M.; Lin, Y.; Forrester, T.; Mockbee, C.; Smith, I.; Di Leo, A.; Johnston, S. Abstract GS6-02: The benefit of abemaciclib in prognostic subgroups: An exploratory analysis of combined data from the MONARCH 2 and 3 studies. Cancer Res. 2018, 78, GS6-02. [Google Scholar] [CrossRef]
- Dean, J.L.; McClendon, A.K.; Hickey, T.E.; Butler, L.M.; Tilley, W.D.; Witkiewicz, A.K.; Knudsen, E.S. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle 2012, 11, 2756–2761. [Google Scholar] [CrossRef]
- Condorelli, R.; Spring, L.; O’Shaughnessy, J.; Lacroix, L.; Bailleux, C.; Scott, V.; Dubois, J.; Nagy, R.J.; Lanman, R.B.; Iafrate, A.J.; et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 2018, 29, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Liu, Y.; Zhu, Z.; Loi, S.; Colleoni, M.; Loibl, S.; DeMichele, A.; Harbeck, N.; André, F.; Zhang, Z.; et al. Abstract CT039: Cyclin E1 (CCNE1) expression associates with benefit from palbociclib in metastatic breast cancer (MBC) in the PALOMA3 trial. Cancer Res. 2018, 78, CT039. [Google Scholar] [CrossRef]
- Finn, R.; Liu, Y.; Martin, M.; Rugo, H.; Dieras, V.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Zhu, Z.; Lu, D.; et al. Abstract P2-09-10: Comprehensive gene expression biomarker analysis of CDK 4/6 and endocrine pathways from the PALOMA-2 study. Cancer Res. 2018, 78, P2-09-10. [Google Scholar] [CrossRef]
- Finn, R.; Jiang, Y.; Rugo, H.; Moulder, S.L.; Im, S.-A.; Gelmon, K.A.; Dieras, V.; Martin, M.; Joy, A.A.; Toi, M.; et al. Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER + /HER2– advanced breast cancer (ABC). Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Clark, A.S.; McAndrew, N.P.; Troxel, A.; Feldman, M.; Lal, P.; Rosen, M.; Burrell, J.; Redlinger, C.; Gallagher, M.; Bradbury, A.R.; et al. Combination Paclitaxel and Palbociclib: Results of a Phase I Trial in Advanced Breast Cancer. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Litchfield, L.M.; Webster, Y.; Chio, L.-C.; Wong, S.S.; Stewart, T.R.; Dowless, M.; Dempsey, J.; Zeng, Y.; Torres, R.; et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell 2017, 32, 761–776.e6. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.E.; Im, S.-A.; Gelmon, K.A.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.L.; et al. PALOMA-2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC). J. Clin. Oncol. 2016, 34, 507. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Pedersini, R.; Cabiddu, M.; Borgonovo, K.; Parati, M.C.; Ghilardi, M.; Amoroso, V.; Berruti, A.; Barni, S. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: An adjusted indirect analysis of randomized controlled trials. Breast Cancer Res. Treat. 2019, 174, 597–604. [Google Scholar] [CrossRef] [PubMed]
| Palbociclib | Ribociclib | Abemaciclib | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Monotherapy [46] | +letrozole PALOMA-2 [47] | +fulvestrant PALOMA-3 [48] | Monotherapy [49] | +letrozole MONALEESA-2 [43] | +fulvestrant MONALEESA-3 [50] | Monotherapy MONARCH-1 [44] | +fulvestrant MONARCH-2 [45] | +letrozole/anastrozole MONARCH-3 [51] | |
| Adverse Event (any grade) | Neutropenia (92%) | Neutropenia (80%) | Neutropenia (80%) | Neutropenia (46%) | Neutropenia (75%) | Neutropenia (70%) | Neutropenia (85%) | Neutropenia (46%) | Neutropenia (41%) |
| Leukopenia (100%) | Leukopenia (40%) | Leukopenia (50%) | Leukopenia (43%) | Leukopenia (33%) | Nausea (45%) | Leukopenia (90%) | Diarrhea (86%) | Diarrhea (81%) | |
| Thrombocytopenia (76%) | Nausea (35%) | Infections (42%) | Thrombocytopenia (30%) | Nausea (50%) | Fatigue (32%) | Thrombocytopenia (76%) | Nausea (45%) | Nausea (39%) | |
