Megakaryocytes in Bone Metastasis: Protection or Progression?
Abstract
:1. Introduction
2. Megakaryocytes: Relationship with the Bone Marrow Extracellular Matrix
3. Megakaryocyte Maturation and Platelet Production
3.1. Role of Tumour Suppressor p53 and Megakaryopoiesis
3.2. Platelets and Cancer
4. Role of Megakaryocytes in Bone Homeostasis
5. Role of MKs in Bone Metastasis
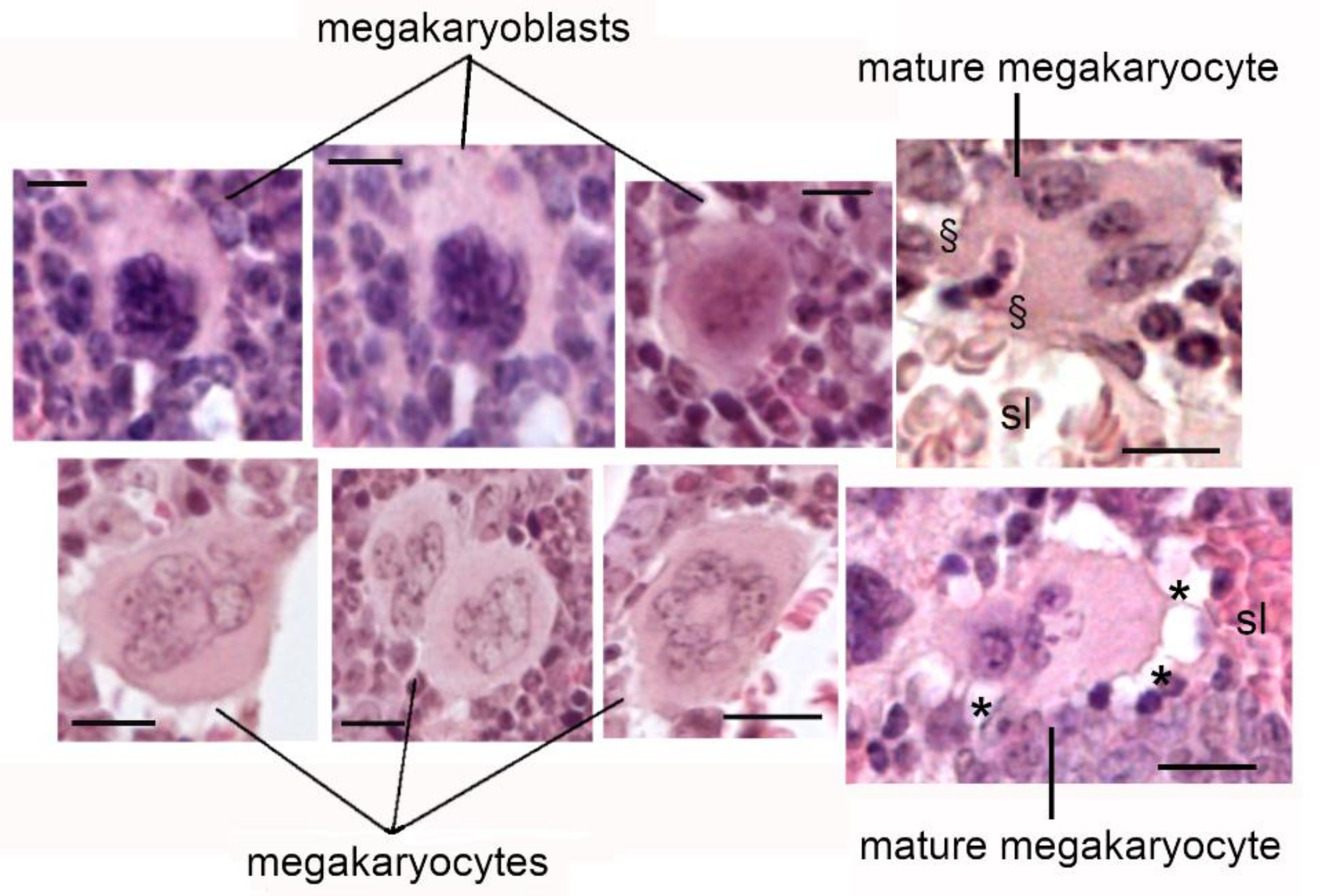
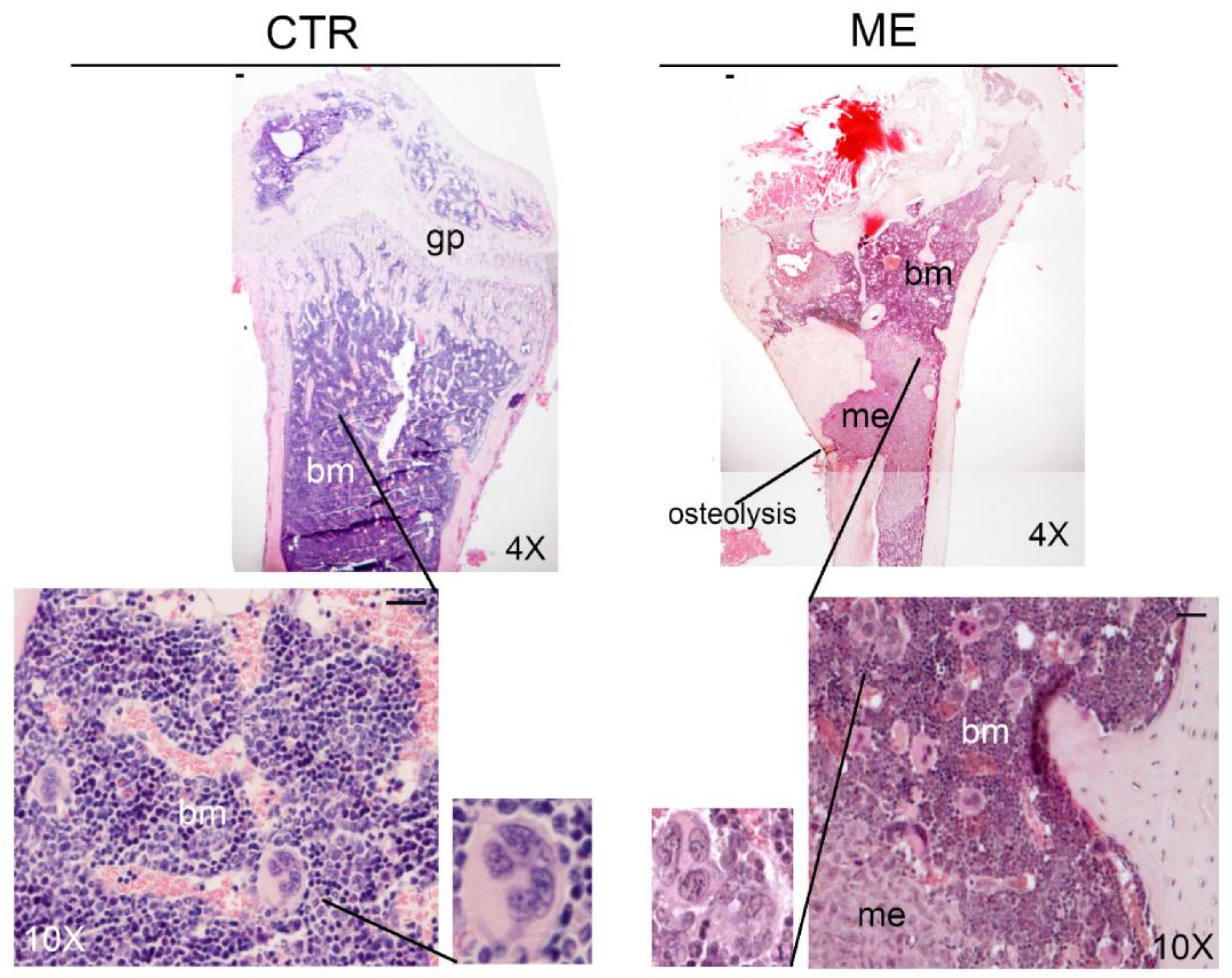
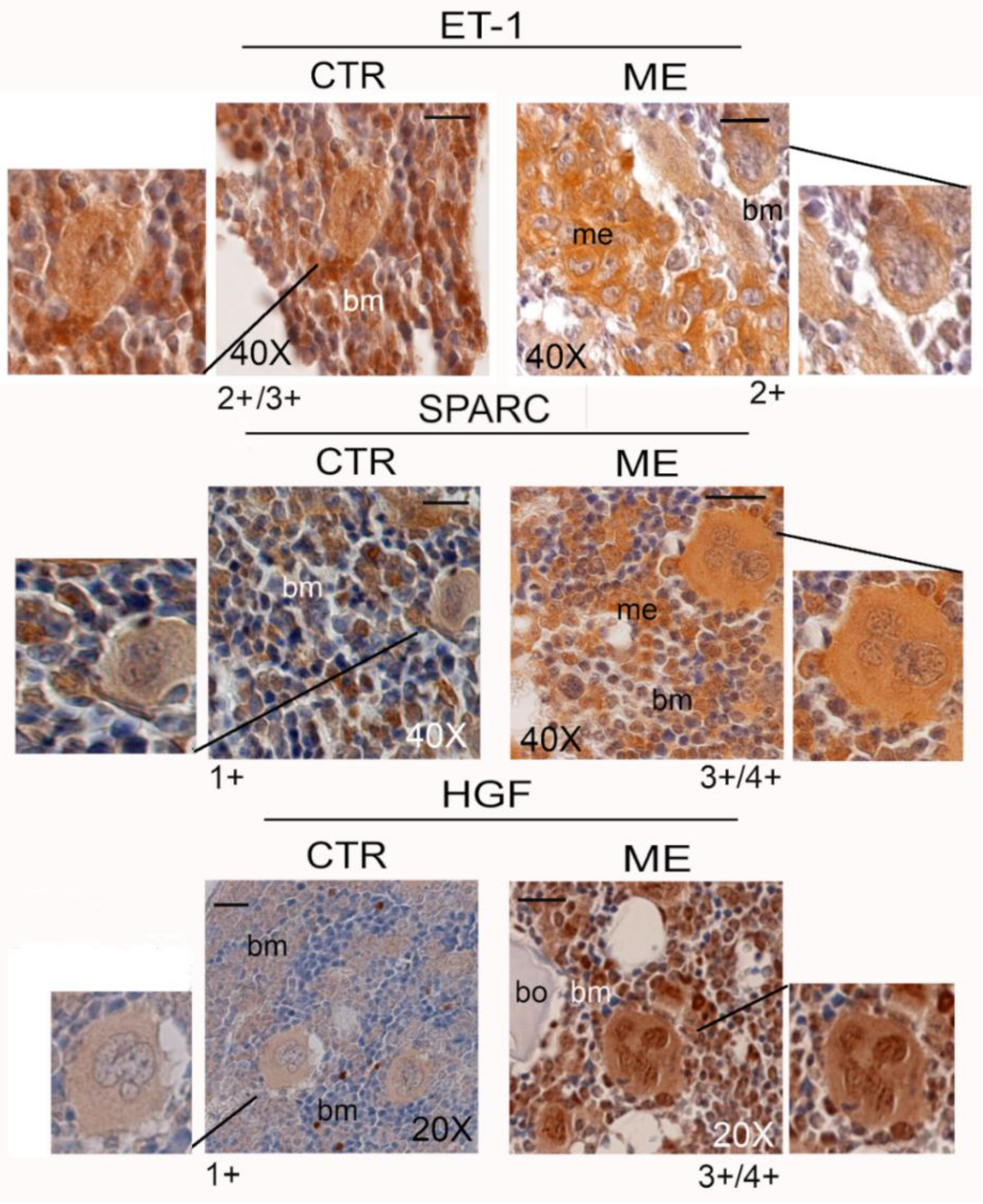
6. Megakaryocytes in the Bone Metastatic Environment of Xenografted Mice with Human Breast Cancer Cells
7. Conclusions and Perspectives
Supplementary Materials
Funding
Conflicts of Interest
References
- Thiede, M.A.; Smock, S.L.; Petersen, D.N.; Grasser, W.A.; Thompson, D.D.; Nishimoto, S.K. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology 1994, 135, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Kelm, R.J., Jr.; Hair, G.A.; Mann, K.G.; Grant, B.W. Characterization of human osteoblast and megakaryocyte-derived osteonectin (SPARC). Blood 1992, 80, 3112–3119. [Google Scholar]
- Breton-Gorius, J.; Clezardin, P.; Guichard, J.; Debili, N.; Malaval, L.; Vainchenker, W.; Cramer, E.M.; Delmas, P.D. Localization of platelet osteonectin at the internal face of the alpha-granule membranes in platelets and megakaryocytes. Blood 1992, 79, 936–941. [Google Scholar] [PubMed]
- Chenu, C.; Delmas, P.D. Platelets contribute to circulating levels of bone sialoprotein in human. J. Bone Miner Res. 1992, 7, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.D.; Balena, R.; Masarachia, P.; Seedor, J.G.; Cartwright, M.E. The effects of three different demineralization agents on osteopontin localization in adult rat bone using immunohistochemistry. Histochemistry 1993, 99, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Koh, A.J.; Wang, Z.; Soki, F.N.; Park, S.I.; Pienta, K.J.; McCauley, L.K. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J. Bone Miner Res. 2011, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, A.; Baek, K.H.; Lynch, R.C.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.E., Jr.; Ryeom, S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood 2010, 115, 4605–4613. [Google Scholar] [CrossRef]
