Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Homogeneous Coated and Nanopatterned Surfaces
2.2. Surface Functionalization with Integrin Ligands
2.3. Surface Immobilization of BMP-6
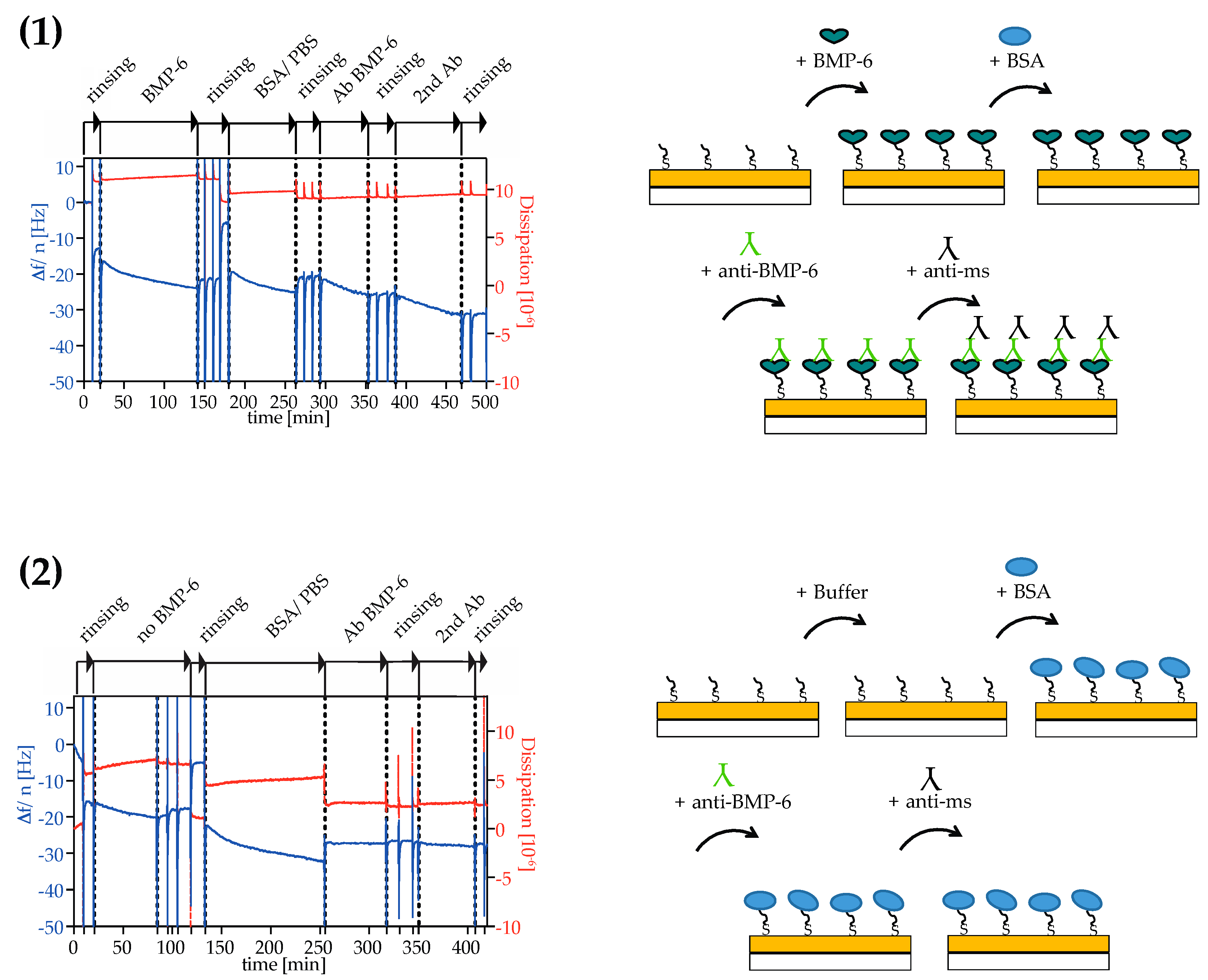
2.4. Analysis of Surface Binding of BMP-6
2.5. Cell Culture
2.6. Western Blot Analysis of Protein Expression
2.7. Immunostaining and Microscopy
2.8. Statistical Analysis
3. Results
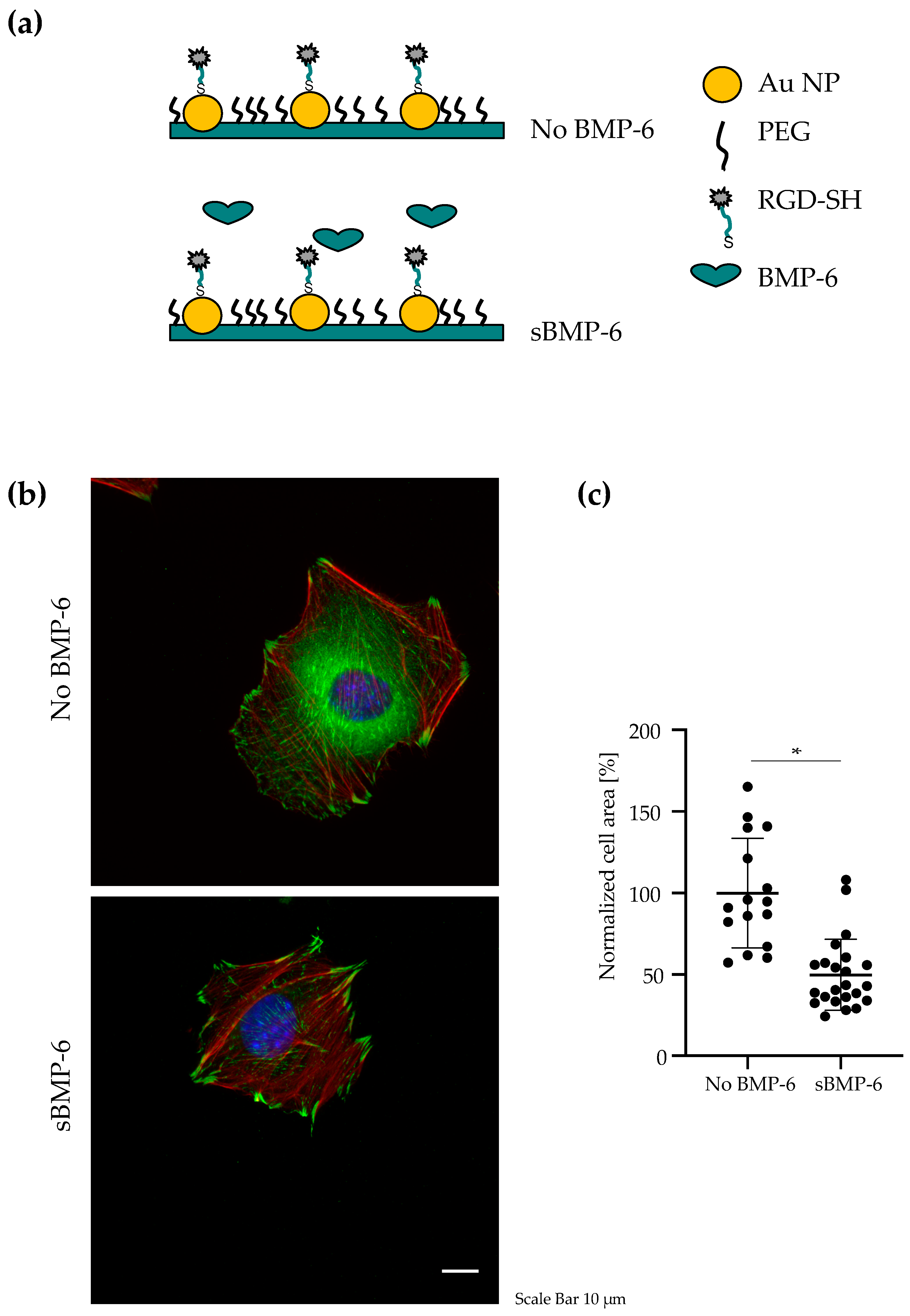
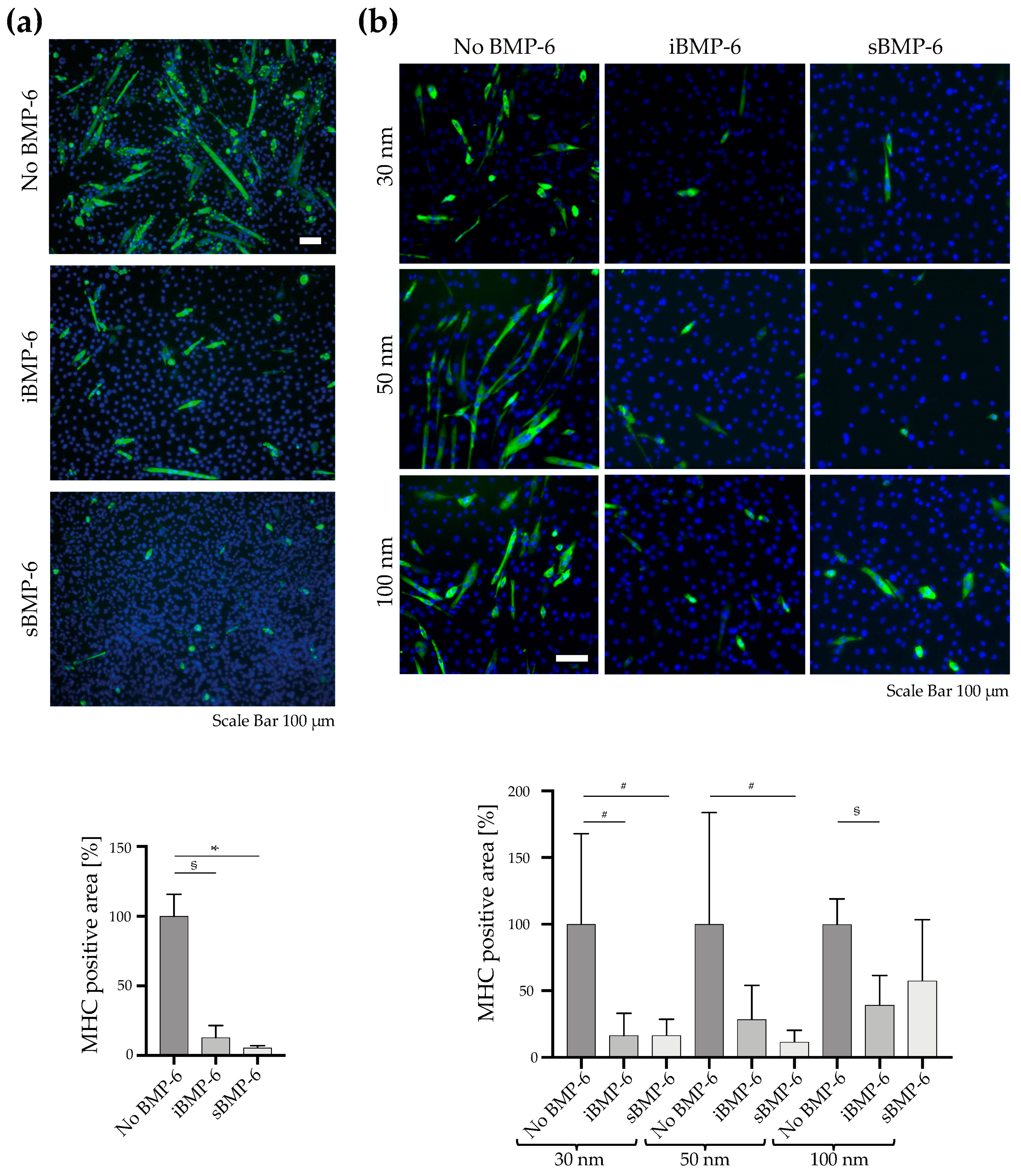
3.1. BMP-6 Influences Cell Spreading and Focal Adhesion Assembly
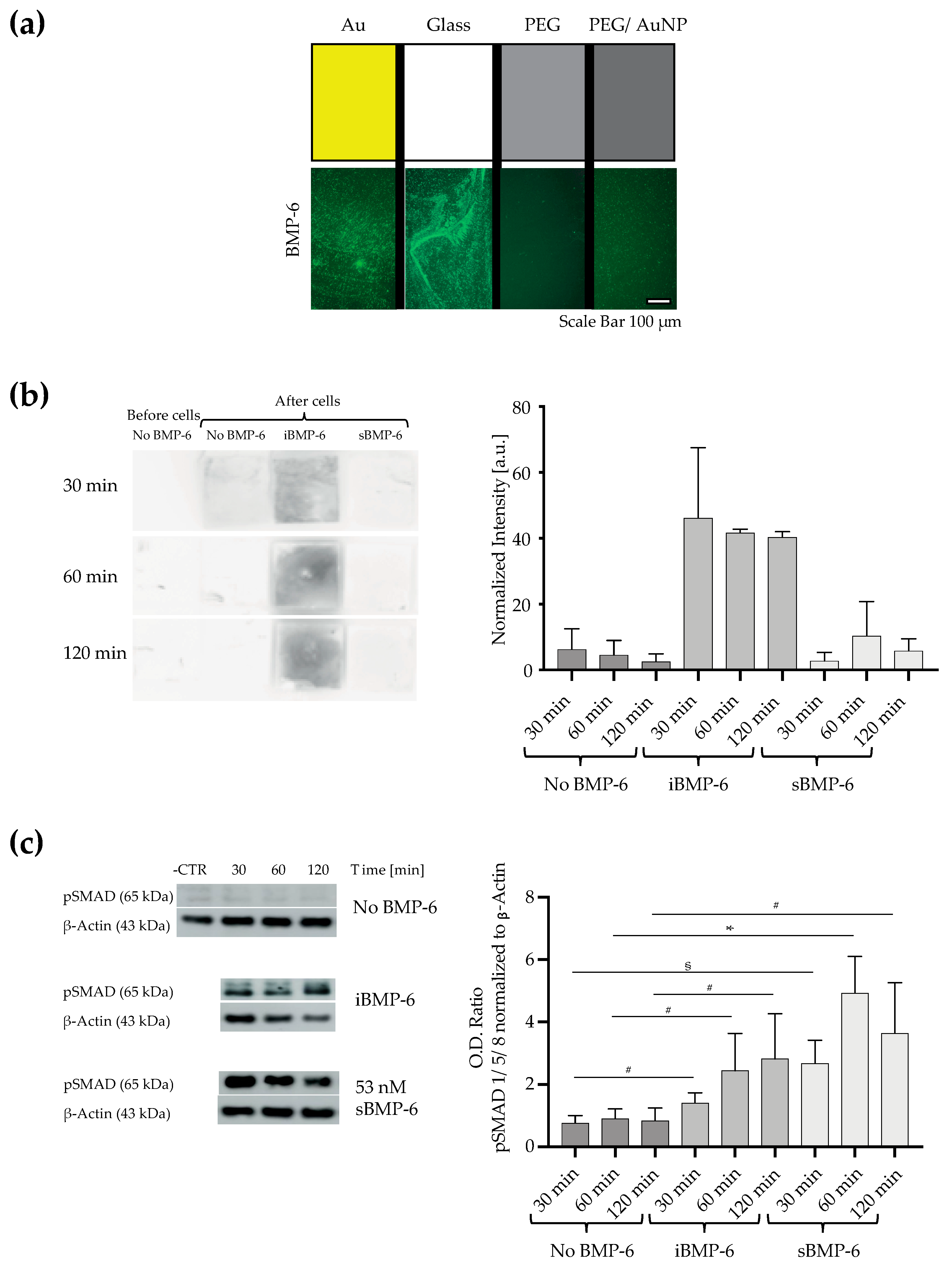
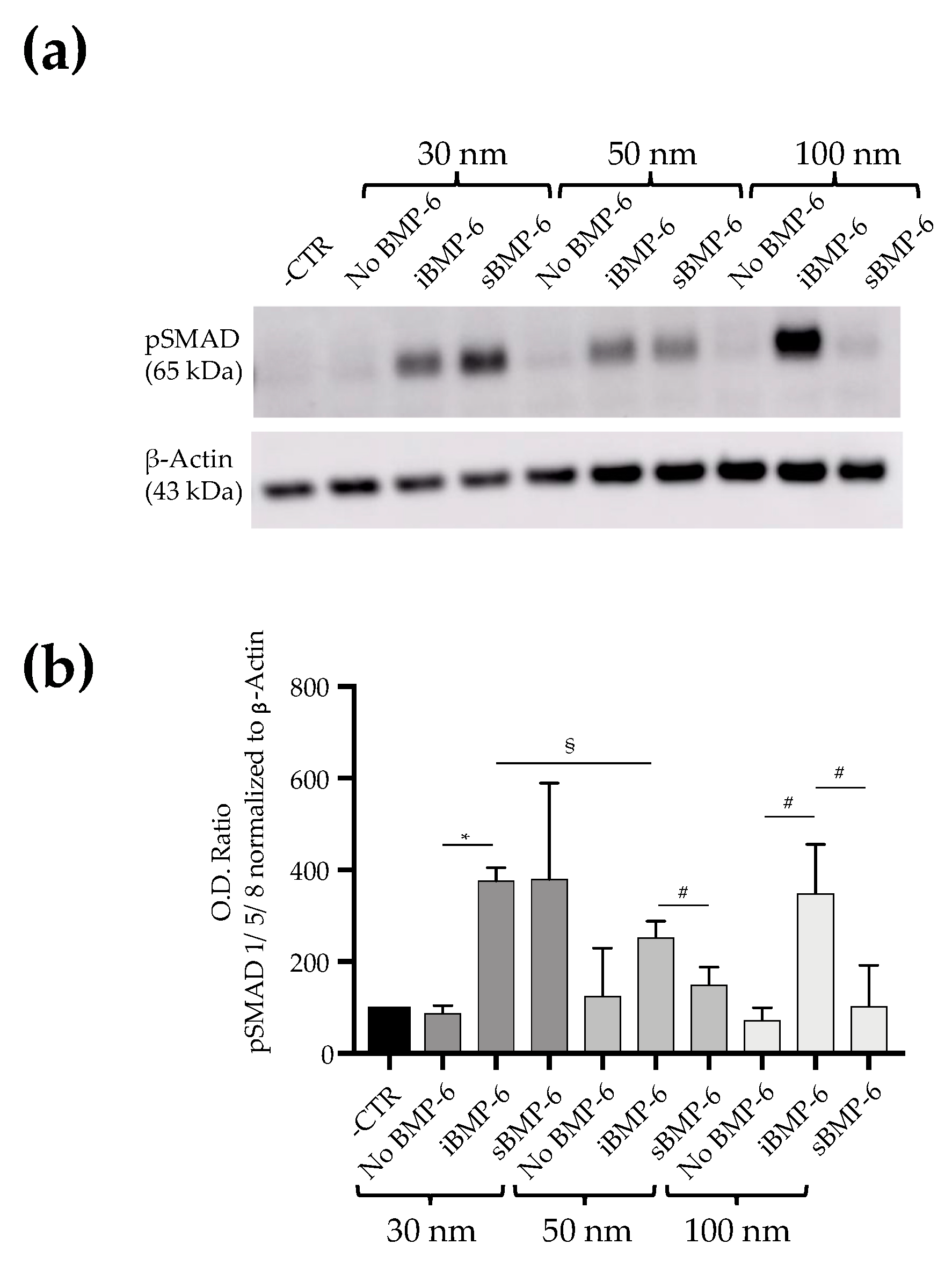
3.2. Surface Immobilized BMP-6 is Not Internalized by Cells and Triggers Smad Signaling
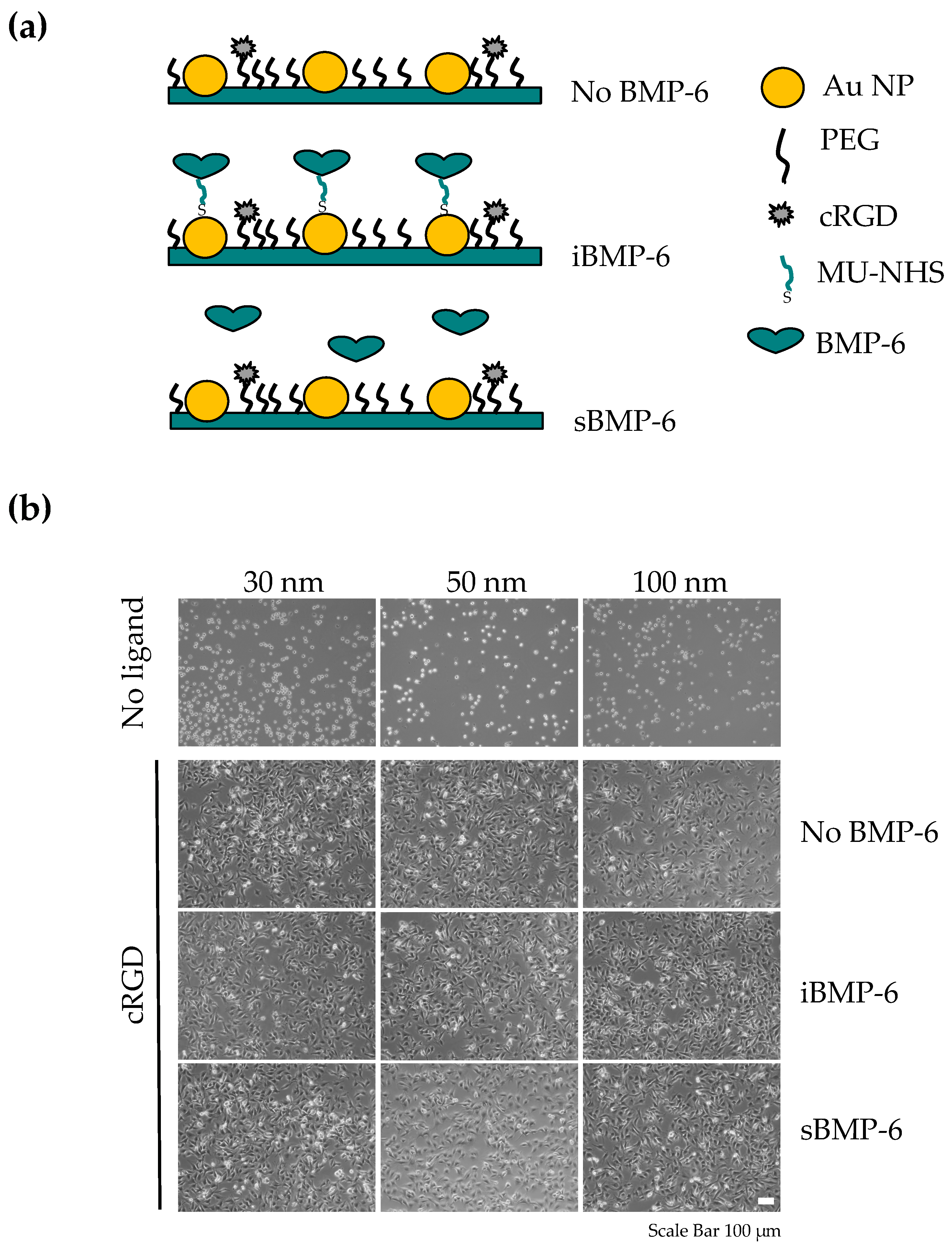
3.3. Surface Copresentation of Immobilized BMP-6 and RGD Ligands Promotes Adhesion and Induces BMP-Mediated Cell Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wagner, D.O.; Sieber, C.; Bhushan, R.; Börgermann, J.H.; Graf, D.; Knaus, P. BMPs: From Bone to Body Morphogenetic Proteins; American Association for the Advancement of Science: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Vukicevic, S.; Grgurevic, L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, S.; Oppermann, H.; Verbanac, D.; Jankolija, M.; Popek, I.; Curak, J.; Brkljacic, J.; Pauk, M.; Erjavec, I.; Francetic, I. The clinical use of bone morphogenetic proteins revisited: A novel biocompatible carrier device OSTEOGROW for bone healing. Int. Orthop. 