Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment
Abstract
:1. Introduction
2. Cysteine Cathepsins: Structure, Function, and Regulation
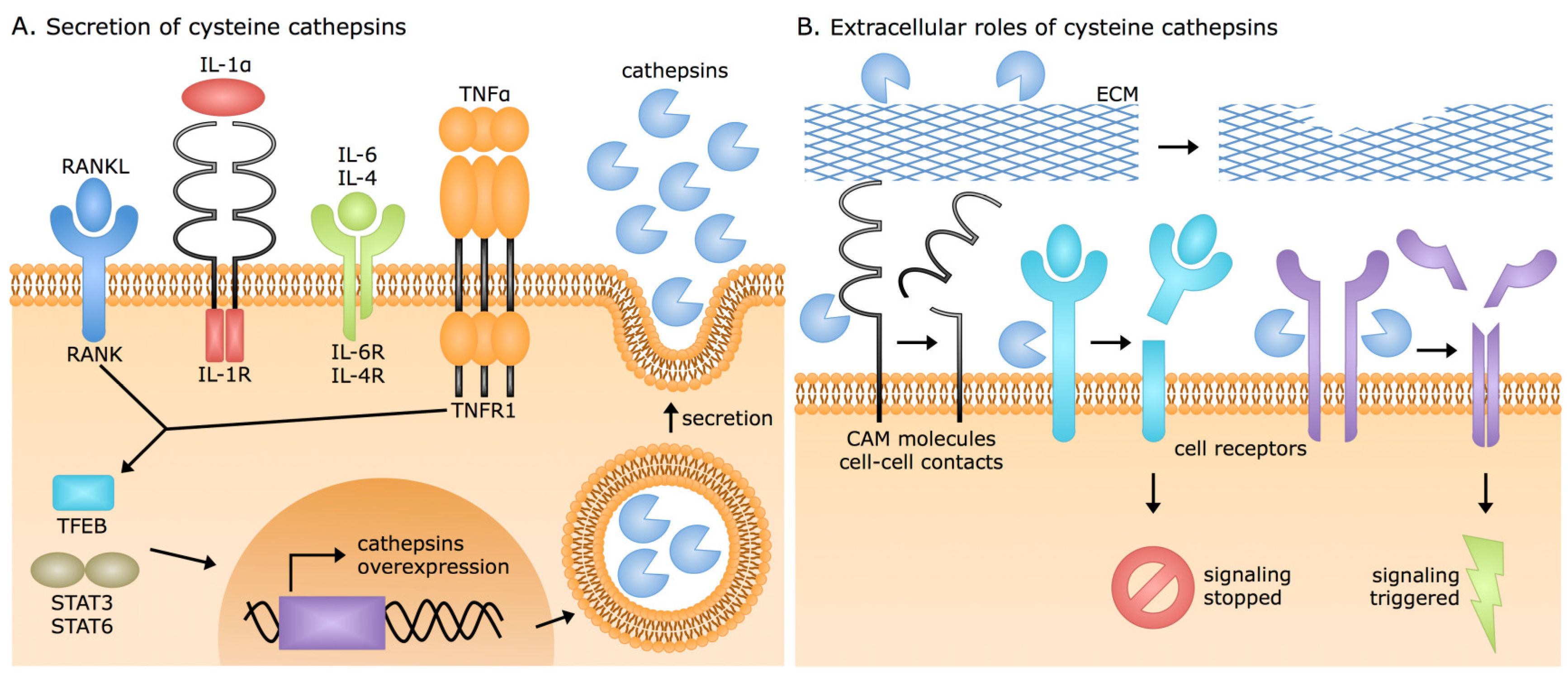
3. Extracellular Cysteine Cathepsin Origins
4. ECM Proteolysis and the Cathepsins
4.1. Cathepsins in Cancer
4.2. Cysteine Cathepsins and Tissue Remodeling
4.3. Cysteine Cathepsins in Inflammation
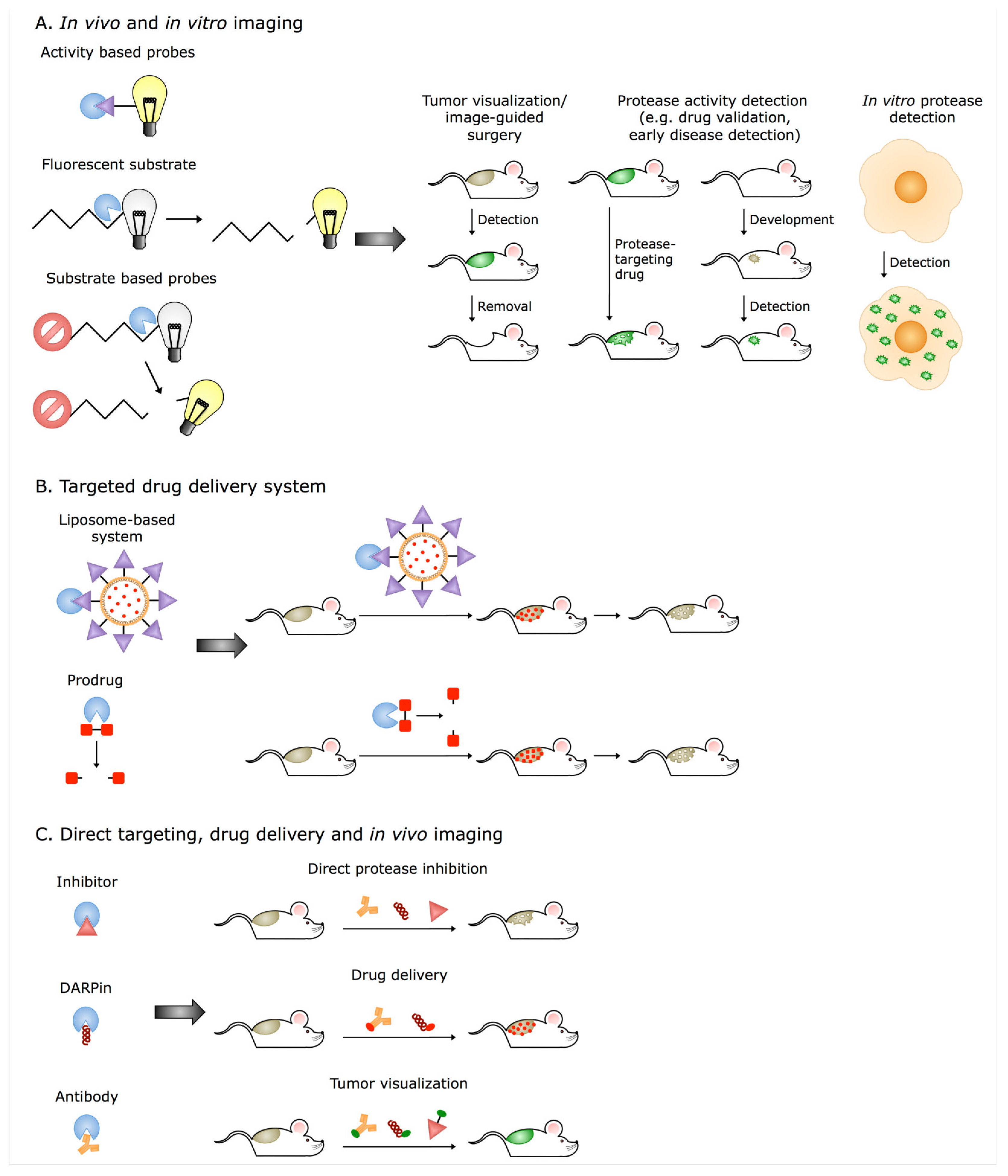
5. Extracellular Cathepsins and Their Translation into Clinical Applications
6. Concluding Remarks and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Turk, V.; Turk, B.; Turk, D. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 2001, 20, 4629–4633. [Google Scholar] [CrossRef]
- Rossi, A.; Deveraux, Q.; Turk, B.; Sali, A. Comprehensive search for cysteine cathepsins in the human genome. Biol. Chem. 2004, 385, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, R.; Vizovisek, M.; Turk, D.; Turk, B.; Fonovic, M. Protease cleavage site fingerprinting by label-free in-gel degradomics reveals pH-dependent specificity switch of legumain. EMBO J. 2017, 36, 2455–2465. [Google Scholar] [CrossRef]
- Vizovisek, M.; Vidmar, R.; Van Quickelberghe, E.; Impens, F.; Andjelkovic, U.; Sobotic, B.; Stoka, V.; Gevaert, K.; Turk, B.; Fonovic, M. Fast profiling of protease specificity reveals similar substrate specificities for cathepsins K, L and S. Proteomics 2015, 15, 2479–2490. [Google Scholar] [CrossRef]
- Biniossek, M.L.; Nagler, D.K.; Becker-Pauly, C.; Schilling, O. Proteomic identification of protease cleavage sites characterizes prime and non-prime specificity of cysteine cathepsins B, L, and S. J. Proteome Res. 2011, 10, 5363–5373. [Google Scholar] [CrossRef]
- Choe, Y.; Leonetti, F.; Greenbaum, D.C.; Lecaille, F.; Bogyo, M.; Bromme, D.; Ellman, J.A.; Craik, C.S. Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J. Biol. Chem. 2006, 281, 12824–12832. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef]
- Brix, K.; Linke, M.; Tepel, C.; Herzog, V. Cysteine proteinases mediate extracellular prohormone processing in the thyroid. Biol. Chem. 2001, 382, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Turk, D.; Turk, B. The Future of Cysteine Cathepsins in Disease Management. Trends Pharm. Sci. 2017, 38, 873–898. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef]
- Gocheva, V.; Joyce, J.A. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 2007, 6, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Wilson, S. Role of Cysteine Cathepsins in Extracellular Proteolysis. In Extracellular Matrix Degradation; Parks, W.C., Mecham, R.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–51. [Google Scholar] [CrossRef]
- Vizovisek, M.; Fonovic, M.; Turk, B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix Biol. 2019, 75–76, 141–159. [Google Scholar] [CrossRef]
- Repnik, U.; Starr, A.E.; Overall, C.M.; Turk, B. Cysteine Cathepsins Activate ELR Chemokines and Inactivate Non-ELR Chemokines. J. Biol. Chem. 2015, 290, 13800–13811. [Google Scholar] [CrossRef] [PubMed]
- Ainscough, J.S.; Macleod, T.; McGonagle, D.; Brakefield, R.; Baron, J.M.; Alase, A.; Wittmann, M.; Stacey, M. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36γ. Proc. Natl. Acad. Sci. USA 2017, 114, E2748–E2757. [Google Scholar] [CrossRef]
- Breznik, B.; Motaln, H.; Lah Turnsek, T. Proteases and cytokines as mediators of interactions between cancer and stromal cells in tumours. Biol. Chem. 2017, 398, 709–719. [Google Scholar] [CrossRef]
- Ohashi, K.; Naruto, M.; Nakaki, T.; Sano, E. Identification of interleukin-8 converting enzyme as cathepsin L. Biochim. Biophys. Acta 2003, 1649, 30–39. [Google Scholar] [CrossRef]
- Hira, V.V.; Verbovsek, U.; Breznik, B.; Srdic, M.; Novinec, M.; Kakar, H.; Wormer, J.; der Swaan, B.V.; Lenarcic, B.; Juliano, L.; et al. Cathepsin K cleavage of SDF-1alpha inhibits its chemotactic activity towards glioblastoma stem-like cells. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Sobotic, B.; Vizovisek, M.; Vidmar, R.; Van Damme, P.; Gocheva, V.; Joyce, J.A.; Gevaert, K.; Turk, V.; Turk, B.; Fonovic, M. Proteomic Identification of Cysteine Cathepsin Substrates Shed from the Surface of Cancer Cells. Mol. Cell. Proteom. 2015, 14, 2213–2228. [Google Scholar] [CrossRef]
- Clark, A.K.; Grist, J.; Al-Kashi, A.; Perretti, M.; Malcangio, M. Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 2012, 64, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Yip, P.K.; Malcangio, M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J. Neurosci. 2009, 29, 6945–6954. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.; Guncar, G.; Podobnik, M.; Turk, B. Revised definition of substrate binding sites of papain-like cysteine proteases. Biol. Chem. 1998, 379, 137–147. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef] [PubMed]
- Turk, B.; Turk, D.; Salvesen, G.S. Regulating cysteine protease activity: Essential role of protease inhibitors as guardians and regulators. Curr. Pharm. Des. 2002, 8, 1623–1637. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.H.; Dodds, R.A.; James, I.E.; Connor, J.R.; Debouck, C.; Richardson, S.; Lee-Rykaczewski, E.; Coleman, L.; Rieman, D.; Barthlow, R.; et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J. Biol. Chem. 1996, 271, 12511–12516. [Google Scholar] [CrossRef] [PubMed]
- Riese, R.J.; Mitchell, R.N.; Villadangos, J.A.; Shi, G.P.; Palmer, J.T.; Karp, E.R.; De Sanctis, G.T.; Ploegh, H.L.; Chapman, H.A. Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Investig. 1998, 101, 2351–2363. [Google Scholar] [CrossRef]
- Nakagawa, T.Y.; Brissette, W.H.; Lira, P.D.; Griffiths, R.J.; Petrushova, N.; Stock, J.; McNeish, J.D.; Eastman, S.E.; Howard, E.D.; Clarke, S.R.; et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity 1999, 10, 207–217. [Google Scholar] [CrossRef]
