Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Hormone Treatment
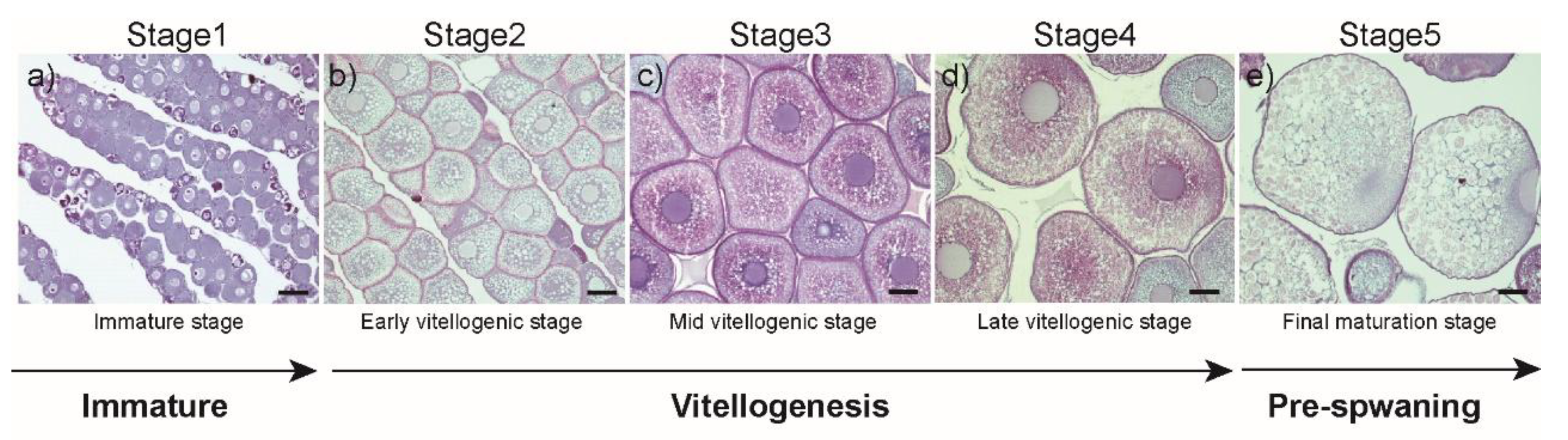
2.2. Histological Procedures and Specimen Collection and Classification
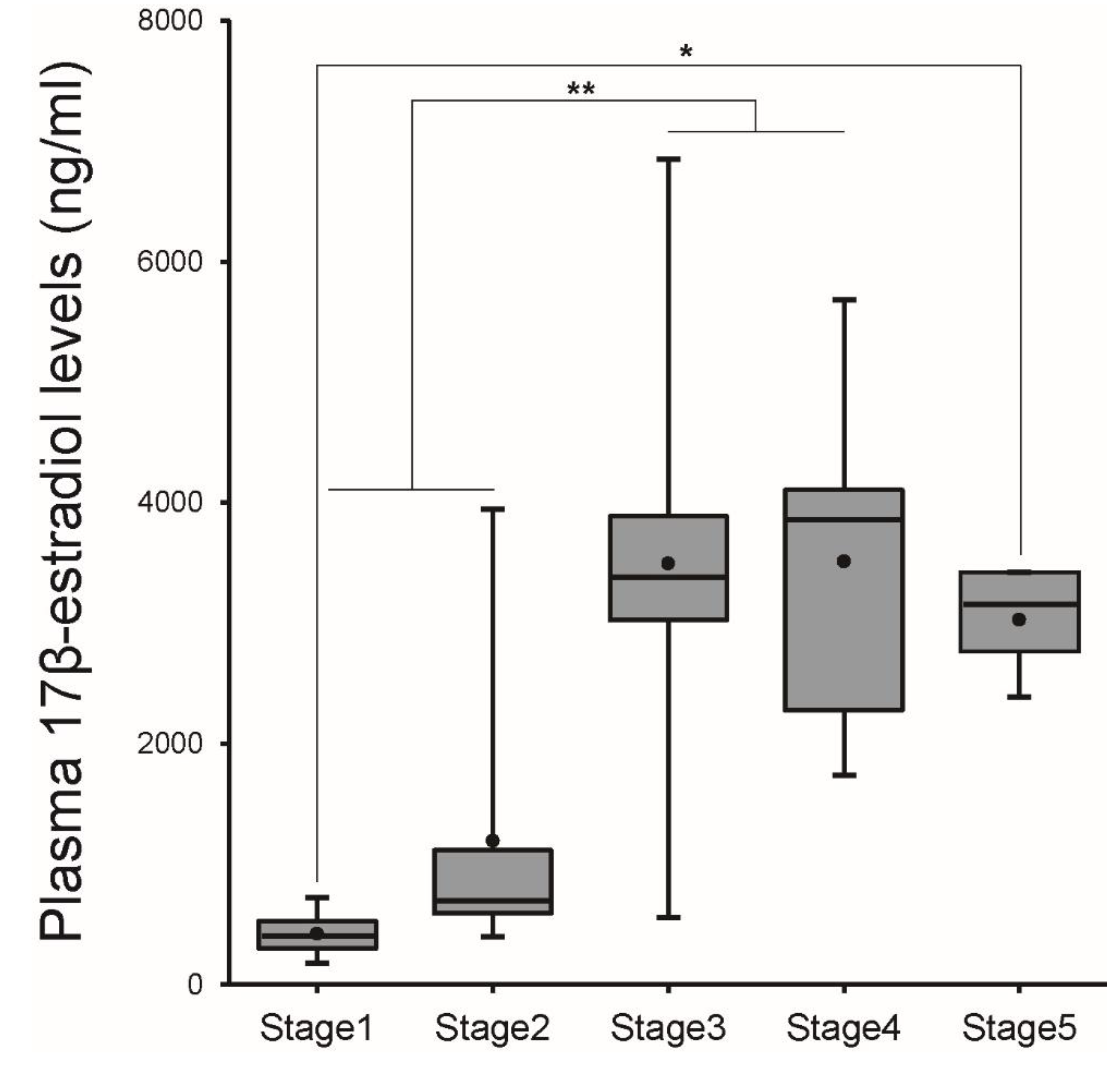
2.3. Plasma Steroid Hormone Assay
2.4. RNA Extraction and cDNA Synthesis
2.5. Real-Time qRT-PCR (qPCR)
2.6. Fluorescence In-Situ Hybridization (FISH)
2.7. Statistical Analysis
3. Results
3.1. Changes in Morphometric Parameters and Plasma E2 Levels during Ovarian Development
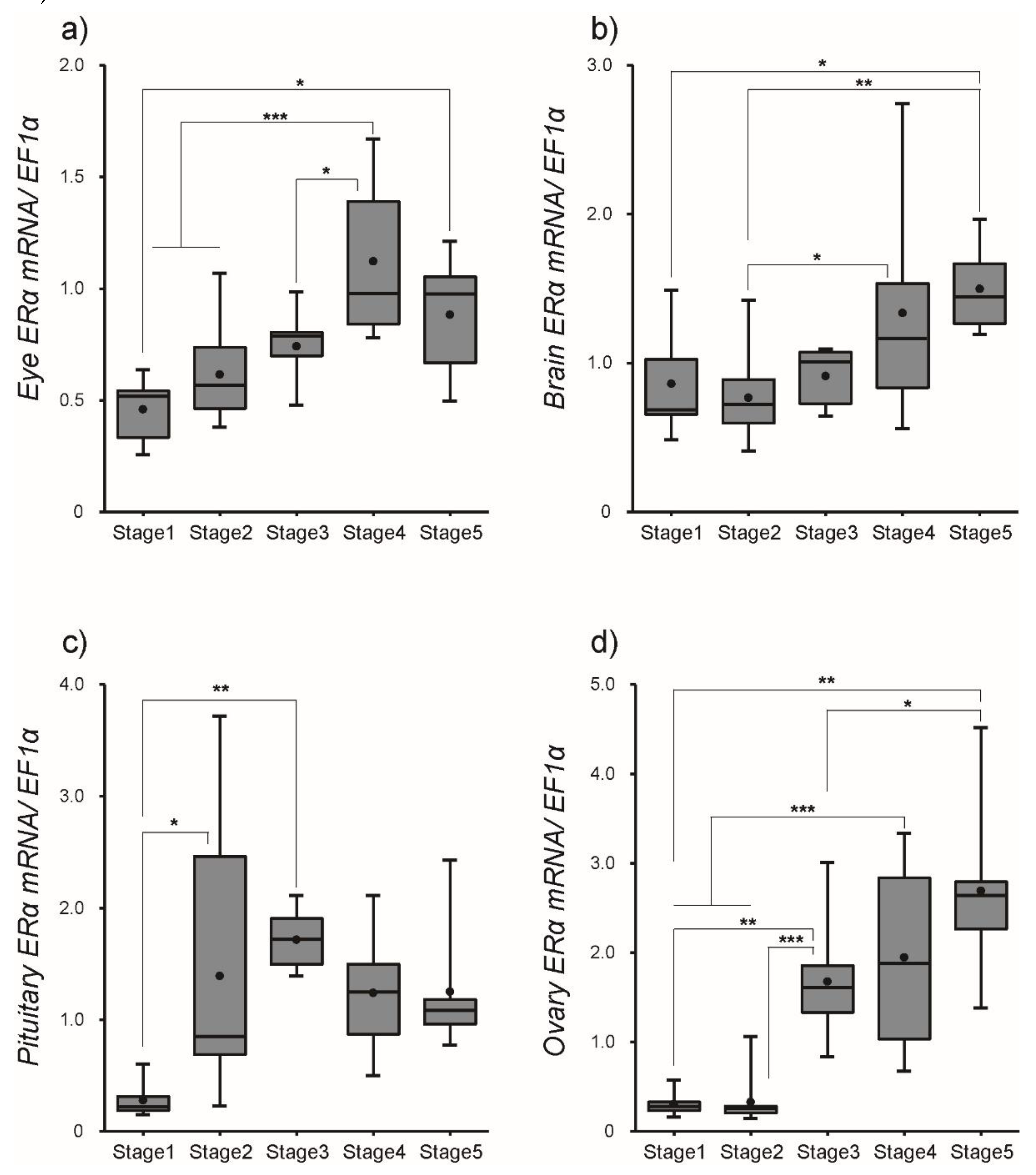
3.2. Changes in ER Transcript Levels within Tissues
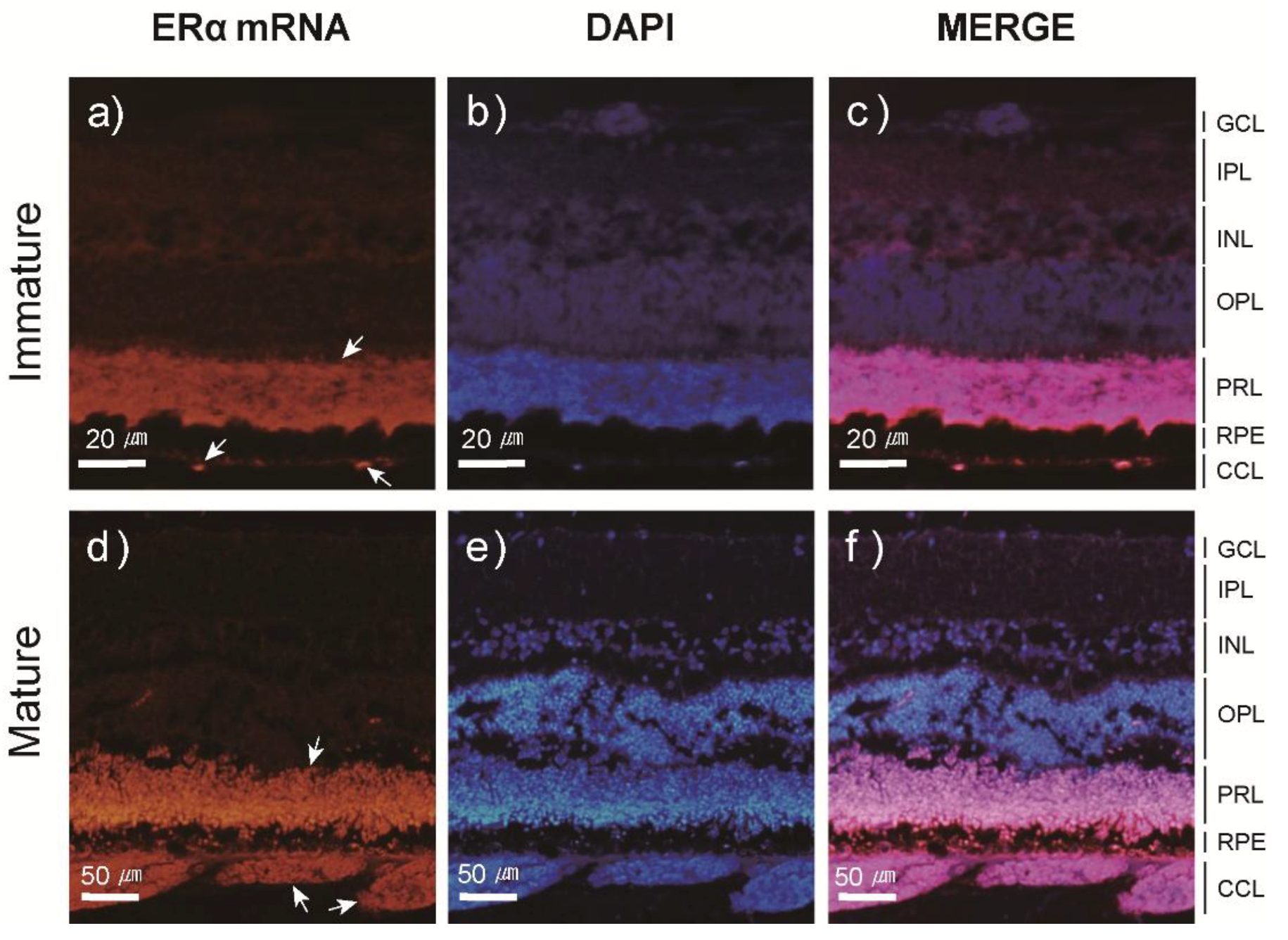
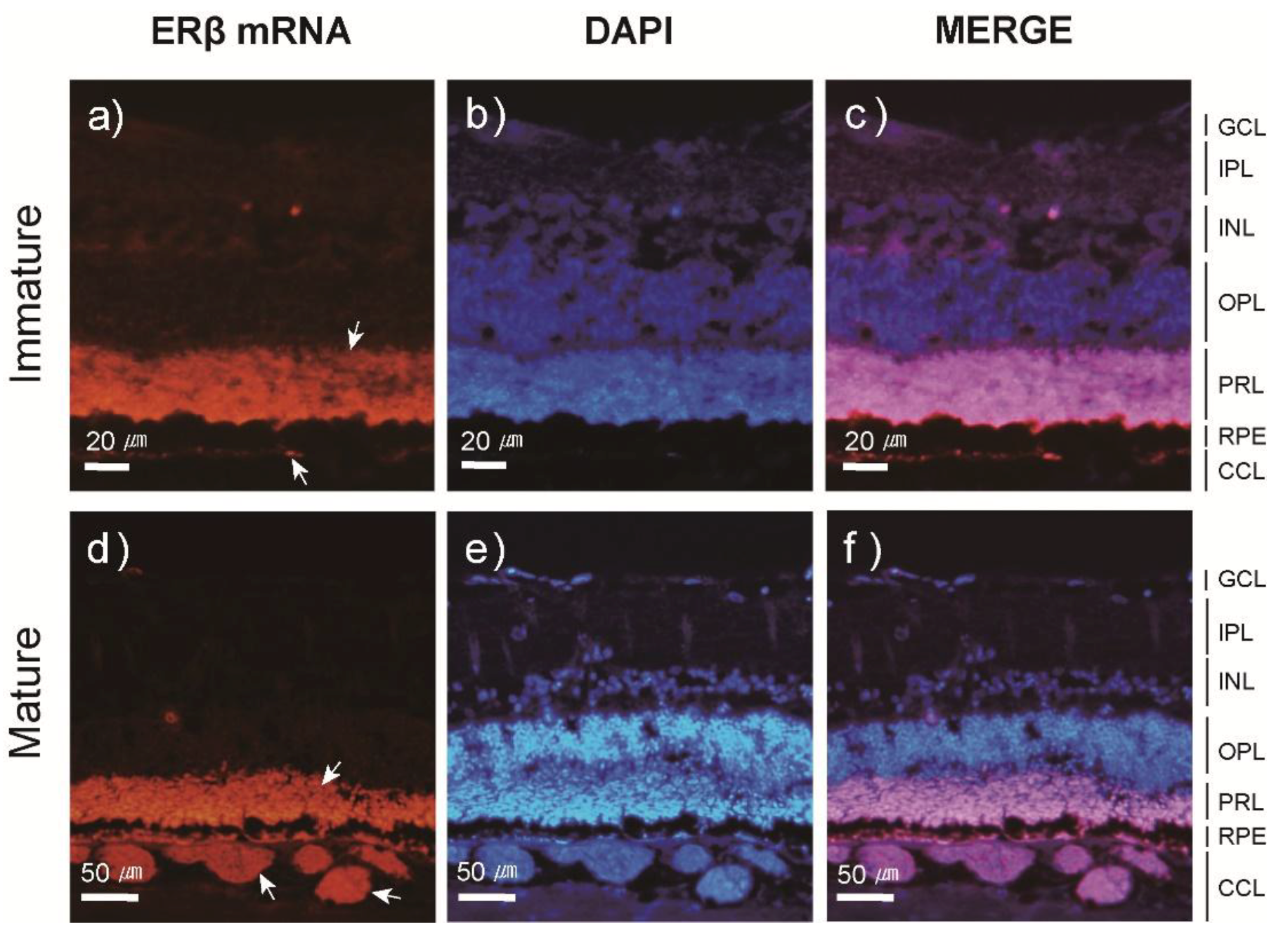
3.3. Localization and Expression Patterns of ER mRNA in the Japanese Eel Eye
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaube, R.; Rawat, A.; Joy, K.P. Molecular cloning and characterization of brain and ovarian cytochrome P450 aromatase genes in the catfish Heteropneustes fossilis: Sex, tissue and seasonal variation in, and effects of gonadotropin on gene expression. Gen. Comp. Endocrinol. 2015, 221, 120–133. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, X.C.; Lin, H.R. Seasonal changes of serum sex steroids concentration and aromatase activity of gonad and brain in red-spotted grouper (Epinephelus akaara). Anim. Rep. Sci. 2007, 99, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hojo, Y.; Hattori, T.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.; Mukai, H.; Morrison, J.H.; Janssen, W.G.M.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef]
- Holloway, C.C.; Clayton, D.F. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat. Neurosci. 2001, 4, 170–175. [Google Scholar] [CrossRef]