| Anemia (70%) | Fatigue (35%) | Fatigue (40%) | Fatigue (45%) | Infections (50%) | Diarrhea (29%) | Anemia (69%) | Fatigue (40%) | Fatigue (40%) | |
| Lymphopenia (65%) | Arthralgia (33%) | Nausea (32%) | Nausea (42%) | Fatigue (37%) | Leukopenia (28%) | Diarrhea (90%) | Abdominal pain (35%) | Infections (39%) | |
| Alopecia (33%) | Diarrhea (35%) | Vomiting (27%) | Fatigue (65%) | ||||||
| Alopecia (33%) | Constipation (25%) | Nausea (65%) | |||||||
| Arthralgia (24%) | Decreased appetite (45%) | ||||||||
| Cough (22%) | Abdominal pain (40%) | ||||||||
| Headache (22%) | Vomiting (35%) | ||||||||
| Alopecia (19%) | |||||||||
| Rash (18%) | |||||||||
| Anemia (17%) | |||||||||
| Adverse Event (grade 3–4) | Neutropenia (54%) | Neutropenia (66%) | Neutropenia (65%) | Neutropenia (27%) | Neutropenia (59%) | Neutropenia (53%) | Neutropenia (27%) | Neutropenia (24%) | Neutropenia (21%) |
| Leukopenia (51%) | Leukopenia (25%) | Leukopenia (28%) | Leukopenia (17%) | Leukopenia (21%) | Leukopenia (14%) | Leukopenia (28%) | |||
| Lymphopenia (30%) | Fatigue (2%) | Anemia (3%) | Diarrhea (20%) | ||||||
| Nausea (2%) | Fatigue (2%) | ||||||||
| Back Pain (2%) | |||||||||
| Nausea (1%) | |||||||||
| Constipation (1%) | |||||||||
| Headache (1%) | |||||||||
| Study | Phase | Arms | Study Population | Primary Endpoint | Available Results |
|---|---|---|---|---|---|
| PENELOPE-B (NCT01864746) is an open-label, randomized clinical investigation, with 1250 participants | 3 | Adjuvant ET ± palbociclib in a 28-day cycle for 13 cycles | ER+/HER2− patients with residual disease and high risk of relapse after more than 16 weeks of neoadjuvant CT | iDFS | No results posted |
| PASTOR (NCT02599714) is an international, multicenter, randomized clinical trial with 54 participants | 1b/2 | Vistusertib + Palbociclib + Fulvestrant Placebo + Palbociclib + Fulvestrant | ER+ locally advanced or MBC postmenopausal patients pretreated with hormonal therapy | PFS | No results posted |
| PALOMA-2 (NCT01740427) is a randomized, multicenter clinical trial with 666 participants | 3 | Palbociclib + Letrozole Placebo + Letrozole | Postmenopausal women with ER+/HER2− advanced BC not previously treated | PFS | 24.8 months (22.1 to NA*) in Palbociclib + Letrozole arm; 14.5 months (12.9 to 17.1) in Placebo + Letrozole arm. * The value was not available because there were not enough disease progression events in the treatment group at the time of analysis, due to drug benefit. |
| NEOPAL (NCT02400567) is an open-label, multicenter, international, randomized clinical trial with 125 participants | 2 | 3_FEC (Fluorouracil-Epirubicin-Cyclophosphamide) Letrozole + Palbociclib | Luminal BC in postmenopausal women | RCB | No results posted |
| PALLET (NCT02296801) is an open-label, randomized clinical trial with 306 participants | 2 | Letrozole Palbociclib + Letrozole Letrozole then Letrozole + Palbociclib Palbociclib then Letrozole + Palbociclib | Early invasive BC with ER+/HER2− not previously treated | Ki-67 proliferation cCR | No results posted |
| PALOMA-3 (NCT01942135) is a double-blind, randomized, placebo-controlled clinical trial with 521 participants | 3 | Palbociclib + Fulvestrant Placebo + Fulvestrant | ER+/HER2− MBC with progression after prior endocrine therapy | PFS | 9.2 months (7.5 to NA*) in Palbociclib + Fulvestrant arm; 3.8 months (3.5 to 5.5) in Placebo + Fulvestrant arm. * This parameter is not estimable when the Kaplan Meier-based curve representing the upper confidence limits for survival function lies above 50%. |