2014, 38, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, S.; Bennet, S.J.; Arora, M. Bone morphogenetic protein-7: Review of signalling and efficacy in fracture healing. J. Orthop. Transl. 2016, 4, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Grgurevic, L.; Erjavec, I.; Dumic-Cule, I.; Bordukalo-Niksic, T.; Pauk, M.; Trkulja, V.; Maticic, D.; Pecin, M.; Lipar, M.; Peric, M. Osteogrow: A novel bone graft substitute for orthopedic reconstruction. In Bone Morphogenetic Proteins: Systems Biology Regulators; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–228. [Google Scholar] [CrossRef]
- Ebisawa, T.; Tada, K.; Kitajima, I.; Tojo, K.; Sampath, T.K.; Kawabata, M.; Miyazono, K.; Imamura, T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999, 112, 3519–3527. [Google Scholar] [PubMed]
- Seckinger, A.; Meissner, T.; Moreaux, J.; Goldschmidt, H.; Fuhler, G.M.; Benner, A.; Hundemer, M.; Rème, T.; Shaughnessy, J.D., Jr.; Barlogie, B. Bone morphogenic protein 6: A member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis. Oncogene 2009, 28, 3866. [Google Scholar] [CrossRef] [PubMed]
- Sagorny, K.; Chapellier, M.; Laperrousaz, B.; Maguer-Satta, V. BMP et cancer: Le Yin et le Yang des cellules souches. Médecine/Sciences 2012, 28, 416–422. [Google Scholar] [CrossRef]
- Sovershaev, T.A.; Unruh, D.; Sveinbjornsson, B.; Fallon, J.T.; Hansen, J.B.; Bogdanov, V.Y.; Sovershaev, M.A. A novel role of bone morphogenetic protein-7 in the regulation of adhesion and migration of human monocytic cells. Thromb. Res. 2016, 147, 24–31. [Google Scholar] [CrossRef]
- Garciadiego-Cazares, D.; Aguirre-Sanchez, H.I.; Abarca-Buis, R.F.; Kouri, J.B.; Velasquillo, C.; Ibarra, C. Regulation of alpha5 and alphaV Integrin Expression by GDF-5 and BMP-7 in Chondrocyte Differentiation and Osteoarthritis. PLoS ONE 2015, 10, e0127166. [Google Scholar] [CrossRef]
- Brigaud, I.; Agniel, R.; Leroy-Dudal, J.; Kellouche, S.; Ponche, A.; Bouceba, T.; Mihailescu, N.; Sopronyi, M.; Viguier, E.; Ristoscu, C.; et al. Synergistic effects of BMP-2, BMP-6 or BMP-7 with human plasma fibronectin onto hydroxyapatite coatings: A comparative study. Acta Biomater. 2017, 55, 481–492. [Google Scholar] [CrossRef]
- Brkljacic, J.; Pauk, M.; Erjavec, I.; Cipcic, A.; Grgurevic, L.; Zadro, R.; Inman, G.J.; Vukicevic, S. Exogenous heparin binds and inhibits bone morphogenetic protein 6 biological activity. Int. Orthop. 2013, 37, 529–541. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta BBA Proteins Proteom. 