- Jackson, W., 3rd; Sosnoski, D.M.; Ohanessian, S.E.; Chandler, P.; Mobley, A.; Meisel, K.D.; Mastro, A.M. Role of Megakaryocytes in Breast Cancer Metastasis to Bone. Cancer Res. 2017, 77, 1942–1954. [Google Scholar] [CrossRef] [Green Version]
- Psaila, B.; Lyden, D.; Roberts, I. Megakaryocytes, malignancy and bone marrow vascular niches. J. Thromb. Haemost. 2012, 10, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Malara, A.; Currao, M.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Gruppi, C.; Pallotta, I.; Spedden, E.; Tenni, R.; Raspanti, M.; Kaplan, D.; Tira, M.E.; Staii, C.; Balduini, A. Extracellular matrix structure and nano-mechanics determine megakaryocyte function. Blood 2011, 118, 4449–4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiva, O.; Leon, C.; Kah Ng, S.; Mangin, P.; Gachet, C.; Ravid, K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am. J. Hematol. 2018, 93, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Gruppi, C.; Rebuzzini, P.; Visai, L.; Perotti, C.; Moratti, R.; Balduini, C.; Tira, M.E.; Balduini, A. Megakaryocyte-matrix interaction within bone marrow: New roles for fibronectin and factor XIII-A. Blood 2011, 117, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Petrey, A.C.; Obery, D.R.; Kessler, S.P.; Flamion, B.; de la Motte, C.A. Hyaluronan depolymerization by megakaryocyte hyaluronidase-2 is required for thrombopoiesis. Am. J. Pathol. 2016, 186, 2390–2403. [Google Scholar] [CrossRef]
- Kashiwakura, I.; Teramachi, T.; Kakizaki, I.; Takagi, Y.; Takahashi, T.A.; Takagaki, K. The effects of glycosaminoglycans on thrombopoietin-induced megakaryocytopoiesis. Haematologica 2006, 91, 445–451. [Google Scholar] [PubMed]
- Abbonante, V.; Di Buduo, C.A.; Gruppi, C.; De Maria, C.; Spedden, E.; De Acutis, A.; Staii, C.; Raspanti, M.; Vozzi, G.; Kaplan, D.L.; et al. A new path to platelet production through matrix sensing. Haematologica 2017, 102, 1150–1160. [Google Scholar] [CrossRef] [Green Version]