| PALOMA-4 (NCT02297438) is a multicenter, randomized, double-blind clinical trial with 339 participants | 3 | Palbociclib + Letrozole Placebo + Letrozole | Untreated Asian postmenopausal women with ER+/HER2− advanced BC | PFS | No results posted |
| PEARL (NCT02028507) is an international, open-label, randomized clinical trial with 596 participants | 3 | Palbociclib + Exemestane or Fulvestrant Capecitabine | ER+/HER2− MBC with resistance to aromatase inhibitors | PFS | No results posted |
| NCT02630693 is an open-label, randomized clinical trial with 180 participants | 2 | Palbociclib 100 mg daily + Fulvestrant or Tamoxifen or another aromatase inhibitor Palbociclib 125 mg daily + Fulvestrant or Tamoxifen or another aromatase inhibitor | ER+/HER2− advanced or MBC | PFS | Results submitted but not publicly available |
| PARSIFAL (NCT02491983) is an open-label, multicenter and randomized clinical trial with 486 participants | 2 | Palbociclib + Fulvestrant Palbociclib + Letrozole | ER+/HER2− MBC | PFS | No results posted |
| MONALEESA-2 (NCT01958021) is randomized, double-blind and placebo-controlled clinical trial with 670 participants | 3 | Ribociclib + Letrozole Placebo + Letrozole | Postmenopausal women with ER+/HER2− advanced BC with no prior therapy | PFS | NA* (19.3 months to NA) in LEE011 + Letrozole arm; 14.7 months (13.0 to 16.5) in Placebo + Letrozole arm. * N/A = not estimable as median PFS was not reached in the ribociclib arm. |
| MONALEESA-3 (NCT02422615) is a double-blind, placebo-controlled and randomized clinical trial with 780 participants | 3 | Ribociclib + Fulvestrant Placebo + Fulvestrant | Postmenopausal women and men with ER+/HER2− advanced BC with only 1 line or no prior endocrine therapy | PFS | 20.5 months (18.5 to 23.5) in Ribociclib + Fulvestrant arm. 12.8 months (10.9 to 16.3) in Placebo + Fulvestrant arm. |
| MONALEESA-7 (NCT02278120) is a multicenter, double-blind, placebo-controlled and randomized clinical trial with 672 participants | 3 | Ribociclib + NSAI or Tamoxifen + Goserelin Placebo + NSAI or Tamoxifen + Goserelin | Premenopausal women with ER+/HER2− advanced BC | PFS | 23.8 * months (19.2 to NA) in LEE011 + NSAI/Tamoxifen + Goserelin arm; 13.0 months (11.0 to 16.4) in Placebo + NSAI/Tamoxifen + Goserelin arm. * N/A = The value was not available due to insufficient number of participants with events in the treatment arm to enable estimation of the upper limit of the confidence interval. |
| MONARCH2 (NCT02107703) is a randomized, double-blind, placebo-controlled clinical trial with 669 participants | 3 | Abemaciclib + Fulvestrant Placebo + Fulvestrant | ER+/HER2− locally advanced or MBC | PFS | 16.4 months (14.4 to 19.3) in Abemaciclib + Fulvestrant arm; 9.3 months (7.4 to 11.4) in Placebo + Fulvestrant arm |
| MONARCH3 (NCT02246621) is a randomized, double-blind, placebo-controlled clinical trial with 493 participants | 3 | Abemaciclib + NSAI Placebo + NSAI | ER+/HER2− locoregionally recurrent or MBC not previously treated in postmenopausal women | PFS | NA * (NA to NA) in Abemaciclib + NSAI arm. * (Median of PFS not reached due to a number of events not yet attained); 14.73 months (11.11 to 17.46) in Placebo + NSAI arm. |
| MONARCHplus (NCT02763566) is a randomized, double-blind, placebo-controlled clinical trial with 450 participants | 3 | Abemaciclib + NSAI Placebo + NSAI Abemaciclib + Fulvestrant Placebo + Fulvestrant | ER+/HER2− locoregionally recurrent or MBC postmenopausal women | PFS | No results posted |
