Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk
Abstract
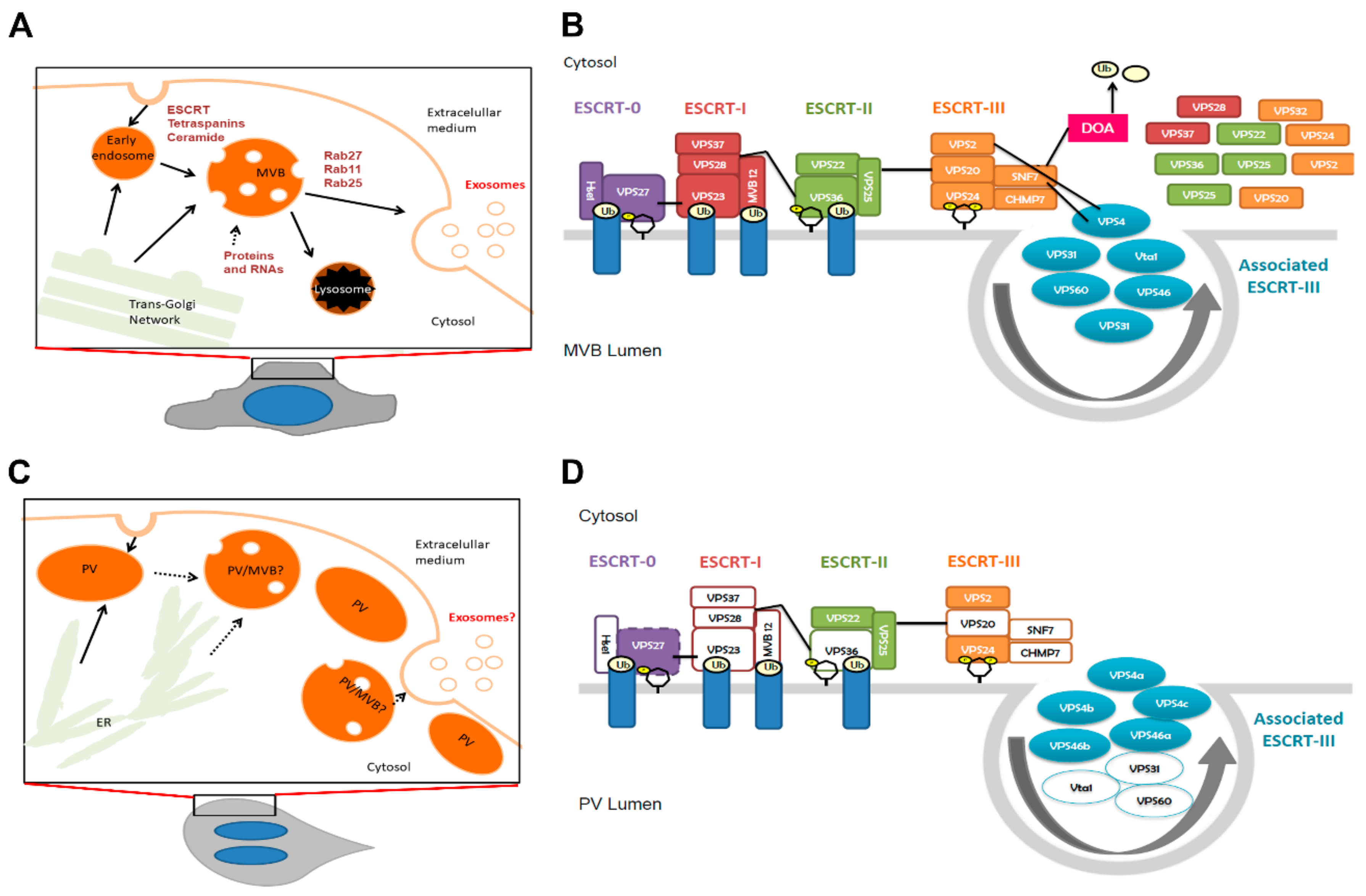
1. Introduction
2. Materials and Methods