- Trotter, T.N.; Yang, Y. Matricellular proteins as regulators of cancer metastasis to bone. Matrix Biol. 2016, 52, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Ru, Y.-X.; Dong, S.-X.; Liang, H.-Y.; Zhao, S.-X. Platelet production of megakaryocytes: A review with original observations on human in vivo cells and bone marrow. Ultrastruct. Pathol. 2016, 40, 163–170. [Google Scholar] [CrossRef]
- Brown, E.; Carlin, L.M.; Nerlov, C.; Lo Celso, C.; Poole, A.W. Multiple membrane extrusion sites drive megakaryocyte migration into bone marrow blood vessels. Life Sci. Alliance 2018, 1, e201800061. [Google Scholar] [CrossRef]
- Kaushansky, K. Thrombopoietin: The primary regulator of platelet production. Blood 1995, 86, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Papadantonakis, N.; Ravid, K. Development of megakaryocytes. In Molecular Basis of Hematopoiesis; Wicrema, A., Kee, B., Eds.; Springer: New York, NY, USA, 2009; pp. 95–126. [Google Scholar]
- Luff, S.A.; Kao, C.Y.; Papoutsakis, E.T. Role of p53 and transcription-independent p53-induced apoptosis in shear-stimulated megakaryocytic maturation, particle generation, and platelet biogenesis. PLoS ONE 2018, 13, e0203991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Woulfe, D.S.; Papoutsakis, E.T. Shear enhances thromopoiesis and formation of microparticles that induce megakaryocytic differentiation of stem cells. Blood 2014, 124, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Stegner, D.; van Eeuwijk, J.M.M.; Angay, O.; Gorelashvili, M.G.; Semeniak, D.; Pinnecker, J.; Schmithausen, P.; Meyer, I.; Friedrich, M.; Dütting, S.; et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat. Commun. 2017, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, M.; Wang, Y.; Tao, C.; Wang, Z.; Yang, J.; Chen, Z.; Zou, Z.; Li, M.; Liu, A.; Jia, C.; et al. Blood 2017, 129, 3196–3209. [CrossRef] [PubMed]