| nextMONARCH 1 (NCT02747004) is a randomized, open-label clinical trial with 225 participants | 2 | Abemaciclib + Tamoxifen Abemaciclib Abemaciclib + Prophylactic Loperamide | ER+/HER2− MBC previously treated | PFS | No results posted |
| monarcHER (NCT02675231) is an open-label, randomized clinical trial, with 225 participants | 2 | Abemaciclib + trastuzumab + fulvestrant Abemaciclib + trastuzumab Trastuzumab + CT | ER+/HER2− locally advanced or metastatic breast cancer patients | PFS | No results posted |
| Study | Phase | Arms | Study Population | Primary Endpoint |
|---|---|---|---|---|
| PALLAS (NCT02513394) is an open-label, randomized clinical trial, with an estimated number of 4600 patients. | 3 | Adjuvant ET (≥5 years) ± palbociclib (for 2 years) | ER+/HER2− Patients at stage II or III | iDFS |
| PATRICIA (NCT02448420) is an open-label, randomized clinical trial, with an estimated number of 138 patients | 2 | Palbociclib + trastuzumab ± letrozole Palbociclib + trastuzumab | HER2+/ER± locally advanced or metastatic BC post-menopausal women treated previously with CT and trastuzumab | PFS |
| PATINA (NCT02947685) is an open-label, randomized clinical trial, with an estimated number of 496 patients | 3 | Palbociclib + anti-HER2 + endocrine therapy | HER2+/ER+ MBC patients who received anti-HER2-based induction of CT before enrollment | PFS |
| NCT02592746 is an open-label, randomized clinical trial, with an estimated number of 182 patients | 2 | Palbociclib + Exemestane + GnRH agonist Capecitabine | ER+ Premenopausal Women with MBC | PFS |
| PADA-1 (NCT03079011), is an open-label, randomized clinical trial, with an estimated number of 800 patients | 3 | A: Palbociclib + AI (Letrozole, Anastrozole, Exemestane) B: Palbociclib + Fulvestrant Selection: Palbociclib + AI (Letrozole, Anastrozole, Exemestane) | ER+/HER2− MBC | Safety until randomization Efficacy from randomization |
| T-DM1 (NCT03530696) is an open-label, randomized clinical trial, with an estimated number of 132 patients | 2 | T-DM1 + Palbociclib T-DM1 | HER2+ MBC | PFS |
| SAFIA (NCT03447132) is a multicenter, international, randomized clinical trial, with an estimated number of 400 patients | 3 | Fulvestrant + Palbociclib Fulvestrant + Placebo | Operable Luminal BC responding to Fulvestrant | pCR |
| PACE (NCT03147287) is an open-label, randomized clinical trial, with an estimated number of 220 patients | 2 | Fulvestrant Fulvestrant + Palbociclib Palbociclib + Fulvestrant + Avelumab | Metastatic ER+/HER2− BC previously stopped responding to palbociclib and endocrine therapy | PFS |
| NCT02384239 is an open-label, randomized clinical trial, with an estimated number of 70 patients | 2 | Palbociclib 100 mg plus fulvestrant or tamoxifen Palbociclib 125 mg + fulvestrant or tamoxifen | ER+ in MBC patients previously exposed to inhibitors of the PI3K Pathway | Tumor progression |
| PASIPHAE (NCT03322215) is an open-label, multicenter international and randomized clinical trial, with an estimated number of 196 patients | 2 | Palbociclib + Fulvestrant Capecitabine | Metastatic ER+/HER2− BC with progressive disease after endocrine treatment | PFS |
| PATHWAY (NCT03423199) is a multicenter, international, randomized clinical trial, with an estimated number of 180 patients | 3 | Palbociclib + Tamoxifen ± Goserelin Placebo + Tamoxifen ± Goserelin | ER+/HER2− Advanced or metastatic breast cancer patients, regardless of menopausal status | PFS |
| NCT02913430 is an open-label, randomized clinical trial, with an estimated number of 150 patients | 2 | Fulvestrant + Palbociclib Tamoxifen + Palbociclib | ER+ with MBC with 2 or 3 prior lines of endocrine therapy and up to one line of CT for MBC | PFS |