- Bromme, D.; Li, Z.; Barnes, M.; Mehler, E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry 1999, 38, 2377–2385. [Google Scholar] [CrossRef]
- Shi, G.P.; Villadangos, J.A.; Dranoff, G.; Small, C.; Gu, L.; Haley, K.J.; Riese, R.; Ploegh, H.L.; Chapman, H.A. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 1999, 10, 197–206. [Google Scholar] [CrossRef]
- Wex, T.; Buhling, F.; Wex, H.; Gunther, D.; Malfertheiner, P.; Weber, E.; Bromme, D. Human cathepsin W, a cysteine protease predominantly expressed in NK cells, is mainly localized in the endoplasmic reticulum. J. Immunol. 2001, 167, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Guncar, G.; Pungercic, G.; Klemencic, I.; Turk, V.; Turk, D. Crystal structure of MHC class II-associated p41 Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J. 1999, 18, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Turk, B.; Dolenc, I.; Turk, V.; Bieth, J.G. Kinetics of the pH-induced inactivation of human cathepsin L. Biochemistry 1993, 32, 375–380. [Google Scholar] [CrossRef]
- Turk, B.; Bieth, J.G.; Bjork, I.; Dolenc, I.; Turk, D.; Cimerman, N.; Kos, J.; Colic, A.; Stoka, V.; Turk, V. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol. Chem. Hoppe Seyler 1995, 376, 225–230. [Google Scholar] [CrossRef]
- Kirschke, H.; Wiederanders, B.; Bromme, D.; Rinne, A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem. J. 1989, 264, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Okamoto, K.; Wang, B.B.; Biroc, S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J. Biol. Chem. 1996, 271, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.C.; Nantes, I.L.; Rizzi, C.C.; Júdice, W.A.; Chagas, J.R.; Juliano, L.; Nader, H.B.; Tersariol, I.L. Cysteine proteinase activity regulation. A possible role of heparin and heparin-like glycosaminoglycans. J. Biol. Chem. 1999, 274, 30433–30438. [Google Scholar] [CrossRef]
- Caglic, D.; Pungercar, J.R.; Pejler, G.; Turk, V.; Turk, B. Glycosaminoglycans facilitate procathepsin B activation through disruption of propeptide-mature enzyme interactions. J. Biol. Chem. 2007, 282, 33076–33085. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yasuda, Y.; Li, W.; Bogyo, M.; Katz, N.; Gordon, R.E.; Fields, G.B.; Bromme, D. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J. Biol. Chem. 2004, 279, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Li, Z.; Greenbaum, D.; Bogyo, M.; Weber, E.; Brömme, D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J. Biol. Chem. 2004, 279, 36761–36770. [Google Scholar] [CrossRef]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef]
- Sukhova, G.K.; Shi, G.P.; Simon, D.I.; Chapman, H.A.; Libby, P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Investig. 1998, 102, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Jordans, S.; Jenko-Kokalj, S.; Kühl, N.M.; Tedelind, S.; Sendt, W.; Brömme, D.; Turk, D.; Brix, K. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009, 10, 23. [Google Scholar] [CrossRef]
- Godat, E.; Hervé-Grvépinet, V.; Veillard, F.; Lecaille, F.; Belghazi, M.; Brömme, D.; Lalmanach, G. Regulation of cathepsin K activity by hydrogen peroxide. Biol. Chem. 2008, 389, 1123–1126. [Google Scholar] [CrossRef]
- Rozhin, J.; Sameni, M.; Ziegler, G.; Sloane, B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994, 54, 6517–6525. [Google Scholar] [PubMed]
- Olson, O.C.; Joyce, J.A. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 2015, 15, 712–729. [Google Scholar] [CrossRef]
- Wu, H.; Du, Q.; Dai, Q.; Ge, J.; Cheng, X. Cysteine Protease Cathepsins in Atherosclerotic Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 25, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Gukovskaya, A.; Tseng, J.; Grinstein, S. Activation of vacuolar-type proton pumps by protein kinase C. Role in neutrophil pH regulation. J. Biol. Chem. 1992, 267, 22740–22746. [Google Scholar]
- Reddy, V.Y.; Zhang, Q.Y.; Weiss, S.J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 1995, 92, 3849–3853. [Google Scholar] [CrossRef]
- Dames, P.; Zimmermann, B.; Schmidt, R.; Rein, J.; Voss, M.; Schewe, B.; Walz, B.; Baumann, O. cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc. Natl. Acad. Sci. USA 2006, 103, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, X.; Xiang, M.X.; Alanne-Kinnunen, M.; Wang, J.A.; Chen, H.; He, A.; Sun, X.; Lin, Y.; Tang, T.T.; et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J. Clin. Investig. 2011, 121, 3564–3577. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Mandelin, J.; Li, T.F.; Salo, J.; Lassus, J.; Liljeström, M.; Hukkanen, M.; Takagi, M.; Virtanen, I.; Santavirta, S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002, 46, 953–960. [Google Scholar] [CrossRef]
- Naghavi, M.; John, R.; Naguib, S.; Siadaty, M.S.; Grasu, R.; Kurian, K.C.; van Winkle, W.B.; Soller, B.; Litovsky, S.; Madjid, M.; et al. pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis 2002, 164, 27–35. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Yan, D.; Wang, H.W.; Bowman, R.L.; Joyce, J.A. STAT3 and STAT6 Signaling Pathways Synergize to Promote Cathepsin Secretion from Macrophages via IRE1α Activation. Cell Rep. 2016, 16, 2914–2927. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, P.A.; Staniszewska, A.D.; Li, W.; Omidvar, N.; Kedjouar, B.; Turkson, J.; Poli, V.; Flavell, R.A.; Clarkson, R.W.; Watson, C.J. Stat3 controls lysosomal-mediated cell death in vivo. Nat. Cell Biol. 2011, 13, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Caglič, D.; Repnik, U.; Jedeszko, C.; Kosec, G.; Miniejew, C.; Kindermann, M.; Vasiljeva, O.; Turk, V.; Wendt, K.U.; Sloane, B.F.; et al. The proinflammatory cytokines interleukin-1α and tumor necrosis factor α promote the expression and secretion of proteolytically active cathepsin S from human chondrocytes. Biol. Chem. 2013, 394, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Cavallo-Medved, D.; Rudy, D.; Anbalagan, A.; Moin, K.; Sloane, B.F. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cell. Physiol. Biochem. 2010, 25, 315–324. [Google Scholar] [CrossRef]