- Shivdasani, R.A. Molecular and transcriptional regulation of megakaryocyte differentiation. Stem Cells 2001, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kacena, M.A.; Shivdasani, R.A.; Wilson, K.; Xi, Y.; Troiano, N.; Nazarian, A.; Gundberg, C.M.; Bouxsein, M.L.; Lorenzo, J.A.; Horowitz, M.C. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J. Bone Miner Res. 2004, 19, 652–660. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Rondina, M.T.; Weyrich, A.S. Regualation of the genetic code in megakaryocytes and platelets. J. Thromb. Haemost. 2015, 13, S26–S32. [Google Scholar] [CrossRef]
- Fuhrken, P.G.; Apostolidis, P.A.; Lindsey, S.; Miller, W.M.; Papoutsakis, E.T. Tumor suppressor protein p53 regulates megakaryocytic polyploidization and apoptosis. J. Biol. Chem. 2008, 283, 15559–15560. [Google Scholar] [CrossRef]
- Apostolidis, P.A.; Lindsey, S.; Miller, W.M.; Papoutsakis, E.T. Proposed megakaryocytic regulon of p53, the genes engaged to control cell cycle and apoptosis during megakaryocytic differentiation. Physiol. Genomics 2012, 44, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The platelet lifeline to cancer: Challenges and opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Menter, D.G.; Tucker, S.C.; Kopetz, S.; Sood, A.K.; Crissman, J.D.; Honn, K.V. Platelets and cancer: A casual or causal relationship: Revisited. Cancer Metastasis Rev. 2014, 33, 231–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Calverley, D.C.; Phang, T.L.; Choudhury, Q.G.; Gao, B.; Oton, A.B.; Weyant, M.J.; Geraci, M.W. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin. Translat. Sci. 2010, 3, 227–232. [Google Scholar] [CrossRef]
- Nilsson, R.J.; Balaj, L.; Hulleman, E.; van Rijn, S.; Pegtel, D.M.; Walraven, M.; Widmark, A.; Gerritsen, W.R.; Verheul, H.M.; Vandertop, W.P.; et al. Blood platelets contain tumor-derived RNA biomarkers. Blood 2011, 118, 3680–3683. [Google Scholar] [CrossRef] [Green Version]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef]
- Best, M.G.; Wesseling, P.; Wurdinger, T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res. 2018, 78, 3407–3412. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Xu, R. Human cancer and platelet interaction, a potential therapeutic target. Int. J. Mol. Sci. 2018, 19, E1246. [Google Scholar] [CrossRef] [PubMed]
