Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes
Abstract
1. Introduction
2. APOA4 Gene and ApoA-IV Proteins
2.1. ApoA-IV Protein Structure and Post-Translational Modifications
2.2. Genetic Variants of ApoA-IV in Human Population
3. ApoA-IV Function in Cells
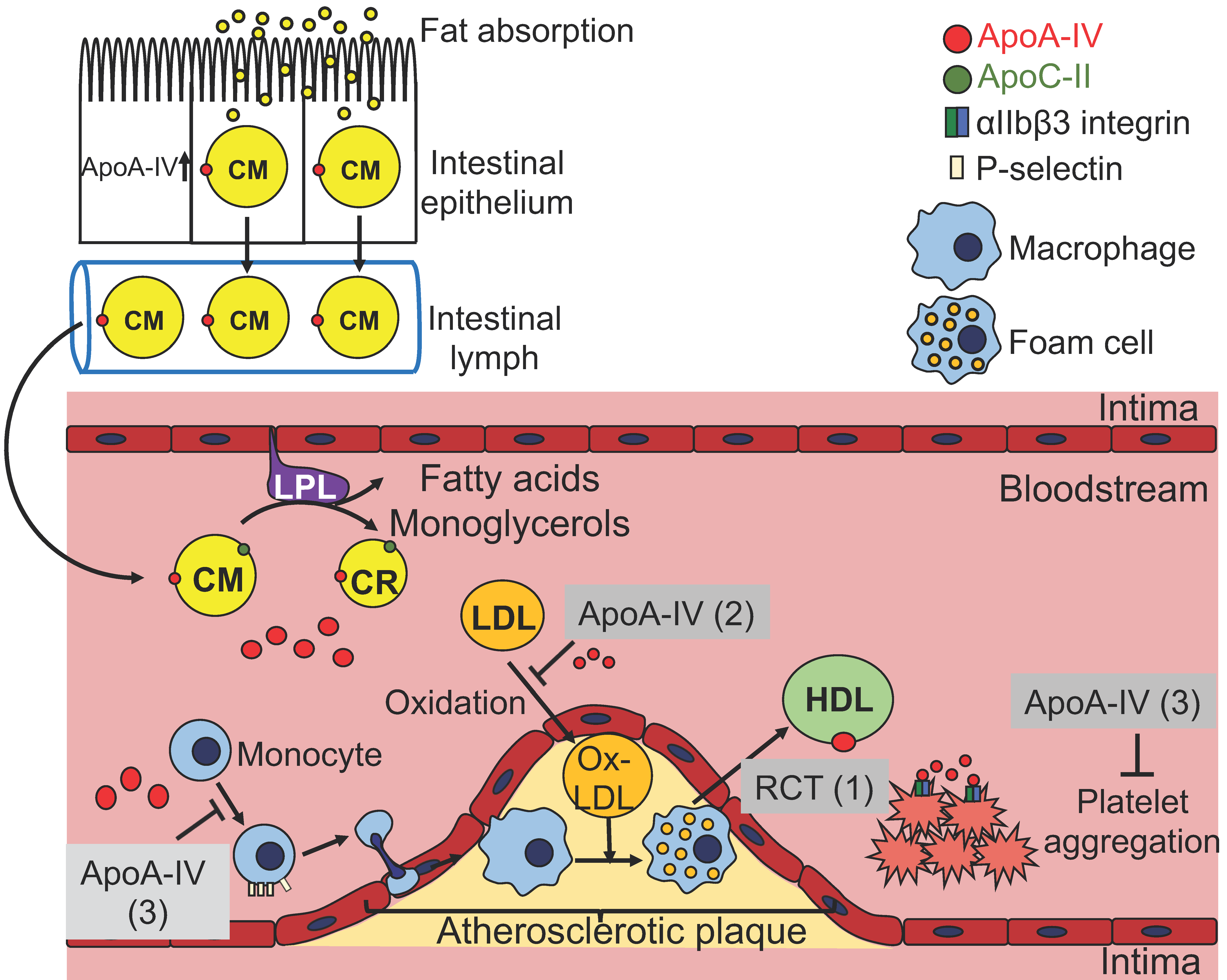
3.1. ApoA-IV and Lipoprotein Metabolism
3.2. ApoA-IV and Reverse Cholesterol Transport
3.3. ApoA-IV and Atherosclerosis
3.4. ApoA-IV and Lipoprotein Oxidation
3.5. ApoA-IV Inhibits Platelet Aggregation and Thrombosis
3.6. ApoA-IV and Glucose Metabolism
3.7. ApoA-IV and Food Intake
4. ApoA-IV Interacting Partners
4.1. ApoA-IV Binding to Cell Surface of Several Cell Lines
4.2. ApoA-IV Binding to NR1D1 and NR4A1 in Human Hepatic Carcinoma Cell Line
4.3. ApoA-IV Binding to αIIbβ3 Integrin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feingold, K.R.; Grunfeld, C. Introduction to Lipids and Lipoproteins. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Utermann, G.; Beisiegel, U. Apolipoprotein A-IV: A protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur. J. Biochem. 1979, 99, 333–343. [Google Scholar] [CrossRef]
- Green, P.H.; Glickman, R.M.; Riley, J.W.; Quinet, E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J. Clin. Investig. 1980, 65, 911–919. [Google Scholar] [CrossRef]
- Wu, A.L.; Windmueller, H.G. Identification of circulating apolipoproteins synthesized by rat small intestine in vivo. J. Biol. Chem. 1978, 253, 2525–2528. [Google Scholar]
- Goldberg, I.J.; Scheraldi, C.A.; Yacoub, L.K.; Saxena, U.; Bisgaier, C.L. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J. Biol. Chem. 1990, 265, 4266–4272. [Google Scholar]
- Lu, S.; Yao, Y.; Meng, S.; Cheng, X.; Black, D.D. Overexpression of apolipoprotein A-IV enhances lipid transport in newborn swine intestinal epithelial cells. J. Biol. Chem. 2002, 277, 31929–31937. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Gallagher, J.W.; Fabritius, M.A.; Shelness, G.S. ApoA-IV modulates the secretory trafficking of apoB and the size of triglyceride-rich lipoproteins. J. Lipid Res. 2012, 53, 736–743. [Google Scholar] [CrossRef]
- Steinmetz, A.; Utermann, G. Activation of lecithin: Cholesterol acyltransferase by human apolipoprotein A-IV. J. Biol. Chem. 1985, 260, 2258–2264. [Google Scholar]
- Stein, O.; Stein, Y.; Lefevre, M.; Roheim, P.S. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim. Biophys. Acta 1986, 878, 7–13. [Google Scholar] [CrossRef]
- Steinmetz, A.; Barbaras, R.; Ghalim, N.; Clavey, V.; Fruchart, J.C.; Ailhaud, G. Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J. Biol. Chem. 1990, 265, 7859–7863. [Google Scholar]
- Duverger, N.; Tremp, G.; Caillaud, J.M.; Emmanuel, F.; Castro, G.; Fruchart, J.C.; Steinmetz, A.; Denefle, P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science 1996, 273, 966–968. [Google Scholar] [CrossRef]
- Cohen, R.D.; Castellani, L.W.; Qiao, J.H.; Van Lenten, B.J.; Lusis, A.J.; Reue, K. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J. Clin. Investig. 1997, 99, 1906–1916. [Google Scholar] [CrossRef] [PubMed]
- Ostos, M.A.; Conconi, M.; Vergnes, L.; Baroukh, N.; Ribalta, J.; Girona, J.; Caillaud, J.M.; Ochoa, A.; Zakin, M.M. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1023–1028. [Google Scholar] [CrossRef]