- Troen, B.R. The regulation of cathepsin K gene expression. Ann. N. Y. Acad. Sci. 2006, 1068, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ruettger, A.; Schueler, S.; Mollenhauer, J.A.; Wiederanders, B. Cathepsins B, K, and L are regulated by a defined collagen type II peptide via activation of classical protein kinase C and p38 MAP kinase in articular chondrocytes. J. Biol. Chem. 2007, 283, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kondo, C.; Katunuma, N. An Active 32-kDa Cathepsin L Is Secreted Directly from HT 1080 Fibrosarcoma Cells and Not via Lysosomal Exocytosis. PLoS ONE 2015, 10, e0145067. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Webster, P.; Ortego, J.; Andrews, N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997, 137, 93–104. [Google Scholar] [CrossRef]
- Ichinose, S.; Usuda, J.; Hirata, T.; Inoue, T.; Ohtani, K.; Maehara, S.; Kubota, M.; Imai, K.; Tsunoda, Y.; Kuroiwa, Y.; et al. Lysosomal cathepsin initiates apoptosis, which is regulated by photodamage to Bcl-2 at mitochondria in photodynamic therapy using a novel photosensitizer, ATX-s10 (Na). Int. J. Oncol. 2006, 29, 349–355. [Google Scholar] [CrossRef]
- Chwieralski, C.E.; Welte, T.; Bühling, F. Cathepsin-regulated apoptosis. Apoptosis 2006, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Kuzuya, M.; Nakamura, K.; Di, Q.; Liu, Z.; Sasaki, T.; Kanda, S.; Jin, H.; Shi, G.P.; Murohara, T.; et al. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin alphanubeta3. Am. J. Pathol. 2006, 168, 685–694. [Google Scholar] [CrossRef]
- Obermajer, N.; Svajger, U.; Bogyo, M.; Jeras, M.; Kos, J. Maturation of dendritic cells depends on proteolytic cleavage by cathepsin X. J. Leukoc. Biol. 2008, 84, 1306–1315. [Google Scholar] [CrossRef]
- Obermajer, N.; Premzl, A.; Zavasnik Bergant, T.; Turk, B.; Kos, J. Carboxypeptidase cathepsin X mediates beta2-integrin-dependent adhesion of differentiated U-937 cells. Exp. Cell Res. 2006, 312, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.D.; Rizzi, C.C.; Nantes, I.L.; Stefe, I.; Turk, B.; Carmona, A.K.; Nader, H.B.; Juliano, L.; Tersariol, I.L. Cathepsin X binds to cell surface heparan sulfate proteoglycans. Arch. Biochem. Biophys. 2005, 436, 323–332. [Google Scholar] [CrossRef]
- Sloane, B.F.; Rozhin, J.; Johnson, K.; Taylor, H.; Crissman, J.D.; Honn, K.V. Cathepsin B: Association with plasma membrane in metastatic tumors. Proc. Natl. Acad. Sci. USA 1986, 83, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Finley, R.L.; Waisman, D.M.; Sloane, B.F. Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J. Biol. Chem. 2000, 275, 12806–12812. [Google Scholar] [CrossRef] [PubMed]
- Cavallo-Medved, D.; Dosescu, J.; Linebaugh, B.E.; Sameni, M.; Rudy, D.; Sloane, B.F. Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia 2003, 5, 507–519. [Google Scholar] [CrossRef]
- Campo, E.; Muñoz, J.; Miquel, R.; Palacín, A.; Cardesa, A.; Sloane, B.F.; Emmert-Buck, M.R. Cathepsin B expression in colorectal carcinomas correlates with tumor progression and shortened patient survival. Am. J. Pathol. 1994, 145, 301–309. [Google Scholar]
- Hazen, L.G.; Bleeker, F.E.; Lauritzen, B.; Bahns, S.; Song, J.; Jonker, A.; Van Driel, B.E.; Lyon, H.; Hansen, U.; Köhler, A.; et al. Comparative localization of cathepsin B protein and activity in colorectal cancer. J. Histochem. Cytochem. 2000, 48, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2009, 341, 126–140. [Google Scholar] [CrossRef]
- Ozbek, S.; Balasubramanian, P.G.; Chiquet-Ehrismann, R.; Tucker, R.P.; Adams, J.C. The evolution of extracellular matrix. Mol. Biol. Cell 2010, 21, 4300–4305. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Werb, Z. ECM and cell surface proteolysis: Regulating cellular ecology. Cell 1997, 91, 439–442. [Google Scholar] [CrossRef]
- Fonovic, M.; Turk, B. Cysteine cathepsins and extracellular matrix degradation. Biochim. Biophys. Acta 2014, 1840, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44–46, 1–6. [Google Scholar] [CrossRef]
- Liu, C.L.; Guo, J.; Zhang, X.; Sukhova, G.K.; Libby, P.; Shi, G.P. Cysteine protease cathepsins in cardiovascular disease: From basic research to clinical trials. Nat. Rev. Cardiol. 2018, 15, 351–370. [Google Scholar] [CrossRef]
- Nakao, S.; Zandi, S.; Sun, D.; Hafezi-Moghadam, A. Cathepsin B-mediated CD18 shedding regulates leukocyte recruitment from angiogenic vessels. FASEB J. 2018, 32, 143–154. [Google Scholar] [CrossRef]
- Mai, J.; Sameni, M.; Mikkelsen, T.; Sloane, B.F. Degradation of extracellular matrix protein tenascin-C by cathepsin B: An interaction involved in the progression of gliomas. Biol. Chem. 2002, 383, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Prudova, A.; Gocheva, V.; Auf dem Keller, U.; Eckhard, U.; Olson, O.C.; Akkari, L.; Butler, G.S.; Fortelny, N.; Lange, P.F.; Mark, J.C.; et al. TAILS N-Terminomics and Proteomics Show Protein Degradation Dominates over Proteolytic Processing by Cathepsins in Pancreatic Tumors. Cell Rep. 2016, 16, 1762–1773. [Google Scholar] [CrossRef]
- Guinec, N.; Dalet-Fumeron, V.; Pagano, M. “In vitro” study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin, and release of gelatinase activities from basement membrane fibronectin. Biol. Chem. Hoppe Seyler 1993, 374, 1135–1146. [Google Scholar]