- Kacena, M.A.; Gundberg, C.M.; Horowitz, M.C. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone 2006, 39, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.A.; Bord, S.; Ireland, D.; Compston, J.E. Osteoclast formation and bone resorption are inhibited by megakaryocytes. Bone 2006, 39, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Ciovacco, W.A.; Cheng, Y.H.; Horowitz, M.C.; Kacena, M.A. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J. Cell Biochem. 2010, 109, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.B.; Zhang, J.; Waits, C.; Skikne, B.; Garimella, R.; Anderson, H.C. Localization of bone morphogenetic proteins (BMPs)-2, -4, and -6 within megakaryocytes and platelets. Bone 2004, 35, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Bianchi, L.; Cellai, C.; Paoletti, F.; Rana, R.A.; Lorenzini, R.; Migliaccio, G.; Migliaccio, A.R. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood 2002, 100, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Wickenhauser, C.; Hillienhof, A.; Jungheim, K.; Lorenzen, J.; Ruskowski, H.; Hansmann, M.L.; Thiele, J.; Fischer, R. Detection and quantification of transforming growth factor beta (TGF-beta) and platelet-derived growth factor (PDGF) release by normal human megakaryocytes. Leukemia 1995, 9, 310–315. [Google Scholar] [PubMed]
- Yang, J.G.; Wang, L.L.; Ma, D.C. Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases. Br. J. Haematol. 2018, 180, 321–334. [Google Scholar] [CrossRef]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M.A. Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia. Int. J. Mol. Sci. 2016, 17, 706. [Google Scholar] [CrossRef]
- Psaila, B.; Kaplan, R.N.; Port, E.R.; Lyden, D. Priming the ‘soil’ for breast cancer metastasis: The pre-metastatic niche. Breast Dis. 2007, 26, 65–74. [Google Scholar] [CrossRef]