| ImmunoADAPT (NCT03573648) is an open-label, randomized clinical trial, with an estimated number of 40 patients | 2 | Tamoxifen + Avelumab Tamoxifen + Palbociclib + Avelumab | Early Stage ER+ BC | cCR |
| PELOPS (NCT02764541) is an open-label, randomized neoadjuvant clinical trial, with an estimated number of 180 patients | 2 | Tamoxifen + Endocrine Therapy Letrozole + Endocrine Therapy Tamoxifen + Endocrine Therapy + Palbociclib Letrozole + Endocrine therapy + Palbociclib | Invasive Lobular Carcinoma and Invasive Ductal Carcinoma | Anti-proliferative activity (Ki67) pCR |
| MORPHEUS (NCT03280563) is an open-label, multicenter, randomized clinical trial, with an estimated number of 111 patients | 1b/2 | Fulvestrant Atezolizumab + Entinostat Atezolizumab + Fulvestrant Atezolizumab + Ipatasertib Atezolizumab + Ipatasertib+ Fulvestrant Atezolizumab + Bevacizumab + Endocrine therapy Mandatory on-treatment biopsy | ER+/HER2− BC with progression during or following treatment with CDK 4/6 inhibitor in the first or second line setting | % of patients with objective response |
| MAINTAIN (NCT02632045) is a randomized clinical trial, with an estimated number of 132 patients | 2 | Ribociclib + Fulvestrant Placebo + Fulvestrant | Metastatic ER+/HER2− BC with progression after Aromatase Inhibitor + CDK4/6 therapy | Percentage of progression-free at 24 weeks |
| KENDO (NCT03227328) is an open-label, multicenter, randomized clinical trial, with an estimated number of 150 patients | 2 | CDK4/6 Inhibitor + Endocrine therapy Standard CT + Endocrine therapy | Advanced ER+/HER2− BC | PFS |
| PREDIX LumB (NCT02603679) is an open-label, randomized clinical trial, with an estimated number of 200 patients | 2 | Paclitaxel Tamoxifen + Palbociclib Aromatase In + Palbociclib Goserelin + Aromatase In + Palbociclib | ER+ tumors with high proliferation or low proliferation with metastatic nodes | Radiological objective response rate |
| PREDIX LumA (NCT02592083) is an open-label, randomized clinical trial, with an estimated number of 200 patients | 2 | Endocrine treatment Endocrine treatment + Palbociclib | Early stage ER+ BC | Clinical and radiological response |
| SONIA (NCT03425838) is an open-label randomized clinical trial, with an estimated number of 1050 patients | 3 | Non-steroidal aromatase Inhibitor + CDK4/6 inhibitor in first line + Fulvestrant in the 2nd line Non-steroidal aromatase Inhibitor + Fulvestrant + CDK4/6 inhibitor in the 2nd line | ER+/HER2− advanced BC | PFS (after 2 lines of treatment) |
| NCT03128619 is an open-label, randomized clinical trial, with an estimated number of 102 patients | 1b/2 | Copanlisib + Letrozole Copanlisib + Letrozole + Palbociclib | ER+/HER2− Postmenopausal Any stage BC | Change in Ki-67 expression The incidence of dose-limiting toxicities (DLT) |
| FLIPPER (NCT02690480)is an international, multicenter, randomized clinical trial, with an estimated number of 190 patients | 2 | Palbociclib + Fulvestrant Placebo + Fulvestrant | Postmenopausal women with ER+/HER2− MBC after 5 or more years of endocrine therapy in adjuvant setting | PFS |
| TOUCH (NCT03644186) is an open-label, international neoadjuvant clinical trial, with an estimated number of 144 patients | 2 | Paclitaxel + Trastuzumab and Pertuzumab Palbociclib + Letrozole + Trastuzumab and Pertuzumab | ER+/HER2+ early BC | pCR |