- Gineyts, E.; Bonnet, N.; Bertholon, C.; Millet, M.; Pagnon-Minot, A.; Borel, O.; Geraci, S.; Bonnelye, E.; Croset, M.; Suhail, A.; et al. The C-Terminal Intact Forms of Periostin (iPTN) Are Surrogate Markers for Osteolytic Lesions in Experimental Breast Cancer Bone Metastasis. Calcif. Tissue Int. 2018, 103, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, I.; Linebaugh, B.E.; Koblinski, J.E.; Rudy, D.L.; Herroon, M.K.; Olive, M.B.; Sloane, B.F. Bone marrow-derived cathepsin K cleaves SPARC in bone metastasis. Am. J. Pathol. 2009, 175, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Kitamoto, S.; Yang, M.; Grubb, A.; Chapman, H.A.; Kalluri, R.; Shi, G.P. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J. Biol. Chem. 2006, 281, 6020–6029. [Google Scholar] [CrossRef]
- Du, X.; Chen, N.L.; Wong, A.; Craik, C.S.; Brömme, D. Elastin degradation by cathepsin V requires two exosites. J. Biol. Chem. 2013, 288, 34871–34881. [Google Scholar] [CrossRef]
- Fang, W.; He, A.; Xiang, M.X.; Lin, Y.; Wang, Y.; Li, J.; Yang, C.; Zhang, X.; Liu, C.L.; Sukhova, G.K.; et al. Cathepsin K-deficiency impairs mouse cardiac function after myocardial infarction. J. Mol. Cell. Cardiol. 2018, 127, 44–56. [Google Scholar] [CrossRef]
- Kehlet, S.N.; Bager, C.L.; Willumsen, N.; Dasgupta, B.; Brodmerkel, C.; Curran, M.; Brix, S.; Leeming, D.J.; Karsdal, M.A. Cathepsin-S degraded decorin are elevated in fibrotic lung disorders—Development and biological validation of a new serum biomarker. BMC Pulm. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Clark, A.K.; Yip, P.K.; Grist, J.; Gentry, C.; Staniland, A.A.; Marchand, F.; Dehvari, M.; Wotherspoon, G.; Winter, J.; Ullah, J.; et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 2007, 104, 10655–10660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lieu, T.; Barlow, N.; Metcalf, M.; Veldhuis, N.A.; Jensen, D.D.; Kocan, M.; Sostegni, S.; Haerteis, S.; Baraznenok, V.; et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. [Google Scholar] [CrossRef] [PubMed]
- Garsen, M.; Rops, A.L.; Dijkman, H.; Willemsen, B.; van Kuppevelt, T.H.; Russel, F.G.; Rabelink, T.J.; Berden, J.H.; Reinheckel, T.; van der Vlag, J. Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int. 2016, 90, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Mort, J.S.; Magny, M.C.; Lee, E.R. Cathepsin B: An alternative protease for the generation of an aggrecan ‘metalloproteinase’ cleavage neoepitope. Biochem. J. 1998, 335 Pt 3, 491–494. [Google Scholar] [CrossRef]
- Mort, J.S.; Beaudry, F.; Théroux, K.; Emmott, A.A.; Richard, H.; Fisher, W.D.; Lee, E.R.; Poole, A.R.; Laverty, S. Early cathepsin K degradation of type II collagen in vitro and in vivo in articular cartilage. Osteoarthr. Cartil. 2016, 24, 1461–1469. [Google Scholar] [CrossRef]
- Hou, W.S.; Li, Z.; Büttner, F.H.; Bartnik, E.; Brömme, D. Cleavage site specificity of cathepsin K toward cartilage proteoglycans and protease complex formation. Biol. Chem. 2003, 384, 891–897. [Google Scholar] [CrossRef]
- Atley, L.M.; Mort, J.S.; Lalumiere, M.; Eyre, D.R. Proteolysis of human bone collagen by cathepsin K: Characterization of the cleavage sites generating by cross-linked N-telopeptide neoepitope. Bone 2000, 26, 241–247. [Google Scholar] [CrossRef]
- Kafienah, W.; Brömme, D.; Buttle, D.J.; Croucher, L.J.; Hollander, A.P. Human cathepsin K cleaves native type I and II collagens at the N-terminal end of the triple helix. Biochem. J. 1998, 331 Pt 3, 727–732. [Google Scholar] [CrossRef]
- Baumgrass, R.; Williamson, M.K.; Price, P.A. Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B, D, L, H, and S. J. Bone Min. Res. 1997, 12, 447–455. [Google Scholar] [CrossRef]
- Bossard, M.J.; Tomaszek, T.A.; Thompson, S.K.; Amegadzie, B.Y.; Hanning, C.R.; Jones, C.; Kurdyla, J.T.; McNulty, D.E.; Drake, F.H.; Gowen, M.; et al. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J. Biol. Chem. 1996, 271, 12517–12524. [Google Scholar]
- Kubler, A.; Larsson, C.; Luna, B.; Andrade, B.B.; Amaral, E.P.; Urbanowski, M.; Orandle, M.; Bock, K.; Ammerman, N.C.; Cheung, L.S.; et al. Cathepsin K Contributes to Cavitation and Collagen Turnover in Pulmonary Tuberculosis. J. Infect. Dis. 2016, 213, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Sloane, B.F.; Dunn, J.R.; Honn, K.V. Lysosomal cathepsin B: Correlation with metastatic potential. Science 1981, 212, 1151–1153. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Papazoglou, A.; Kruger, A.; Brodoefel, H.; Korovin, M.; Deussing, J.; Augustin, N.; Nielsen, B.S.; Almholt, K.; Bogyo, M.; et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006, 66, 5242–5250. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Baruch, A.; Chehade, K.; Meyer-Morse, N.; Giraudo, E.; Tsai, F.Y.; Greenbaum, D.C.; Hager, J.H.; Bogyo, M.; Hanahan, D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004, 5, 443–453. [Google Scholar] [CrossRef]
- Ruffell, B.; Affara, N.I.; Cottone, L.; Junankar, S.; Johansson, M.; DeNardo, D.G.; Korets, L.; Reinheckel, T.; Sloane, B.F.; Bogyo, M.; et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013, 27, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L. Cystatins and cancer. Front. Biosci. 2009, 14, 463–474. [Google Scholar] [CrossRef]
- Akkari, L.; Gocheva, V.; Quick, M.L.; Kester, J.C.; Spencer, A.K.; Garfall, A.L.; Bowman, R.L.; Joyce, J.A. Combined deletion of cathepsin protease family members reveals compensatory mechanisms in cancer. Genes Dev. 2016, 30, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Schurigt, U.; Sachse, K.; Gajda, M.; Werner, F.; Muller, S.; Vasiljeva, O.; Schwinde, A.; Klemm, N.; Deussing, J.; et al. Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2497–2502. [Google Scholar] [CrossRef]