- Planey, S.L.; Kumar, R.; Arnott, J.A. Estrogen receptors (ERα versus ERβ): Friends or foes in human biology? J. Recept. Signal. Transduct. 2014, 34, 1–5. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Pakdel, F.; Féon, S.; Gac, F.L.; Menn, F.L.; Valotaire, Y. In vivo estrogen induction of hepatic estrogen receptor mRNA and correlation with vitellogenin mRNA in rainbow trout. Mol. Cell. Endocrinol. 1991, 75, 205–212. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Li, S.; Lin, M.; Shi, Y.; Sang, Q.; Liu, M.; Zhang, H.; Lu, D.; Meng, Z.; et al. Molecular colning, characterization and expression profiles of three estrogen receptors in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2011, 172, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Nagler, J.J.; Cavileer, T.D.; Verducci, J.S.; Schultz, I.R.; Hook, S.E.; Hayton, W.L. Estrogen receptor mRNA expression patterns in the liver and ovary of female rainbow trout over a complete reproductive cycle. Gen. Comp. Endocrinol. 2012, 178, 556–561. [Google Scholar] [CrossRef]
- Mu, W.J.; Wen, H.S.; Shi, D.; Yang, Y.P. Molecular cloning and expression analysis of estrogen receptor betas (ERβ1 and ERβ2) during gonad development in the Korean rockfish, Sevastes schlegeli. Gene 2013, 523, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lafont, A.G.; Rousseau, K.; Tomkiewicz, J.; Dufour, S. Three nuclear and two membrane estrogen receptors in basal teleosts, Anguilla sp.: Identification, evolutionary history and differential expression regulation. Gen. Comp. Endocrinol. 2016, 235, 177–191. [Google Scholar] [CrossRef]