- Xu, X.R.; Wang, Y.; Adili, R.; Ju, L.; Spring, C.M.; Jin, J.W.; Yang, H.; Neves, M.A.D.; Chen, P.; Yang, Y.; et al. Apolipoprotein A-IV binds αIIbβ3 integrin and inhibits thrombosis. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Kindel, T.L.; Corbin, K.L.; Nunemaker, C.S.; Obici, S.; Woods, S.C.; Davidson, W.S.; Tso, P. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc. Natl. Acad. Sci. USA 2012, 109, 9641–9646. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Wang, F.; Kohan, A.B.; Haas, M.K.; Yang, Q.; Lou, D.; Obici, S.; Davidson, W.S.; Tso, P. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. J. Biol. Chem. 2014, 289, 2396–2404. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Wang, F.; Ji, Y.; Davidso, N.W.; Li, Z.; Tso, P. Interaction of ApoA-IV with NR4A1 and NR1D1 Represses G6Pase and PEPCK Transcription: Nuclear Receptor-Mediated Downregulation of Hepatic Gluconeogenesis in Mice and a Human Hepatocyte Cell Line. PLoS ONE 2015, 10, e0142098. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Xu, M.; Howles, P.; Tso, P. ApoA-IV improves insulin sensitivity and glucose uptake in mouse adipocytes via PI3K-Akt Signaling. Sci. Rep. 2017, 7, 41289. [Google Scholar] [CrossRef]
- Fujimoto, K.; Fukagawa, K.; Sakata, T.; Tso, P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J. Clin. Investig. 1993, 91, 1830–1833. [Google Scholar] [CrossRef]
- Shen, L.; Lo, C.C.; Woollett, L.A.; Liu, M. Apolipoprotein A-IV exerts its anorectic action through a PI3K/Akt signaling pathway in the hypothalamus. Biochem. Biophys. Res. Commun. 2017, 494, 152–157. [Google Scholar] [CrossRef]
- Kronenberg, F.; Stuhlinger, M.; Trenkwalder, E.; Geethanjali, F.S.; Pachinger, O.; von Eckardstein, A.; Dieplinger, H. Low apolipoprotein A-IV plasma concentrations in men with coronary artery disease. J. Am. Coll. Cardiol. 2000, 36, 751–757. [Google Scholar] [CrossRef]
- Rao, R.; Roche, A.; Febres, G.; Bessler, M.; Tso, P.; Korner, J. Circulating Apolipoprotein A-IV presurgical levels are associated with improvement in insulin sensitivity after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2017, 13, 468–473. [Google Scholar] [CrossRef][Green Version]
- Kronenberg, F.; Kuen, E.; Ritz, E.; Konig, P.; Kraatz, G.; Lhotta, K.; Mann, J.F.; Muller, G.A.; Neyer, U.; Riegel, W.; et al. Apolipoprotein A-IV serum concentrations are elevated in patients with mild and moderate renal failure. J. Am. Soc. Nephrol. 2002, 13, 461–469. [Google Scholar] [PubMed]
- Boes, E.; Fliser, D.; Ritz, E.; Konig, P.; Lhotta, K.; Mann, J.F.; Muller, G.A.; Neyer, U.; Riegel, W.; Riegler, P.; et al. Apolipoprotein A-IV predicts progression of chronic kidney disease: The mild to moderate kidney disease study. J. Am. Soc. Nephrol. 2006, 17, 528–536. [Google Scholar] [CrossRef] [PubMed]
- von Toerne, C.; Huth, C.; de Las Heras Gala, T.; Kronenberg, F.; Herder, C.; Koenig, W.; Meisinger, C.; Rathmann, W.; Waldenberger, M.; Roden, M.; et al. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: The KORA F4 study. Diabetologia 2016, 59, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.W.; Hung, Y.C.; Wu, T.H.; Chen, M.H.; Yeh, C.T.; Pan, T.L. Proteome-based identification of apolipoprotein A-IV as an early diagnostic biomarker in liver fibrosis. Oncotarget 2017, 8, 88951–88964. [Google Scholar] [CrossRef]