- Akkari, L.; Gocheva, V.; Kester, J.C.; Hunter, K.E.; Quick, M.L.; Sevenich, L.; Wang, H.W.; Peters, C.; Tang, L.H.; Klimstra, D.S.; et al. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev. 2014, 28, 2134–2150. [Google Scholar] [CrossRef]
- Dennemarker, J.; Lohmuller, T.; Mayerle, J.; Tacke, M.; Lerch, M.M.; Coussens, L.M.; Peters, C.; Reinheckel, T. Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene 2010, 29, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Zeng, W.; Ke, D.; Klimstra, D.; Reinheckel, T.; Peters, C.; Hanahan, D.; Joyce, J.A. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006, 20, 543–556. [Google Scholar] [CrossRef]
- Small, D.M.; Burden, R.E.; Jaworski, J.; Hegarty, S.M.; Spence, S.; Burrows, J.F.; McFarlane, C.; Kissenpfennig, A.; McCarthy, H.O.; Johnston, J.A.; et al. Cathepsin S from both tumor and tumor-associated cells promote cancer growth and neovascularization. Int. J. Cancer 2013, 133, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, O.; Korovin, M.; Gajda, M.; Brodoefel, H.; Bojic, L.; Kruger, A.; Schurigt, U.; Sevenich, L.; Turk, B.; Peters, C.; et al. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene 2008, 27, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Bengsch, F.; Buck, A.; Gunther, S.C.; Seiz, J.R.; Tacke, M.; Pfeifer, D.; von Elverfeldt, D.; Sevenich, L.; Hillebrand, L.E.; Kern, U.; et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene 2014, 33, 4474–4484. [Google Scholar] [CrossRef]
- Mitrovic, A.; Pecar Fonovic, U.; Kos, J. Cysteine cathepsins B and X promote epithelial-mesenchymal transition of tumor cells. Eur. J. Cell Biol. 2017, 96, 622–631. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, Y.; Guan, X.Y. Overexpression of cathepsin Z contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. PLoS ONE 2011, 6, e24967. [Google Scholar] [CrossRef]
- Tripathi, R.; Fiore, L.S.; Richards, D.L.; Yang, Y.; Liu, J.; Wang, C.; Plattner, R. Abl and Arg mediate cysteine cathepsin secretion to facilitate melanoma invasion and metastasis. Sci. Signal. 2018, 11, eaao0422. [Google Scholar] [CrossRef]
- Gopinathan, A.; Denicola, G.M.; Frese, K.K.; Cook, N.; Karreth, F.A.; Mayerle, J.; Lerch, M.M.; Reinheckel, T.; Tuveson, D.A. Cathepsin B promotes the progression of pancreatic ductal adenocarcinoma in mice. Gut 2012, 61, 877–884. [Google Scholar] [CrossRef]
- Maacha, S.; Hong, J.; von Lersner, A.; Zijlstra, A.; Belkhiri, A. AXL Mediates Esophageal Adenocarcinoma Cell Invasion through Regulation of Extracellular Acidification and Lysosome Trafficking. Neoplasia 2018, 20, 1008–1022. [Google Scholar] [CrossRef]
- Rempel, S.A.; Rosenblum, M.L.; Mikkelsen, T.; Yan, P.S.; Ellis, K.D.; Golembieski, W.A.; Sameni, M.; Rozhin, J.; Ziegler, G.; Sloane, B.F. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994, 54, 6027–6031. [Google Scholar] [PubMed]
- Breznik, B.; Limback, C.; Porcnik, A.; Blejec, A.; Krajnc, M.K.; Bosnjak, R.; Kos, J.; Van Noorden, C.J.F.; Lah, T.T. Localization patterns of cathepsins K and X and their predictive value in glioblastoma. Radiol. Oncol. 2018, 52, 433–442. [Google Scholar] [CrossRef]
- Breznik, B.; Limbaeck Stokin, C.; Kos, J.; Khurshed, M.; Hira, V.V.V.; Bosnjak, R.; Lah, T.T.; Van Noorden, C.J.F. Cysteine cathepsins B, X and K expression in peri-arteriolar glioblastoma stem cell niches. J. Mol. Histol. 2018, 49, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Wang, R.; Sun, A.; Wei, J.; Peng, K.; Dai, Q.; Yang, W.; Lin, Q. The E3 ubiquitin ligase NEDD4 mediates cell migration signaling of EGFR in lung cancer cells. Mol. Cancer 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Vasiljeva, O.; Turk, B. Dual contrasting roles of cysteine cathepsins in cancer progression: Apoptosis versus tumour invasion. Biochimie 2008, 90, 380–386. [Google Scholar] [CrossRef]
- Bruchard, M.; Mignot, G.; Derangere, V.; Chalmin, F.; Chevriaux, A.; Vegran, F.; Boireau, W.; Simon, B.; Ryffel, B.; Connat, J.L.; et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013, 19, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Butinar, M.; Prebanda, M.T.; Rajkovic, J.; Jeric, B.; Stoka, V.; Peters, C.; Reinheckel, T.; Kruger, A.; Turk, V.; Turk, B.; et al. Stefin B deficiency reduces tumor growth via sensitization of tumor cells to oxidative stress in a breast cancer model. Oncogene 2014, 33, 3392–3400. [Google Scholar] [CrossRef]
- Zavrsnik, J.; Butinar, M.; Prebanda, M.T.; Krajnc, A.; Vidmar, R.; Fonovic, M.; Grubb, A.; Turk, V.; Turk, B.; Vasiljeva, O. Cystatin C deficiency suppresses tumor growth in a breast cancer model through decreased proliferation of tumor cells. Oncotarget 2017, 8, 73793–73809. [Google Scholar] [CrossRef]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef]
- Fukuda, S.; Schmid-Schonbein, G.W. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc. Natl. Acad. Sci. USA 2003, 100, 13152–13157. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Kanasaki, K.; Gocheva, V.; Blum, G.; Harper, J.; Moses, M.A.; Shih, S.C.; Nagy, J.A.; Joyce, J.; Bogyo, M.; et al. VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009, 69, 4537–4544. [Google Scholar] [CrossRef]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Bay-Jensen, A.C.; Karsdal, M.A. Nidogen-1 Degraded by Cathepsin S can be Quantified in Serum and is Associated with Non-Small Cell Lung Cancer. Neoplasia 2017, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Dreier, L.; Bryant, R.A.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Veillard, F.; Saidi, A.; Burden, R.E.; Scott, C.J.; Gillet, L.; Lecaille, F.; Lalmanach, G. Cysteine cathepsins S and L modulate anti-angiogenic activities of human endostatin. J. Biol. Chem. 2011, 286, 37158–37167. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaissé, J.M. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Yamachika, E.; Nakanishi, M.; Ninomiya, T.; Nakatsuji, K.; Kobayashi, Y.; Fujii, T.; Iida, S. Cathepsin K inhibitor causes changes in crystallinity and crystal structure of newly-formed mandibular bone in rats. Br. J. Oral Maxillofac. Surg. 2018, 56, 732–738. [Google Scholar] [CrossRef]