- Nelson, E.R.; Wiehler, W.B.; Cole, W.C.; Habibi, H.R. Homologous regulationof estrogen receptor subtypes in goldfish (Carassius auratus). Col. Reprod. Dev. 2007, 74, 1105–1112. [Google Scholar] [CrossRef]
- Nagler, J.J.; Krisfalusi, M.; Cyr, D.G. Quantification of rainbow trout (Oncorhynchus mykiss) estrogen receptor-alpha messenger RNA and its expression in the ovary during the reproductive cycle. J. Mol. Endocrinol. 2000, 25, 243–251. [Google Scholar] [CrossRef]
- Tsukamoto, K. Discovery of the spawning area for Japanese eel. Nature 1992, 356, 789–791. [Google Scholar] [CrossRef]
- Aoyama, J. Life history and evolution of migration in catadromous eels (Genus Anguilla). Aqua-Bio. Sci. Monogr. 2009, 2, 1–42. [Google Scholar]
- Aoyama, J.; Watanabe, S.; Miller, M.J.; Mochioka, N.; Otake, T.; Yoshinaga, T.; Tsukamoto, K. Spawning sites of the Japanese eel in relation to oceanographic structure and the West Mariana Ridge. PLoS ONE 2014, 9, e88759. [Google Scholar] [CrossRef]
- Kagawa, H. Oogenesis in Teleost Fish. Aqua-Bio. Sci. Monogr. 2013, 6, 99–127. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hayakawa, Y.; Park, W.; Banda, A.; Yoshizaki, G.; Kumamaru, K.; Kagawa, H.; Kaki, H.; Nagaya, H.; Sohn, Y.C. Production of recombinant Japanese eel gonadotropins by baculovirus in silkworm larvae. Gen. Comp. Endocrinol. 2010, 167, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Liao, I.C.; Huang, Y.S.; He, J.T.; Chang, C.W.; Tzeng, W.N. Synchronous change of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 2003, 219, 783–796. [Google Scholar] [CrossRef]