- Dieplinger, H.; Ankerst, D.P.; Burges, A.; Lenhard, M.; Lingenhel, A.; Fineder, L.; Buchner, H.; Stieber, P. Afamin and apolipoprotein A-IV: Novel protein markers for ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, S.K. Apolipoprotein multigene family: Tandem organization of human apolipoprotein AI, CIII, and AIV genes. Proc. Natl. Acad. Sci. USA 1985, 82, 6374–6378. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, H.N.; Sammels, M.G.; Leegwater, A.C.; Levels, J.H.; Reitsma, P.H.; Boers, W.; Chamuleau, R.A. Apolipoprotein A-V: A novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 2001, 276, 44512–44520. [Google Scholar] [CrossRef]
- Williams, S.C.; Bruckheimer, S.M.; Lusis, A.J.; LeBoeuf, R.C.; Kinniburgh, A.J. Mouse apolipoprotein A-IV gene: Nucleotide sequence and induction by a high-lipid diet. Mol. Cell. Biol. 1986, 6, 3807–3814. [Google Scholar] [CrossRef]
- Lamina, C.; Friedel, S.; Coassin, S.; Rueedi, R.; Yousri, N.A.; Seppala, I.; Gieger, C.; Schonherr, S.; Forer, L.; Erhart, G.; et al. A genome-wide association meta-analysis on apolipoprotein A-IV concentrations. Hum. Mol. Genet. 2016, 25, 3635–3646. [Google Scholar] [CrossRef]
- Ou, H.J.; Huang, G.; Liu, W.; Ma, X.L.; Wei, Y.; Zhou, T.; Pan, Z.M. Relationship of the APOA5/A4/C3/A1 gene cluster and APOB gene polymorphisms with dyslipidemia. Genet. Mol. Res. 2015, 14, 9277–9290. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Jimenez, F.; Ruano, J.; Perez-Martinez, P.; Fuentes, F.; Criado-Garcia, J.; Parnell, L.D.; Garcia-Rios, A.; Ordovas, J.M.; Lopez-Miranda, J. Effects of variations in the APOA1/C3/A4/A5 gene cluster on different parameters of postprandial lipid metabolism in healthy young men. J. Lipid Res. 2010, 51, 63–73. [Google Scholar] [CrossRef]
- Liu, Y.; Ordovas, J.M.; Gao, G.; Province, M.; Straka, R.J.; Tsai, M.Y.; Lai, C.Q.; Zhang, K.; Borecki, I.; Hixson, J.E.; et al. Pharmacogenetic association of the APOA1/C3/A4/A5 gene cluster and lipid responses to fenofibrate: The genetics of lipid-lowering drugs and diet network study. Pharmacogenet. Genom. 2009, 19, 161–169. [Google Scholar] [CrossRef]
- Karathanasis, S.K.; Yunis, I.; Zannis, V.I. Structure, evolution, and tissue-specific synthesis of human apolipoprotein AIV. Biochemistry 1986, 25, 3962–3970. [Google Scholar] [CrossRef]
- Staels, B.; van Tol, A.; Verhoeven, G.; Auwerx, J. Apolipoprotein A-IV messenger ribonucleic acid abundance is regulated in a tissue-specific manner. Endocrinology 1990, 126, 2153–2163. [Google Scholar] [CrossRef]
- Kalogeris, T.J.; Tsuchiya, T.; Fukagawa, K.; Wolf, R.; Tso, P. Apolipoprotein A-IV synthesis in proximal jejunum is stimulated by ileal lipid infusion. Am. J. Physiol. 1996, 270, G277–G286. [Google Scholar] [CrossRef]
- Sanecka, A.; Ansems, M.; van Hout-Kuijer, M.A.; Looman, M.W.; Prosser, A.C.; Welten, S.; Gilissen, C.; Sama, I.E.; Huynen, M.A.; Veltman, J.A.; et al. Analysis of genes regulated by the transcription factor LUMAN identifies ApoA4 as a target gene in dendritic cells. Mol. Immunol. 2012, 50, 66–73. [Google Scholar] [CrossRef]