2012, 1824, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.Y.; Park, J.H.; Park, J.B.; Suh, J.S.; Choi, Y.S.; Lee, S.J.; Chung, C.-P.; Park, Y.J. The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role on osteogenesis. Biomaterials 2010, 31, 7226–7238. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Krause, C.; Shi, S.; Patterson, M.; Suto, R.; Grgurevic, L.; Vukicevic, S.; van Dinther, M.; Falb, D.; Ten Dijke, P.; et al. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J. Biol. Chem. 2010, 285, 12169–12180. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.J.; Collyer, J.; Stanfield, M.; Goodman, S.A. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology 1995, 136, 2671–2677. [Google Scholar] [CrossRef]
- Aoki, H.; Fujii, M.; Imamura, T.; Yagi, K.; Takehara, K.; Kato, M.; Miyazono, K. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J. Cell Sci. 2001, 114, 1483–1489. [Google Scholar]
- Blummel, J.; Perschmann, N.; Aydin, D.; Drinjakovic, J.; Surrey, T.; Lopez-Garcia, M.; Kessler, H.; Spatz, J.P. Protein repellent properties of covalently attached PEG coatings on nanostructured SiO(2)-based interfaces. Biomaterials 2007, 28, 4739–4747. [Google Scholar] [CrossRef]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blummel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 2004, 5, 383–388. [Google Scholar] [CrossRef]