- Beullens, K.; Eding, E.H.; Gilson, P.; Ollevier, F.; Komen, J.; Richter, C.J.J. Gonadal differentiation, intersexuality and sex ratios of European eel (Anguilla anguilla L.) maintained in captivity. Aquaculture 1997, 153, 135–150. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Relation of visual change to the onset of sexual maturation in the European eel Anguilla anguilla (L.). Depart. Zool. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Okamura, A.; Yamada, Y.; Yokouchi, K.; Horie, N.; Mikawa, N.; Utoh, T.; Tanaka, S.; Tsukamoto, K. A silvering index for the Japanese eel Anguilla japonica. Environ. Biol. Fishes 2007, 80, 77–89. [Google Scholar] [CrossRef]
- Okamura, A.; Yamada, Y.; Horie, N.; Utoh, T.; Mikawa, N.; Tanaka, S.; Tsukamoto, K. Effects of silvering state on induced maturation and spawning in wild female Japanese eel Anguilla japonica. Fish. Sci. 2008, 74, 642–648. [Google Scholar] [CrossRef]
- Pérez, L.; Peñaranda, D.S.; Dufour, S.; Baloche, S.; Palstra, A.P.; van den Thillart, G.E.; Asturiano, J.F. Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen. Comp. Endocrinol. 2011, 174, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Asahina, K.; Kambegawa, A.; Higashi, T. Development of a microtiter plate enzyme-linked immunosorbent assay for 17α,20β-21-trihydroxy-4-pregnen-3-one, a teleost gonadal steroid. Fish. Sci. 1995, 61, 491–494. [Google Scholar] [CrossRef]
- van den Thillart, G.E.; Dufour, S. Spawning Migration of the European Eel; Springer: New York, NY, USA, 2009; pp. 3–9. [Google Scholar]
- Mordenti, O.; Biase, A.D.; Bastone, G.; Sirri, R.; Zaccaroni, A.; Parmeggiani, A. Controlled reproduction in the wild European eel (Anguilla anguilla): Two populations compared. Aquacult. Int. 2013, 21, 1045–1063. [Google Scholar] [CrossRef]