- Liu, M.; Doi, T.; Shen, L.; Woods, S.C.; Seeley, R.J.; Zheng, S.; Jackman, A.; Tso, P. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1382–R1387. [Google Scholar] [CrossRef]
- Shen, L.; Pearson, K.J.; Xiong, Y.; Lo, C.M.; Tso, P.; Woods, S.C.; Davidson, W.S.; Liu, M. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol. Behav. 2008, 95, 161–167. [Google Scholar] [CrossRef]
- Ghiselli, G.; Krishnan, S.; Beigel, Y.; Gotto, A.M., Jr. Plasma metabolism of apolipoprotein A-IV in humans. J. Lipid Res. 1986, 27, 813–827. [Google Scholar]
- Ohta, T.; Fidge, N.H.; Nestel, P.J. Studies on the in vivo and in vitro distribution of apolipoprotein A-IV in human plasma and lymph. J. Clin. Investig. 1985, 76, 1252–1260. [Google Scholar] [CrossRef]
- Deng, X.; Morris, J.; Dressmen, J.; Tubb, M.R.; Tso, P.; Jerome, W.G.; Davidson, W.S.; Thompson, T.B. The structure of dimeric apolipoprotein A-IV and its mechanism of self-association. Structure 2012, 20, 767–779. [Google Scholar] [CrossRef]
- Tubb, M.R.; Silva, R.A.; Pearson, K.J.; Tso, P.; Liu, M.; Davidson, W.S. Modulation of apolipoprotein A-IV lipid binding by an interaction between the N and C termini. J. Biol. Chem. 2007, 282, 28385–28394. [Google Scholar] [CrossRef]
- Tubb, M.R.; Silva, R.A.; Fang, J.; Tso, P.; Davidson, W.S. A three-dimensional homology model of lipid-free apolipoprotein A-IV using cross-linking and mass spectrometry. J. Biol. Chem. 2008, 283, 17314–17323. [Google Scholar] [CrossRef]
- Deng, X.; Morris, J.; Chaton, C.; Schroder, G.F.; Davidson, W.S.; Thompson, T.B. Small-angle X-ray scattering of apolipoprotein A-IV reveals the importance of its termini for structural stability. J. Biol. Chem. 2013, 288, 4854–4866. [Google Scholar] [CrossRef]
- Pearson, K.; Tubb, M.R.; Tanaka, M.; Zhang, X.Q.; Tso, P.; Weinberg, R.B.; Davidson, W.S. Specific sequences in the N and C termini of apolipoprotein A-IV modulate its conformation and lipid association. J. Biol. Chem. 2005, 280, 38576–38582. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Scanu, A.M. Isolation and characterization of human apolipoprotein A-IV from lipoprotein-depleted serum. J. Lipid Res. 1983, 24, 52–59. [Google Scholar]
- Dai, Y.; Shen, Y.; Li, Q.R.; Ding, F.H.; Wang, X.Q.; Liu, H.J.; Yan, X.X.; Wang, L.J.; Yang, K.; Wang, H.B.; et al. Glycated Apolipoprotein A-IV Induces Atherogenesis in Patients With CAD in Type 2 Diabetes. J. Am. Coll. Cardiol. 2017, 70, 2006–2019. [Google Scholar] [CrossRef]
- Weinberg, R.B. Apolipoprotein A-IV polymorphisms and diet-gene interactions. Curr. Opin. Lipidol. 2002, 13, 125–134. [Google Scholar] [CrossRef]
- Lohse, P.; Kindt, M.R.; Rader, D.J.; Brewer, H.B., Jr. Three genetic variants of human plasma apolipoprotein A-IV. apoA-IV-1(Thr347----Ser), apoA-IV-0(Lys167----Glu,Gln360----His), and apoA-IV-3(Glu165----Lys). J. Biol. Chem. 1991, 266, 13513–13518. [Google Scholar] [PubMed]
- Wong, W.M.; Hawe, E.; Li, L.K.; Miller, G.J.; Nicaud, V.; Pennacchio, L.A.; Humphries, S.E.; Talmud, P.J. Apolipoprotein AIV gene variant S347 is associated with increased risk of coronary heart disease and lower plasma apolipoprotein AIV levels. Circ. Res. 2003, 92, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Hockey, K.J.; Anderson, R.A.; Cook, V.R.; Hantgan, R.R.; Weinberg, R.B. Effect of the apolipoprotein A-IV Q360H polymorphism on postprandial plasma triglyceride clearance. J. Lipid Res. 2001, 42, 211–217. [Google Scholar] [PubMed]