- Previdi, S.; Maroni, P.; Matteucci, E.; Broggini, M.; Bendinelli, P.; Desiderio, M.A. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and b-catenin/Wnt pathways. Eur. J. Cancer 2010, 46, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Bendinelli, P.; Matteucci, E.; Locatelli, A.; Nakamura, T.; Scita, G.; Desiderio, M.A. Osteolytic bone metastasis is hampered by impinging on the interplay among autophagy, anoikis and ossification. Cell Death Dis. 2014, 5, e1005. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.A. Increased numbers of pulmonary megakaryocytes in patients with arterial pulmonary tumour embolism and with lung metastases seen at necropsy. J. Clin. Pathol. 1992, 45, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix. Matrix Biol. 2000, 19, 569–580. [Google Scholar] [CrossRef]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M.A. Epigenetic regulation of HGF/Met receptor axis is critical for the outgrowth of bone metastasis from breast carcinoma Cell Death Dis. Cell Death Dis. 2017, 8, e2578. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Bendinelli, P.; Morelli, D.; Drago, L.; Luzzati, A.; Perrucchini, G.; Bonini, C.; Matteucci, E.; Desiderio, M.A. High SPARC Expression Starting from Dysplasia, Associated with Breast Carcinoma, Is Predictive for Bone Metastasis without Enhancement of Plasma Levels. Int. J. Mol. Sci. 2015, 16, 28108–28122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Esposito, M.; Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015, 93, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroni, P. Megakaryocytes in Bone Metastasis: Protection or Progression? Cells 2019, 8, 134. https://doi.org/10.3390/cells8020134
Maroni P. Megakaryocytes in Bone Metastasis: Protection or Progression? Cells. 2019; 8(2):134. https://doi.org/10.3390/cells8020134
Chicago/Turabian StyleMaroni, Paola. 2019. "Megakaryocytes in Bone Metastasis: Protection or Progression?" Cells 8, no. 2: 134. https://doi.org/10.3390/cells8020134
APA StyleMaroni, P. (2019). Megakaryocytes in Bone Metastasis: Protection or Progression? Cells, 8(2), 134. https://doi.org/10.3390/cells8020134