- Lokman, P.K.; Vermeulen, G.J.; Lambert, J.G.D.; Young, G. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish. Physiol. Biochem. 1998, 19, 325–338. [Google Scholar] [CrossRef]
- Han, U.S.; Liao, I.C.; Tzeng, W.N.; Huang, Y.S.; Yu, Y.L. Serum estradiol-17β and testosterone levels during silvering in wild Japanese eel Anguilla japonica. Comp. Biochem. Physiol. 2003, 136, 912–920. [Google Scholar] [CrossRef]
- Lokman, P.M.; Young, G. In vitro biosynthesis of oestradiol-17β and 17α,20β-dihydroxy-4-pregnen-3-one by vitellogenic ovarian follicles from migrating New Zealand longfinned eels (Anguilla dieffendachii). Aquaculture 1995, 135, 17–26. [Google Scholar] [CrossRef]
- Sudo, R.; Tosaka, R.; Ijiri, S.; Adachi, S.; Aoyama, J.; Tsukamoto, K. 11-ketotestosterone synchronously induces oocyte development and silvering-related changes in the Japanese eel, Anguilla japonica. Zool. Sci. 2012, 29, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, O.; Emmanuele, P.; Casalini, A.; Lokman, P.M.; Zaccaroni, A.; Biase, A.D.; Parmeggiani, A. Effect of aromatable androgen (17-methyltestosterone) on induced maturation of silver European eels (Anguilla Anguilla): Oocyte performance and synchronization. Aquacult. Res. 2018, 49, 442–448. [Google Scholar] [CrossRef]
- Lokman, P.M.; George, K.A.N.; Divers, S.L.; Algie, M.; Young, G. 11-ketotestosterone and IGF-1 increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 2007, 133, 955–967. [Google Scholar] [CrossRef]