- Carrejo, M.H.; Sharrett, R.; Patsch, W.; Boerwinkle, E. No association of apolipoprotein A-IV codon 347 and 360 variation with atherosclerosis and lipid transport in a sample of mixed hyperlipidemics. Genet. Epidemiol. 1995, 12, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Kamboh, M.I.; Hoag, S.; Shetterly, S.M.; Ferrell, R.E.; Hamman, R.F. ApoA-IV polymorphism associated with myocardial infarction in obese NIDDM patients. The San Luis Valley Diabetes Study. Diabetes 1994, 43, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, M.; De Bruin, T.W.; Dallinga-Thie, G.M. Two polymorphisms in the apo A-IV gene and familial combined hyperlipidemia. Atherosclerosis 2001, 158, 369–376. [Google Scholar] [CrossRef]
- Weinberg, R.B. Identification of functional domains in the plasma apolipoproteins by analysis of inter-species sequence variability. J. Lipid Res. 1994, 35, 2212–2222. [Google Scholar] [PubMed]
- Lohse, P.; Kindt, M.R.; Rader, D.J.; Brewer, H.B., Jr. Human plasma apolipoproteins A-IV-0 and A-IV-3. Molecular basis for two rare variants of apolipoprotein A-IV-1. J. Biol. Chem. 1990, 265, 12734–12739. [Google Scholar]
- Menzel, H.J.; Dieplinger, H.; Sandholzer, C.; Karadi, I.; Utermann, G.; Csaszar, A. Apolipoprotein A-IV polymorphism in the Hungarian population: Gene frequencies, effect on lipid levels, and sequence of two new variants. Hum. Mutat. 1995, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Fujimoto, K.; Cardelli, J.A.; Nutting, D.F.; Bergstedt, S.; Tso, P. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. 1990, 259, G709–G719. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nutting, D.F.; Fujimoto, K.; Cardelli, J.A.; Black, D.; Tso, P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J. Lipid Res. 1990, 31, 1613–1625. [Google Scholar]
- Kalogeris, T.J.; Monroe, F.; Demichele, S.J.; Tso, P. Intestinal synthesis and lymphatic secretion of apolipoprotein A-IV vary with chain length of intestinally infused fatty acids in rats. J. Nutr. 1996, 126, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Mansbach, C.M., 2nd. Prechylomicron transport vesicle: Isolation and partial characterization. Am. J. Physiol. 1999, 276, G378–G386. [Google Scholar] [CrossRef]
- Lu, S.; Yao, Y.; Cheng, X.; Mitchell, S.; Leng, S.; Meng, S.; Gallagher, J.W.; Shelness, G.S.; Morris, G.S.; Mahan, J.; et al. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 2006, 281, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, C.M.; Siddiqi, S.A. The biogenesis of chylomicrons. Ann. Rev. Physiol. 2010, 72, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.W.; Weinberg, R.B.; Shelness, G.S. apoA-IV tagged with the ER retention signal KDEL perturbs the intracellular trafficking and secretion of apoB. J. Lipid Res. 2004, 45, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Kohan, A.B.; Wang, F.; Li, X.; Vandersall, A.E.; Huesman, S.; Xu, M.; Yang, Q.; Lou, D.; Tso, P. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride? Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1128–G1135. [Google Scholar] [CrossRef] [PubMed]