- Sharma, V.; Panwar, P.; O’Donoghue, A.J.; Cui, H.; Guido, R.V.; Craik, C.S.; Brömme, D. Structural requirements for the collagenase and elastase activity of cathepsin K and its selective inhibition by an exosite inhibitor. Biochem. J. 2015, 465, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Aguda, A.H.; Panwar, P.; Du, X.; Nguyen, N.T.; Brayer, G.D.; Brömme, D. Structural basis of collagen fiber degradation by cathepsin K. Proc. Natl. Acad. Sci. USA 2014, 111, 17474–17479. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2017, 65, 30–44. [Google Scholar] [CrossRef]
- Yasuda, Y.; Kaleta, J.; Brömme, D. The role of cathepsins in osteoporosis and arthritis: Rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 973–993. [Google Scholar] [CrossRef]
- Wheater, G.; Elshahaly, M.; Tuck, S.P.; Datta, H.K.; van Laar, J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013, 11, 201. [Google Scholar] [CrossRef]
- Mort, J.S.; Recklies, A.D.; Poole, A.R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984, 27, 509–515. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kakegawa, H.; Narita, Y.; Hachiya, Y.; Hayakawa, T.; Kos, J.; Turk, V.; Katunuma, N. Significance of cathepsin B accumulation in synovial fluid of rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2001, 283, 334–339. [Google Scholar] [CrossRef]
- Pozgan, U.; Caglic, D.; Rozman, B.; Nagase, H.; Turk, V.; Turk, B. Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol. Chem. 2010, 391, 571–579. [Google Scholar] [CrossRef]
- Ben-Aderet, L.; Merquiol, E.; Fahham, D.; Kumar, A.; Reich, E.; Ben-Nun, Y.; Kandel, L.; Haze, A.; Liebergall, M.; Kosinska, M.K.; et al. Detecting cathepsin activity in human osteoarthritis via activity-based probes. Arthritis Res. Ther. 2015, 17, 69. [Google Scholar] [CrossRef]
- Weitoft, T.; Larsson, A.; Manivel, V.A.; Lysholm, J.; Knight, A.; Ronnelid, J. Cathepsin S and cathepsin L in serum and synovial fluid in rheumatoid arthritis with and without autoantibodies. Rheumatology 2015, 54, 1923–1928. [Google Scholar] [CrossRef]
- Ruge, T.; Sodergren, A.; Wallberg-Jonsson, S.; Larsson, A.; Arnlov, J. Circulating plasma levels of cathepsin S and L are not associated with disease severity in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 371–373. [Google Scholar] [CrossRef]
- Robert, L.; Robert, A.M.; Jacotot, B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis 1998, 140, 281–295. [Google Scholar] [CrossRef]
- Robert, L.; Robert, A.M.; Fülöp, T. Rapid increase in human life expectancy: Will it soon be limited by the aging of elastin? Biogerontology 2008, 9, 119–133. [Google Scholar] [CrossRef]
- Sukhova, G.K.; Zhang, Y.; Pan, J.H.; Wada, Y.; Yamamoto, T.; Naito, M.; Kodama, T.; Tsimikas, S.; Witztum, J.L.; Lu, M.L.; et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Investig. 2003, 111, 897–906. [Google Scholar] [CrossRef]
- Samokhin, A.O.; Wong, A.; Saftig, P.; Bromme, D. Role of cathepsin K in structural changes in brachiocephalic artery during progression of atherosclerosis in apoE-deficient mice. Atherosclerosis 2008, 200, 58–68. [Google Scholar] [CrossRef]
- Kitamoto, S.; Sukhova, G.K.; Sun, J.; Yang, M.; Libby, P.; Love, V.; Duramad, P.; Sun, C.; Zhang, Y.; Yang, X.; et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation 2007, 115, 2065–2075. [Google Scholar] [CrossRef]
- Fonovic, U.P.; Jevnikar, Z.; Kos, J. Cathepsin S generates soluble CX3CL1 (fractalkine) in vascular smooth muscle cells. Biol. Chem. 2013, 394, 1349–1352. [Google Scholar] [CrossRef]
- Zhao, C.F.; Herrington, D.M. The function of cathepsins B, D, and X in atherosclerosis. Am. J. Cardiovasc. Dis. 2016, 6, 163–170. [Google Scholar]
- Tohda, C.; Tohda, M. Extracellular cathepsin L stimulates axonal growth in neurons. BMC Res. Notes 2017, 10, 613. [Google Scholar] [CrossRef]
- Tran, A.P.; Sundar, S.; Yu, M.; Lang, B.T.; Silver, J. Modulation of Receptor Protein Tyrosine Phosphatase Sigma Increases Chondroitin Sulfate Proteoglycan Degradation through Cathepsin B Secretion to Enhance Axon Outgrowth. J. Neurosci. 2018, 38, 5399–5414. [Google Scholar] [CrossRef]
- Saini, M.G.; Bix, G.J. Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res. 2012, 1438, 65–74. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef]
- Taggart, C.; Mall, M.A.; Lalmanach, G.; Cataldo, D.; Ludwig, A.; Janciauskiene, S.; Heath, N.; Meiners, S.; Overall, C.M.; Schultz, C.; et al. Protean proteases: At the cutting edge of lung diseases. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Perdereau, C.; Godat, E.; Maurel, M.C.; Hazouard, E.; Diot, E.; Lalmanach, G. Cysteine cathepsins in human silicotic bronchoalveolar lavage fluids. Biochim. Biophys. Acta 2006, 1762, 351–356. [Google Scholar] [CrossRef]
- Dongre, A.; Clements, D.; Fisher, A.J.; Johnson, S.R. Cathepsin K in Lymphangioleiomyomatosis: LAM Cell-Fibroblast Interactions Enhance Protease Activity by Extracellular Acidification. Am. J. Pathol. 2017, 187, 1750–1762. [Google Scholar] [CrossRef]
- Squeglia, F.; Ruggiero, A.; Berisio, R. Collagen degradation in tuberculosis pathogenesis: The biochemical consequences of hosting an undesired guest. Biochem. J. 2018, 475, 3123–3140. [Google Scholar] [CrossRef]