- Jeng, S.R.; Pasquier, J.; Yueh, W.S.; Chen, G.R.; Lee, Y.H.; Dufour, S.; Chang, C.F. Differential regulation of the expression of cytochrome P450 aromatase, estrogen and androgen receptor subtypes in the brain–pituitary–ovarian axis of the Japanese eel (Anguilla japonica) reveals steroid dependent and independent mechanisms. Gen. Comp. Endocrinol. 2012, 175, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Habibi, H.R. Molecular cloning of estrogen receptor α and expression pattern of estrogen receptor subtypes in male and female goldfish. Mol. Cell. Endocrinol. 2003, 204, 169–177. [Google Scholar] [CrossRef]
- Ding, W.; Cao, L.; Cao, Z.; Bing, X.; Zhao, F. Molecular characterization and expression profile of the estrogen receptor α gene during different reproductive phases in Monopterus albus. Nature 2016, 6, 27924. [Google Scholar] [CrossRef]
- Hiraki, T.; Takeuchi, A.; Tsumaki, T.; Zempo, B.; Kanda, S.; Oka, Y.; Nagahama, Y.; Okubo, K. Female-specific target sites for both oestrogen and androgen in the teleost brain. Proc. R. Soc. B 2012. [Google Scholar] [CrossRef]
- Lu, H.; Cui, Y.; Jiang, L.; Ge, W. Functional analysis of nuclear estrogen receptors (nERs) in zebrafish reproduction by genome editing approach. Endocrinology 2017, 158, 2292–2308. [Google Scholar] [CrossRef]
- Jansen, H.J.; Liem, M.; Jong-Raadsen, S.A.; Dufour, S.; Weltzien, F.A.; Swinkels, W.; Koelewijn, A.; Palstra, A.P.; Pelster, B.; Spaink, H.P.; et al. Rapid de novo assembly of the European eel genome from nanopore sequencing reads. Sci. Rep. 2017, 7, 7213. [Google Scholar] [CrossRef] [PubMed]
- Henkel, C.V.; Dirks, R.P.; de Wijze, D.L.; Minegishi, Y.; Aoyama, J.; Jansen, H.J.; Turner, B.; Knudsen, B.; Bundgaard, M.; Hvam, K.L.; et al. First draft genome sequence of the Japanese eel, Anguilla japonica. Gene 2012, 511, 195–201. [Google Scholar] [CrossRef]