- Aalto-Setala, K.; Bisgaier, C.L.; Ho, A.; Kieft, K.A.; Traber, M.G.; Kayden, H.J.; Ramakrishnan, R.; Walsh, A.; Essenburg, A.D.; Breslow, J.L. Intestinal expression of human apolipoprotein A-IV in transgenic mice fails to influence dietary lipid absorption or feeding behavior. J. Clin. Investig. 1994, 93, 1776–1786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohan, A.B.; Wang, F.; Li, X.; Bradshaw, S.; Yang, Q.; Caldwell, J.L.; Bullock, T.M.; Tso, P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G628–G636. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Breckenridge, W.C.; Little, J.A.; Steiner, G.; Chow, A.; Poapst, M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N. Engl. J. Med. 1978, 298, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R. An overview of reverse cholesterol transport. Eur. Heart J. 1998, 19 (Suppl. A), A31–A35. [Google Scholar] [CrossRef]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef] [PubMed]
- Glomset, J.A. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 1968, 9, 155–167. [Google Scholar] [PubMed]
- Schwartz, C.C.; VandenBroek, J.M.; Cooper, P.S. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J. Lipid Res. 2004, 45, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Kovanen, P.T.; Goldstein, J.L. Regulation of plasma cholesterol by lipoprotein receptors. Science 1981, 212, 628–635. [Google Scholar] [CrossRef]
- Remaley, A.T.; Stonik, J.A.; Demosky, S.J.; Neufeld, E.B.; Bocharov, A.V.; Vishnyakova, T.G.; Eggerman, T.L.; Patterson, A.P.; Duverger, N.J.; Santamarina-Fojo, S.; et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 2001, 280, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.; Saito, H.; Woods, S.C.; Lund-Katz, S.; Tso, P.; Phillips, M.C.; Davidson, W.S. Structure of human apolipoprotein A-IV: A distinct domain architecture among exchangeable apolipoproteins with potential functional implications. Biochemistry 2004, 43, 10719–10729. [Google Scholar] [CrossRef] [PubMed]
- Glomset, J.A.; Norum, K.R. The metabolic role of lecithin: Cholesterol acyltransferase: Perspectives form pathology. Adv. Lipid Res. 1973, 11, 1–65. [Google Scholar] [PubMed]
- Jonas, A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim. Biophys. Acta 1991, 1084, 205–220. [Google Scholar] [CrossRef]
- Main, L.A.; Ohnishi, T.; Yokoyama, S. Activation of human plasma cholesteryl ester transfer protein by human apolipoprotein A-IV. Biochim. Biophys. Acta 1996, 1300, 17–24. [Google Scholar] [CrossRef]
- Tanigawa, H.; Billheimer, J.T.; Tohyama, J.; Zhang, Y.; Rothblat, G.; Rader, D.J. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 2007, 116, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Inazu, A.; Hesler, C.B.; Agellon, L.B.; Mann, C.; Whitlock, M.E.; Marcel, Y.L.; Milne, R.W.; Koizumi, J.; Mabuchi, H.; et al. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature 1989, 342, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.B.; Anderson, R.A.; Cook, V.R.; Emmanuel, F.; Denefle, P.; Tall, A.R.; Steinmetz, A. Interfacial exclusion pressure determines the ability of apolipoprotein A-IV truncation mutants to activate cholesterol ester transfer protein. J. Biol. Chem. 2002, 277, 21549–21553. [Google Scholar] [CrossRef]