- Cavalcanti-Adam, E.A.; Volberg, T.; Micoulet, A.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007, 92, 2964–2974. [Google Scholar] [CrossRef]
- Schenk, F.C.; Boehm, H.; Spatz, J.P.; Wegner, S.V. Dual-functionalized nanostructured biointerfaces by click chemistry. Langmuir 2014, 30, 6897–6905. [Google Scholar] [CrossRef]
- Huang, J.; Grater, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef]
- Pohl, T.L.; Boergermann, J.H.; Schwaerzer, G.K.; Knaus, P.; Cavalcanti-Adam, E.A. Surface immobilization of bone morphogenetic protein 2 via a self-assembled monolayer formation induces cell differentiation. Acta Biomater. 2012, 8, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Schwab, E.H.; Pohl, T.L.; Haraszti, T.s.; Schwaerzer, G.K.; Hiepen, C.; Spatz, J.P.; Knaus, P.; Cavalcanti-Adam, E.A. Nanoscale control of surface immobilized BMP-2: Toward a quantitative assessment of BMP-mediated signaling events. Nano Lett. 2015, 15, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- McColl, R.; Nkosi, M.; Snyman, C.; Niesler, C. Analysis and quantification of in vitro myoblast fusion using the LADD Multiple Stain. BioTechniques 2016, 61, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Yu, X.; Cohen, D.M.; Wozniak, M.A.; Yang, M.T.; Gao, L.; Eyckmans, J.; Chen, C.S. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem Cells Dev. 2012, 21, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Pohl, T.L.; Schwab, E.H.; Cavalcanti-Adam, E.A. Covalent binding of BMP-2 on surfaces using a self-assembled monolayer approach. J. Vis. Exp. 2013, 78, e50842. [Google Scholar] [CrossRef] [PubMed]
- Hauff, K.; Zambarda, C.; Dietrich, M.; Halbig, M.; Grab, A.L.; Medda, R.; Cavalcanti-Adam, E.A. Matrix-immobilized BMP-2 on microcontact printed fibronectin as an in vitro tool to study BMP-mediated signaling and cell migration. Front. Bioeng. Biotechnol. 2015, 3, 62. [Google Scholar] [CrossRef]
- Fourel, L.; Valat, A.; Faurobert, E.; Guillot, R.; Bourrin-Reynard, I.; Ren, K.; Lafanechère, L.; Planus, E.; Picart, C.; Albiges-Rizo, C. β3 integrin–mediated spreading induced by matrix-bound BMP-2 controls Smad signaling in a stiffness-independent manner. J. Cell. Biol. 2016, 212, 693–706. [Google Scholar] [CrossRef]
- Oberhansl, S.; Castaño, A.; Lagunas, A.; Prats-Alfonso, E.; Hirtz, M.; Albericio, F.; Fuchs, H.; Samitier, J.; Martinez, E. Mesopattern of immobilised bone morphogenetic protein-2 created by microcontact printing and dip-pen nanolithography influence C2C12 cell fate. RSC Adv. 2014, 4, 56809–56815. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011, 7, 2091–2100. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.; Durrieu, M.; Laroche, G. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater. 2016, 36, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Pohl, T.L.; Seckinger, A.; Spatz, J.P.; Cavalcanti-Adam, E.A. Regulation of integrin and growth factor signaling in biomaterials for osteodifferentiation. Beilstein J. Org. Chem. 2015, 11, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, E.; Horn, P.; Haraszti, T.; Wegner, S.V.; Hiepen, C.; Knaus, P.; Richter, R.P.; Cavalcanti-Adam, E.A. Enhanced Biological Activity of BMP-2 Bound to Surface-Grafted Heparan Sulfate. Adv. Biosyst. 2017, 1, 1600041. [Google Scholar] [CrossRef]
- Migliorini, E.; Valat, A.; Picart, C.; Cavalcanti-Adam, E.A. Tuning cellular responses to BMP-2 with material surfaces. Cytokine Growth Factor Rev. 2016, 27, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Salmeron-Sanchez, M.; Dalby, M.J. Synergistic growth factor microenvironments. Chem. Commun. 2016, 52, 13327–13336. [Google Scholar] [CrossRef] [PubMed]
- Tabisz, B.; Schmitz, W.; Schmitz, M.; Luehmann, T.; Heusler, E.; Rybak, J.C.; Meinel, L.; Fiebig, J.E.; Mueller, T.D.; Nickel, J. Site-Directed Immobilization of BMP-2: Two Approaches for the Production of Innovative Osteoinductive Scaffolds. Biomacromolecules 2017, 18, 695–708. [Google Scholar] [CrossRef]