- Beyer, C. Estrogen and the developing mammalian brain. Anat. Embryol. 1999, 199, 379–390. [Google Scholar] [CrossRef]
- Tchoudakova, A.; Pathak, S.; Callard, G.V. Molecular cloning of an estrogen receptor β subtype from the Goldfish. Gen. Comp. Endocrinol. 1999, 113, 388–400. [Google Scholar] [CrossRef]
- Begay, V.; Valotaire, Y.; Ravault, J.P.; Collin, J.P.; Falcon, J. Detection of estogen receptor mRNA in Trout pineal and retina: Estradiol-17β modulates melatonin production by cultured pineal photoreceptor cells. Gen. Comp. Endocrinol. 1994, 93, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, G.; Archer, A.; Gustafsson, J.A.; Lendahl, M.A. Level of 17β-estradiol receptors expressed in embryonic and adult Zebrafish following in vivo treatment of natural or synthetic ligands. PLoS ONE 2010, 5, e9678. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Mandai, M.; Suzuma, I.; Kobayashi, H.; Okinami, S. Expression of estrogen receptor in the choroidal neovascular membranes in highly myopic eyes. Retina 2002, 22, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Ogueta, S.B.; Schwartz, S.D.; Yamashita, C.K.; Farber, D.B. Estrogen receptor in the human eye: Influence of gender and age on gene expression. Ophthalmol. Visual. Sci. 1999, 40, 1906–1911. [Google Scholar]
- Kobayashi, K.; Kobayashi, H.; Ueda, M.; Honda, Y. Estrogen receptor expression in bovine and rat retina. Invest. Ophthalmol. Vis. Sci. 1998, 39, 2105–2110. [Google Scholar] [PubMed]
- Munaut, C.; Lambert, V.; Noël, A.; Frankenne, F.; Deprez, M.; Foidart, J.M.; Rakic, J.M. Presence of oestrogen receptor type β in human retina. Br. J. Ophthalmol. 2001, 85, 877–882. [Google Scholar] [CrossRef]