- Qin, X.; Swertfeger, D.K.; Zheng, S.; Hui, D.Y.; Tso, P. Apolipoprotein AIV: A potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 1998, 274, H1836–H1840. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Bicchiega, V.; Curatola, G. Effect of human Apo AIV against lipid peroxidation of very low density lipoproteins. Chem. Phys. Lipids 2002, 114, 45–54. [Google Scholar] [CrossRef]
- Heinecke, J.W. Oxidants and antioxidants in the pathogenesis of atherosclerosis: Implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Investig. 1991, 88, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Lipoprotein oxidation in cardiovascular disease: Chief culprit or innocent bystander? J. Exp. Med. 2006, 203, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, C.P.; Parthasarathy, S.; Steinberg, D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J. Lipid Res. 1988, 29, 745–753. [Google Scholar]
- Spaulding, H.L.; Saijo, F.; Turnage, R.H.; Alexander, J.S.; Aw, T.Y.; Kalogeris, T.J. Apolipoprotein A-IV attenuates oxidant-induced apoptosis in mitotic competent, undifferentiated cells by modulating intracellular glutathione redox balance. Am. J. Physiol. Cell Physiol. 2006, 290, C95–C103. [Google Scholar] [CrossRef]
- Pias, E.K.; Aw, T.Y. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ. 2002, 9, 1007–1016. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Glutathione and apoptosis. Free Radic. Res. 2008, 42, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Vowinkel, T.; Mori, M.; Krieglstein, C.F.; Russell, J.; Saijo, F.; Bharwani, S.; Turnage, R.H.; Davidson, W.S.; Tso, P.; Granger, D.N.; et al. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Investig. 2004, 114, 260–269. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Pena, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Geng, J.-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch. Immunol. Ther. Exp. 2006, 54, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bledzka, K.; Smyth, S.S.; Plow, E.F. Integrin alphaIIbbeta3: From discovery to efficacious therapeutic target. Circ. Res. 2013, 112, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Piotrowicz, R.S.; Orchekowski, R.P.; Nugent, D.J.; Yamada, K.Y.; Kunicki, T.J. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J. Cell Biol. 1988, 106, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.; Modderman, P.W.; Hogervorst, F. Laminin receptor on platelets is the integrin VLA-6. Nature 1988, 336, 487–489. [Google Scholar] [CrossRef]
- Staatz, W.D.; Rajpara, S.M.; Wayner, E.A.; Carter, W.G.; Santoro, S.A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J. Cell Biol. 1989, 108, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renstrom, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Straub, S.G.; Sharp, G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes/Metab. Re. Rev. 2002, 18, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; James, D.E. Moving GLUT4: The biogenesis and trafficking of GLUT4 storage vesicles. Diabetes 1997, 46, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Adelmant, G.; Begue, A.; Stehelin, D.; Laudet, V. A functional Rev-erb alpha responsive element located in the human Rev-erb alpha promoter mediates a repressing activity. Proc. Natl. Acad. Sci. USA 1996, 93, 3553–3558. [Google Scholar] [CrossRef]