| NEOLBC (NCT03283384) is an open-label randomized clinical trial, with an estimated number of 100 patients | 2 | Letrozole Standard CT Ribociclib + Letrozole | Postmenopausal ER+/HER2− Stage II/III Luminal BC | Difference in complete cell cycle arrest (Ki-67 < 1%) |
| RIBBIT (NCT03462251) is a prospective, randomized, open-label multicenter clinical trial, with an estimated number of 160 patients | 3 | Ribociclib + Aromatase Inhibitor Paclitaxel ± Bevacizumab | Postmenopausal women ER+/HER2− Advanced BC with visceral metastases and no prior therapy for advanced disease | PFS |
| AMICA (NCT03555877) is a multicenter, prospective, randomized, open-label clinical trial, with an estimated number of 150 patients | 2 | Anti-hormonal treatment + ribociclib Anti-hormonal treatment | ER+/HER2− BC patients with disease control (at least stable disease) after 1st line CT | PFS |
| NCT03671330 is a randomized, placebo-controlled clinical trial, with an estimated number of 315 patients | 2 | Ribociclib Ribociclib Placebo | Chinese postmenopausal women with ER+/HER2− Advanced BC | PFS |
| CORALLEEN (NCT03248427) is a two-arm, multicentric, randomized clinical trial, with an estimated number of 94 patients | 2 | Ribociclib + Letrozole CT (doxorubicin and cyclophosphamide) + paclitaxel | Postmenopausal women with primary operable Luminal B ER+/HER2− BC | Residual Cancer Burden (RCB) |
| FELINE (NCT02712723) is a randomized clinical trial, with an estimated number of 120 patients | 2 | Placebo + Letrozole Ribociclib + Letrozole | ER+/HER2− Early BC | Rate of Pre-operative Endocrine Prognostic Index (PEPI) score 0 at surgery |
| LEADER (NCT03285412) is an open-label randomized clinical trial, with an estimated number of 120 patients | 2 | Ribociclib Intermittent (21 days) + Endocrine Therapy daily Ribociclib (28 days) + Endocrine Therapy daily | ER+ Early stage BC | Number of patients who complete 12 months of treatment |
| NCT03703466 is an open-label, randomized clinical trial, with an estimated number of 60 patients | 2 | Abemaciclib + a meal Abemaciclib without a meal Abemaciclib without regard to food | ER+/HER2− MBC previously treated | % of participants with severe diarrhoea with grade 3, 2 % of participants with dose reduction, dose interruption and discontinue treatment due to diarrhoea % of participants utilizing antidiarrheal/s |
| MonarchE (NCT03155997) is an open-label, randomized clinical trial, with an estimated number of 3580 patients | 3 | Adjuvant ET ± abemaciclib | ER+/HER2− Node-positive high-risk breast tumors (≥ 4 lymph nodes, tumor > 5 cm, grade 3 or central Ki67 ≥ 20%) | iDFS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobhani, N.; D’Angelo, A.; Pittacolo, M.; Roviello, G.; Miccoli, A.; Corona, S.P.; Bernocchi, O.; Generali, D.; Otto, T. Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells 2019, 8, 321. https://doi.org/10.3390/cells8040321
Sobhani N, D’Angelo A, Pittacolo M, Roviello G, Miccoli A, Corona SP, Bernocchi O, Generali D, Otto T. Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells. 2019; 8(4):321. https://doi.org/10.3390/cells8040321
Chicago/Turabian StyleSobhani, Navid, Alberto D’Angelo, Matteo Pittacolo, Giandomenico Roviello, Anna Miccoli, Silvia Paola Corona, Ottavia Bernocchi, Daniele Generali, and Tobias Otto. 2019. "Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer" Cells 8, no. 4: 321. https://doi.org/10.3390/cells8040321
APA StyleSobhani, N., D’Angelo, A., Pittacolo, M., Roviello, G., Miccoli, A., Corona, S. P., Bernocchi, O., Generali, D., & Otto, T. (2019). Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells, 8(4), 321. https://doi.org/10.3390/cells8040321