| Gene (Accession No.) | Primer | Sequence | Product Size (bp) |
|---|---|---|---|
| Ef1α (MH020210) | Forward | 5′-TCACCCTGGGAGTAAAGCAG-3′ | 222 |
| Reverse | 5′-TCCATCCCTTGAACCAGGAC-3′ | ||
| ERα (HM545084) | Forward | 5′-TGATCGCTTGGGCTAAGAAAGT-3′ | 209 |
| Reverse | 5′-GTCGAAAATCTCGGCCATGC-3′ | ||
| ERβ (AB003356) | Forward | 5′-AAGTACACCTGCTGGAGTGC-3′ | 220 |
| Reverse | 5′-AGGCACACATACTCCTCCCT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeon, J.-Y.; Hur, S.-P.; Kim, B.-H.; Byun, J.-H.; Kim, E.-S.; Lim, B.-S.; Lee, B.-I.; Kim, S.-K.; Takemura, A.; Kim, S.-J. Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation. Cells 2019, 8, 310. https://doi.org/10.3390/cells8040310
Hyeon J-Y, Hur S-P, Kim B-H, Byun J-H, Kim E-S, Lim B-S, Lee B-I, Kim S-K, Takemura A, Kim S-J. Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation. Cells. 2019; 8(4):310. https://doi.org/10.3390/cells8040310
Chicago/Turabian StyleHyeon, Ji-Yeon, Sung-Pyo Hur, Byeong-Hoon Kim, Jun-Hwan Byun, Eun-Su Kim, Bong-Soo Lim, Bae-Ik Lee, Shin-Kwon Kim, Akihiro Takemura, and Se-Jae Kim. 2019. "Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation" Cells 8, no. 4: 310. https://doi.org/10.3390/cells8040310
APA StyleHyeon, J.-Y., Hur, S.-P., Kim, B.-H., Byun, J.-H., Kim, E.-S., Lim, B.-S., Lee, B.-I., Kim, S.-K., Takemura, A., & Kim, S.-J. (2019). Involvement of Estrogen and Its Receptors in Morphological Changes in the Eyes of the Japanese Eel, Anguilla japonica, in the Process of Artificially-Induced Maturation. Cells, 8(4), 310. https://doi.org/10.3390/cells8040310