- Timper, K.; Bruning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Lo, C.C.; Langhans, W.; Georgievsky, M.; Arnold, M.; Caldwell, J.L.; Cheng, S.; Liu, M.; Woods, S.C.; Tso, P. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology 2012, 153, 5857–5865. [Google Scholar] [CrossRef]
- Morton, G.J.; Gelling, R.W.; Niswender, K.D.; Morrison, C.D.; Rhodes, C.J.; Schwartz, M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005, 2, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morrison, C.D.; Clegg, D.J.; Olson, R.; Baskin, D.G.; Myers, M.G., Jr.; Seeley, R.J.; Schwartz, M.W. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: A key mediator of insulin-induced anorexia. Diabetes 2003, 52, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morton, G.J.; Stearns, W.H.; Rhodes, C.J.; Myers, M.G., Jr.; Schwartz, M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001, 413, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Innerarity, T.L.; Rall, S.C., Jr.; Weisgraber, K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984, 25, 1277–1294. [Google Scholar] [PubMed]
- Pieters, M.N.; Schouten, D.; Van Berkel, T.J. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim. Biophys. Acta 1994, 1225, 125–134. [Google Scholar] [CrossRef]
- Savion, N.; Gamliel, A.; Tauber, J.P.; Gospodarowicz, D. Free apolipoproteins A-I and A-IV present in human plasma displace high-density lipoprotein on cultured bovine aortic endothelial cells. Eur. J. Biochem. 1987, 164, 435–443. [Google Scholar] [CrossRef]
- Tauber, J.P.; Goldminz, D.; Gospodarowicz, D. Up-regulation in vascular endothelial cells of binding sites of high density lipoprotein induced by 25-hydroxycholesterol. Eur. J. Biochem. 1981, 119, 327–339. [Google Scholar] [CrossRef]
- Dvorin, E.; Gorder, N.L.; Benson, D.M.; Gotto, A.M., Jr. Apolipoprotein A-IV. A determinant for binding and uptake of high density lipoproteins by rat hepatocytes. J. Biol. Chem. 1986, 261, 15714–15718. [Google Scholar] [PubMed]
- Segrest, J.P.; Garber, D.W.; Brouillette, C.G.; Harvey, S.C.; Anantharamaiah, G.M. The amphipathic alpha helix: A multifunctional structural motif in plasma apolipoproteins. Adv. Protein Chem. 1994, 45, 303–369. [Google Scholar]
- Li, W.H.; Tanimura, M.; Luo, C.C.; Datta, S.; Chan, L. The apolipoprotein multigene family: Biosynthesis, structure, structure-function relationships, and evolution. J. Lipid Res. 1988, 29, 245–271. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Ko, C.-W.; Tso, P.; Bhargava, A. Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells 2019, 8, 319. https://doi.org/10.3390/cells8040319
Qu J, Ko C-W, Tso P, Bhargava A. Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells. 2019; 8(4):319. https://doi.org/10.3390/cells8040319
Chicago/Turabian StyleQu, Jie, Chih-Wei Ko, Patrick Tso, and Aditi Bhargava. 2019. "Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes" Cells 8, no. 4: 319. https://doi.org/10.3390/cells8040319
APA StyleQu, J., Ko, C.-W., Tso, P., & Bhargava, A. (2019). Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells, 8(4), 319. https://doi.org/10.3390/cells8040319