- Martino, M.M.; Hubbell, J.A. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar] [CrossRef]
- Liu, H.; Niu, A.; Chen, S.E.; Li, Y.P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 2011, 25, 1914–1921. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posa, F.; Grab, A.L.; Martin, V.; Hose, D.; Seckinger, A.; Mori, G.; Vukicevic, S.; Cavalcanti-Adam, E.A. Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling. Cells 2019, 8, 1646. https://doi.org/10.3390/cells8121646
Posa F, Grab AL, Martin V, Hose D, Seckinger A, Mori G, Vukicevic S, Cavalcanti-Adam EA. Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling. Cells. 2019; 8(12):1646. https://doi.org/10.3390/cells8121646
Chicago/Turabian StylePosa, Francesca, Anna Luise Grab, Volker Martin, Dirk Hose, Anja Seckinger, Giorgio Mori, Slobodan Vukicevic, and Elisabetta Ada Cavalcanti-Adam. 2019. "Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling" Cells 8, no. 12: 1646. https://doi.org/10.3390/cells8121646
APA StylePosa, F., Grab, A. L., Martin, V., Hose, D., Seckinger, A., Mori, G., Vukicevic, S., & Cavalcanti-Adam, E. A. (2019). Copresentation of BMP-6 and RGD Ligands Enhances Cell Adhesion and BMP-Mediated Signaling. Cells, 8(12), 1646. https://doi.org/10.3390/cells8121646