- Srivastava, M.; Steinwede, K.; Kiviranta, R.; Morko, J.; Hoymann, H.G.; Langer, F.; Buhling, F.; Welte, T.; Maus, U.A. Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis. Respir. Res. 2008, 9, 54. [Google Scholar] [CrossRef]
- Buhling, F.; Rocken, C.; Brasch, F.; Hartig, R.; Yasuda, Y.; Saftig, P.; Bromme, D.; Welte, T. Pivotal role of cathepsin K in lung fibrosis. Am. J. Pathol. 2004, 164, 2203–2216. [Google Scholar] [CrossRef]
- Kasabova, M.; Villeret, B.; Gombault, A.; Lecaille, F.; Reinheckel, T.; Marchand-Adam, S.; Couillin, I.; Lalmanach, G. Discordance in cathepsin B and cystatin C expressions in bronchoalveolar fluids between murine bleomycin-induced fibrosis and human idiopathic fibrosis. Respir. Res. 2016, 17, 118. [Google Scholar] [CrossRef]
- Small, D.M.; Brown, R.R.; Doherty, D.F.; Abladey, A.; Zhou-Suckow, Z.; Delaney, R.J.; Kerrigan, L.; Dougan, C.M.; Borensztajn, K.S.; Holsinger, L.; et al. Targeting of Cathepsin S Reduces Cystic Fibrosis-like Lung Disease. Eur. Respir. J. 2019. [Google Scholar] [CrossRef]
- Elmariah, S.B.; Reddy, V.B.; Lerner, E.A. Cathepsin S signals via PAR2 and generates a novel tethered ligand receptor agonist. PLoS ONE 2014, 9, e99702. [Google Scholar] [CrossRef]
- Taleb, S.; Cancello, R.; Clément, K.; Lacasa, D. Cathepsin s promotes human preadipocyte differentiation: Possible involvement of fibronectin degradation. Endocrinology 2006, 147, 4950–4959. [Google Scholar] [CrossRef]
- Douglas, S.A.; Lamothe, S.E.; Singleton, T.S.; Averett, R.D.; Platt, M.O. Human cathepsins K, L, and S: Related proteases, but unique fibrinolytic activity. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1925–1932. [Google Scholar] [CrossRef]
- Ferrall-Fairbanks, M.C.; West, D.M.; Douglas, S.A.; Averett, R.D.; Platt, M.O. Computational predictions of cysteine cathepsin-mediated fibrinogen proteolysis. Protein Sci. 2018, 27, 714–724. [Google Scholar] [CrossRef]
- Ogasawara, S.; Cheng, X.W.; Inoue, A.; Hu, L.; Piao, L.; Yu, C.; Goto, H.; Xu, W.; Zhao, G.; Lei, Y.; et al. Cathepsin K activity controls cardiotoxin-induced skeletal muscle repair in mice. J. Cachexia Sarcopenia Muscle 2017, 9, 160–175. [Google Scholar] [CrossRef]
- Lechner, A.M.; Assfalg-Machleidt, I.; Zahler, S.; Stoeckelhuber, M.; Machleidt, W.; Jochum, M.; Nagler, D.K. RGD-dependent binding of procathepsin X to integrin alphavbeta3 mediates cell-adhesive properties. J. Biol. Chem. 2006, 281, 39588–39597. [Google Scholar] [CrossRef]
- Sina, C.; Lipinski, S.; Gavrilova, O.; Aden, K.; Rehman, A.; Till, A.; Rittger, A.; Podschun, R.; Meyer-Hoffert, U.; Haesler, R.; et al. Extracellular cathepsin K exerts antimicrobial activity and is protective against chronic intestinal inflammation in mice. Gut 2013, 62, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, C.; Tran, N.; Paulus, P.; Ellinghaus, P.; Eble, J.A.; Zacharowski, K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol. Med. 2011, 17, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Libert, C. Inflammation: A nervous connection. Nature 2003, 421, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Yona, S.; Kim, K.W.; Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 2013, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Pislar, A.; Kos, J. Cysteine cathepsins in neurological disorders. Mol. Neurobiol. 2014, 49, 1017–1030. [Google Scholar] [CrossRef]
- Cocchiaro, P.; De Pasquale, V.; Della Morte, R.; Tafuri, S.; Avallone, L.; Pizard, A.; Moles, A.; Pavone, L.M. The Multifaceted Role of the Lysosomal Protease Cathepsins in Kidney Disease. Front. Cell Dev. Biol. 2017, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Sena, B.F.; Figueiredo, J.L.; Aikawa, E. Cathepsin S As an Inhibitor of Cardiovascular Inflammation and Calcification in Chronic Kidney Disease. Front. Cardiovasc. Med. 2017, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, R.; Morko, J.; Uusitalo, H.; Aro, H.T.; Vuorio, E.; Rantakokko, J. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J. Bone Miner. Res. 2001, 16, 1444–1452. [Google Scholar] [CrossRef]
- Gowen, M.; Lazner, F.; Dodds, R.; Kapadia, R.; Feild, J.; Tavaria, M.; Bertoncello, I.; Drake, F.; Zavarselk, S.; Tellis, I. Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J. Bone Miner. Res. 1999, 14, 1654–1663. [Google Scholar] [CrossRef]
- Mullard, A. Merck &Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov. 2016, 15, 669. [Google Scholar] [CrossRef]
- Bromme, D.; Panwar, P.; Turan, S. Cathepsin K osteoporosis trials, pycnodysostosis and mouse deficiency models: Commonalities and differences. Expert Opin. Drug Discov. 2016, 11, 457–472. [Google Scholar] [CrossRef]
- Panwar, P.; Soe, K.; Guido, R.V.; Bueno, R.V.; Delaisse, J.M.; Bromme, D. A novel approach to inhibit bone resorption: Exosite inhibitors against cathepsin K. Br. J. Pharmacol. 2016, 173, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Xue, L.; Soe, K.; Srivastava, K.; Law, S.; Delaisse, J.M.; Bromme, D. An Ectosteric Inhibitor of Cathepsin K Inhibits Bone Resorption in Ovariectomized Mice. J. Bone Miner. Res. 2017, 32, 2415–2430. [Google Scholar] [CrossRef]
- Burden, R.E.; Gormley, J.A.; Jaquin, T.J.; Small, D.M.; Quinn, D.J.; Hegarty, S.M.; Ward, C.; Walker, B.; Johnston, J.A.; Olwill, S.A.; et al. Antibody-mediated inhibition of cathepsin S blocks colorectal tumor invasion and angiogenesis. Clin. Cancer Res. 2009, 15, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.E.; Gormley, J.A.; Kuehn, D.; Ward, C.; Kwok, H.F.; Gazdoiu, M.; McClurg, A.; Jaquin, T.J.; Johnston, J.A.; Scott, C.J.; et al. Inhibition of Cathepsin S by Fsn0503 enhances the efficacy of chemotherapy in colorectal carcinomas. Biochimie 2012, 94, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sanman, L.E.; Bogyo, M. Activity-based profiling of proteases. Annu. Rev. Biochem. 2014, 83, 249–273. [Google Scholar] [CrossRef]
- Withana, N.P.; Saito, T.; Ma, X.; Garland, M.; Liu, C.; Kosuge, H.; Amsallem, M.; Verdoes, M.; Ofori, L.O.; Fischbein, M.; et al. Dual-Modality Activity-Based Probes as Molecular Imaging Agents for Vascular Inflammation. J. Nucl. Med. 2016, 57, 1583–1590. [Google Scholar] [CrossRef]
- Walker, E.; Mann, M.; Honda, K.; Vidimos, A.; Schluchter, M.D.; Straight, B.; Bogyo, M.; Popkin, D.; Basilion, J.P. Rapid visualization of nonmelanoma skin cancer. J. Am. Acad. Derm. 2017, 76, 209–216.e9. [Google Scholar] [CrossRef]
- Withana, N.P.; Ma, X.; McGuire, H.M.; Verdoes, M.; van der Linden, W.A.; Ofori, L.O.; Zhang, R.; Li, H.; Sanman, L.E.; Wei, K.; et al. Non-invasive Imaging of Idiopathic Pulmonary Fibrosis Using Cathepsin Protease Probes. Sci. Rep. 2016, 6, 19755. [Google Scholar] [CrossRef]
- Watzke, A.; Kosec, G.; Kindermann, M.; Jeske, V.; Nestler, H.P.; Turk, V.; Turk, B.; Wendt, K.U. Selective activity-based probes for cysteine cathepsins. Angew. Chem. Int. Ed. Engl. 2008, 47, 406–409. [Google Scholar] [CrossRef]
- Hu, H.Y.; Vats, D.; Vizovisek, M.; Kramer, L.; Germanier, C.; Wendt, K.U.; Rudin, M.; Turk, B.; Plettenburg, O.; Schultz, C. In vivo imaging of mouse tumors by a lipidated cathepsin S substrate. Angew. Chem. Int. Ed. Engl. 2014, 53, 7669–7673. [Google Scholar] [CrossRef]
- Kramer, L.; Renko, M.; Zavrsnik, J.; Turk, D.; Seeger, M.A.; Vasiljeva, O.; Grutter, M.G.; Turk, V.; Turk, B. Non-invasive in vivo imaging of tumour-associated cathepsin B by a highly selective inhibitory DARPin. Theranostics 2017, 7, 2806–2821. [Google Scholar] [CrossRef]
- Garland, M.; Yim, J.J.; Bogyo, M. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef]
- Fang, Y.; Du, F.; Xu, Y.; Meng, H.; Huang, J.; Zhang, X.; Lu, W.; Liu, S.; Yu, J. Enhanced cellular uptake and intracellular drug controlled release of VESylated gemcitabine prodrug nanocapsules. Colloids Surf. B Biointerfaces 2015, 128, 357–362. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, K.; Wang, H.; Liu, Y.; Bao, B.; Fang, Y.; Zhang, X.; Lu, W. Design, Synthesis, and Biological Evaluation of New Cathepsin B-Sensitive Camptothecin Nanoparticles Equipped with a Novel Multifuctional Linker. Bioconj. Chem. 2016, 27, 1267–1275. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Klimpel, D.; Schaschke, N.; Mikac, U.; Vizovisek, M.; Fonovic, M.; Turk, V.; Turk, B.; Vasiljeva, O. Selective targeting of tumor and stromal cells by a nanocarrier system displaying lipidated cathepsin B inhibitor. Angew. Chem. Int. Ed. Engl. 2014, 53, 10077–10081. [Google Scholar] [CrossRef]
- Vizovisek, M.; Vidmar, R.; Drag, M.; Fonovic, M.; Salvesen, G.S.; Turk, B. Protease Specificity: Towards In Vivo Imaging Applications and Biomarker Discovery. Trends Biochem. Sci. 2018, 43, 829–844. [Google Scholar] [CrossRef]
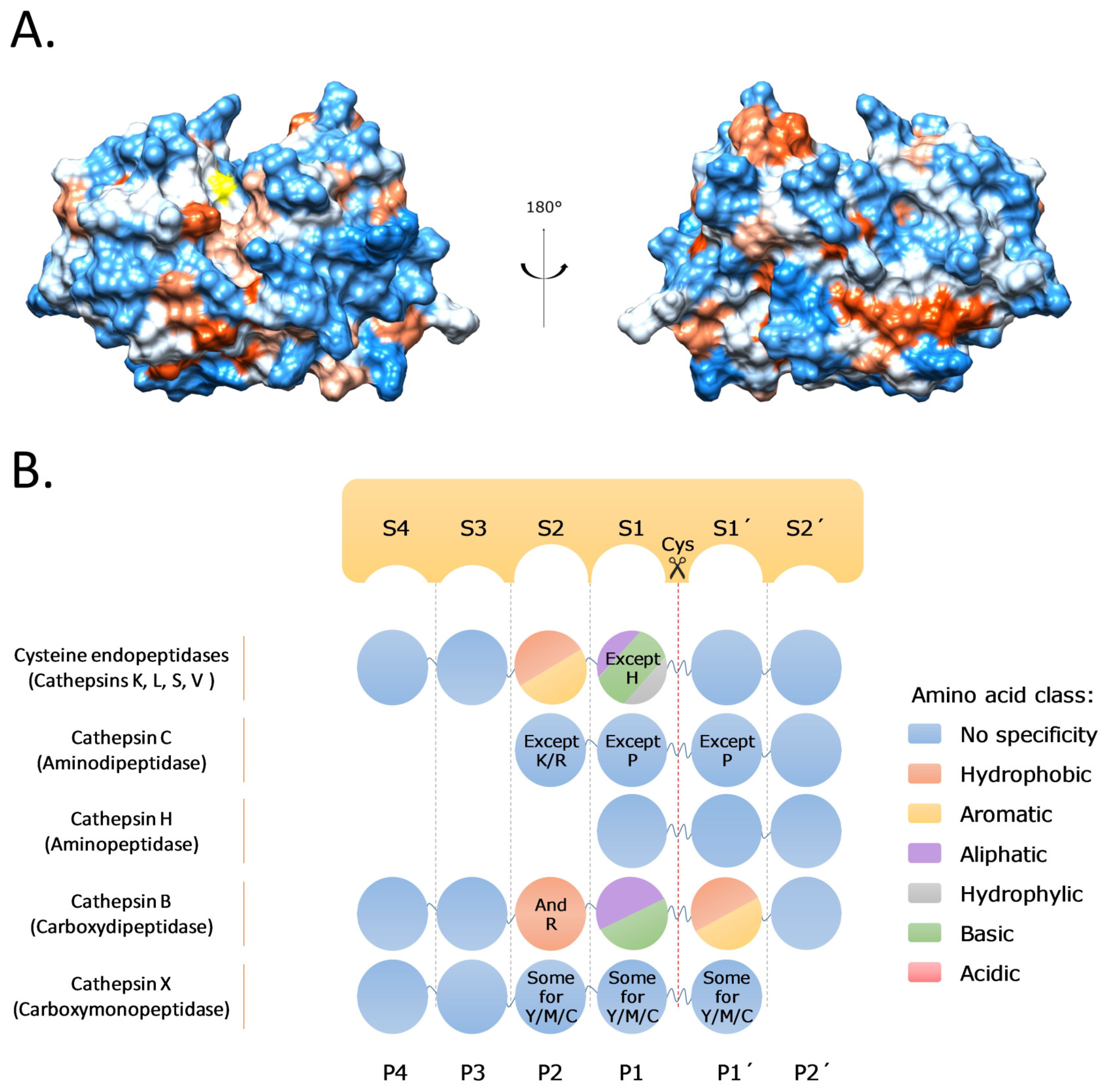
| Cysteine Cathepsins. | Gene Name | Peptidase Activity | Expression |
|---|---|---|---|
| Cathepsin B | CTSB | Carboxydipeptidase, Endopeptidase | Ubiquitous |
| Cathepsin C | CTSC | Aminodipeptidase | Ubiquitous |
| Cathepsin F | CTSF | Endopeptidase | Ubiquitous |
| Cathepsin H | CTSH | Aminopeptidase, Endopeptidase | Ubiquitous |
| Cathepsin K | CTSK | Endopeptidase | Osteoclasts [27] |
| Cathepsin L | CTSL | Endopeptidase | Ubiquitous |
| Cathepsin O | CTSO | Unknown | Ubiquitous |
| Cathepsin S | CTSS | Endopeptidase | Antigen-presenting cells (e.g., dendritic cells, B-cells) [28,29] |
| Cathepsin V | CTSV | Endopeptidase | Thymus, testis [30,31] |
| Cathepsin W | CTSW | Unknown | Natural killer cells, cytotoxic T cells [32] |
| Cathepsin Z (Cathepsin X) | CTSZ | Carboxymonopeptidase | Ubiquitous |
| Disease | Cathepsins Involved | Cleaved Targets | Selected References |
|---|---|---|---|
| Angiogenesis/Leukocyte recruitment | B, K, L, S | ELR (glutamate-leucin-arginin motif) chemokines/non-ELR chemokines, CD18 | [16,85] |
| Cancer | B, K, L, S, X | Tenascin-C, nidogen-1, fibronectin, osteonectin, laminin, periostin, collagen IV, general degradation | [86,87,88,89,90,91] |
| Cardiovascular and kidney diseases (e.g., atherosclerosis, abdominal aortic aneurysm, chronic kidney disease) | K, L, S, V | Elastin, CX3CL, heparanase, collagen I (catK: Gly61-Lys62, Arg144-Gly145, Gln189-Gly190) | [41,92,93] |
| Lung fibrosis | S | Decorin | [94] |
| Neuroinflammation/hyperalgesia | S | CX3CL1, PAR2 | [95,96,97] |
| Osteoarthritis and rheumatoid arthritis | K, B, L, S | Collagen II (catK: Gly61-Lys62, Arg144-Gly145, Gln189-Gly190), aggrecan (catB: Asn341-Phe342, Gly344-Val345, catL: Gly344-Val345) | [98,99,100] |
| Osteoporosis | K, B, L, S, H | Collagen I (catK: Gly61-Lys62, Arg144-Gly145, Gln189-Gly190), osteonectin, osteocalcin (catB: Arg44-Phe45, catL: Gly7-Ala8, Arg43-Arg44, catS: Gly7-Ala8) | [101,102,103,104] |
| Tuberculosis | K | Collagen I (casK: Gly61-Lys62, Arg144-Gly145, Gln189-Gly190) | [105] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. https://doi.org/10.3390/cells8030264
Vidak E, Javoršek U, Vizovišek M, Turk B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells. 2019; 8(3):264. https://doi.org/10.3390/cells8030264
Chicago/Turabian StyleVidak, Eva, Urban Javoršek, Matej Vizovišek, and Boris Turk. 2019. "Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment" Cells 8, no. 3: 264. https://doi.org/10.3390/cells8030264
APA StyleVidak, E., Javoršek, U., Vizovišek, M., & Turk, B. (2019). Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells, 8(3), 264. https://doi.org/10.3390/cells8030264