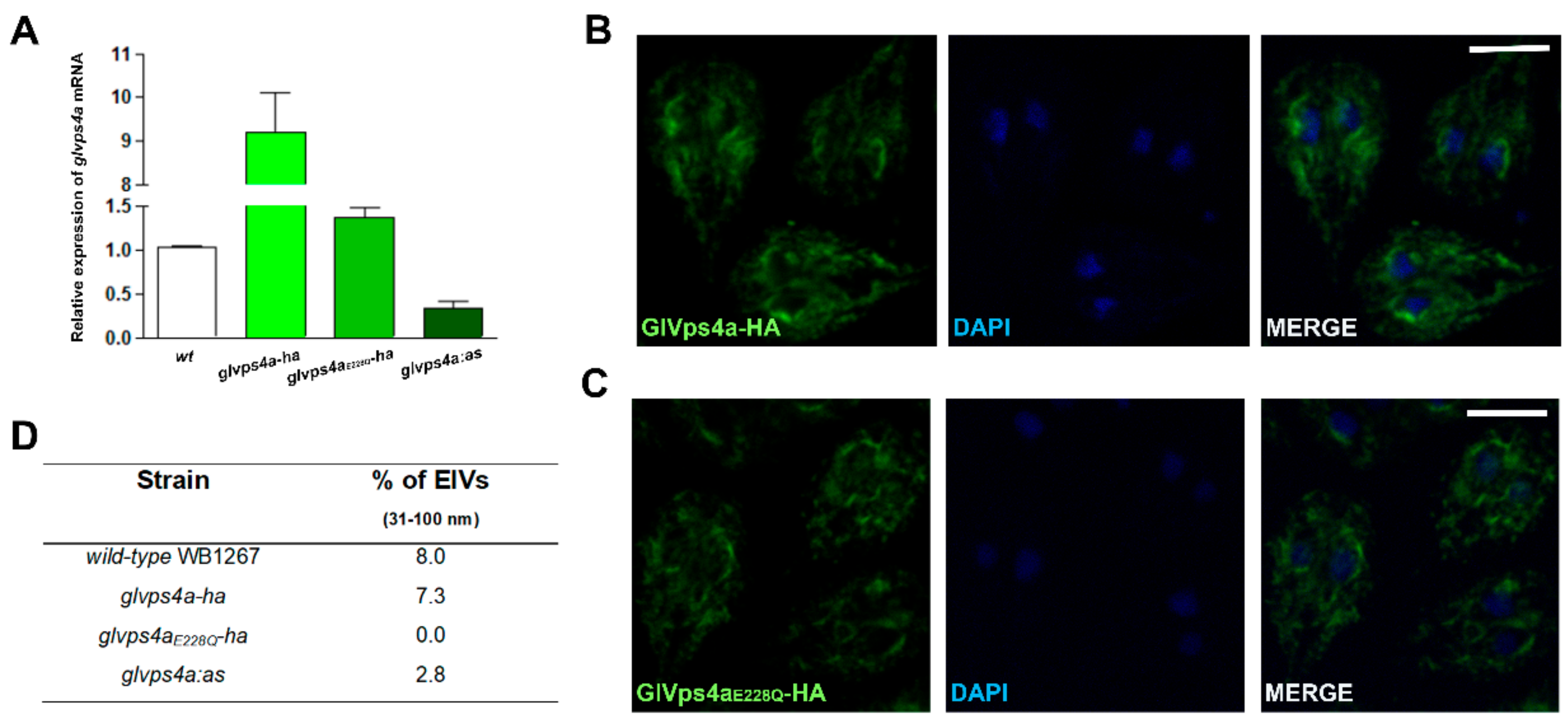
2.1. GlVps4a and Rab11 Expression
2.2. GlVps4a and GlRab11 Downregulation
2.3. Giardia Isolates and Transfection
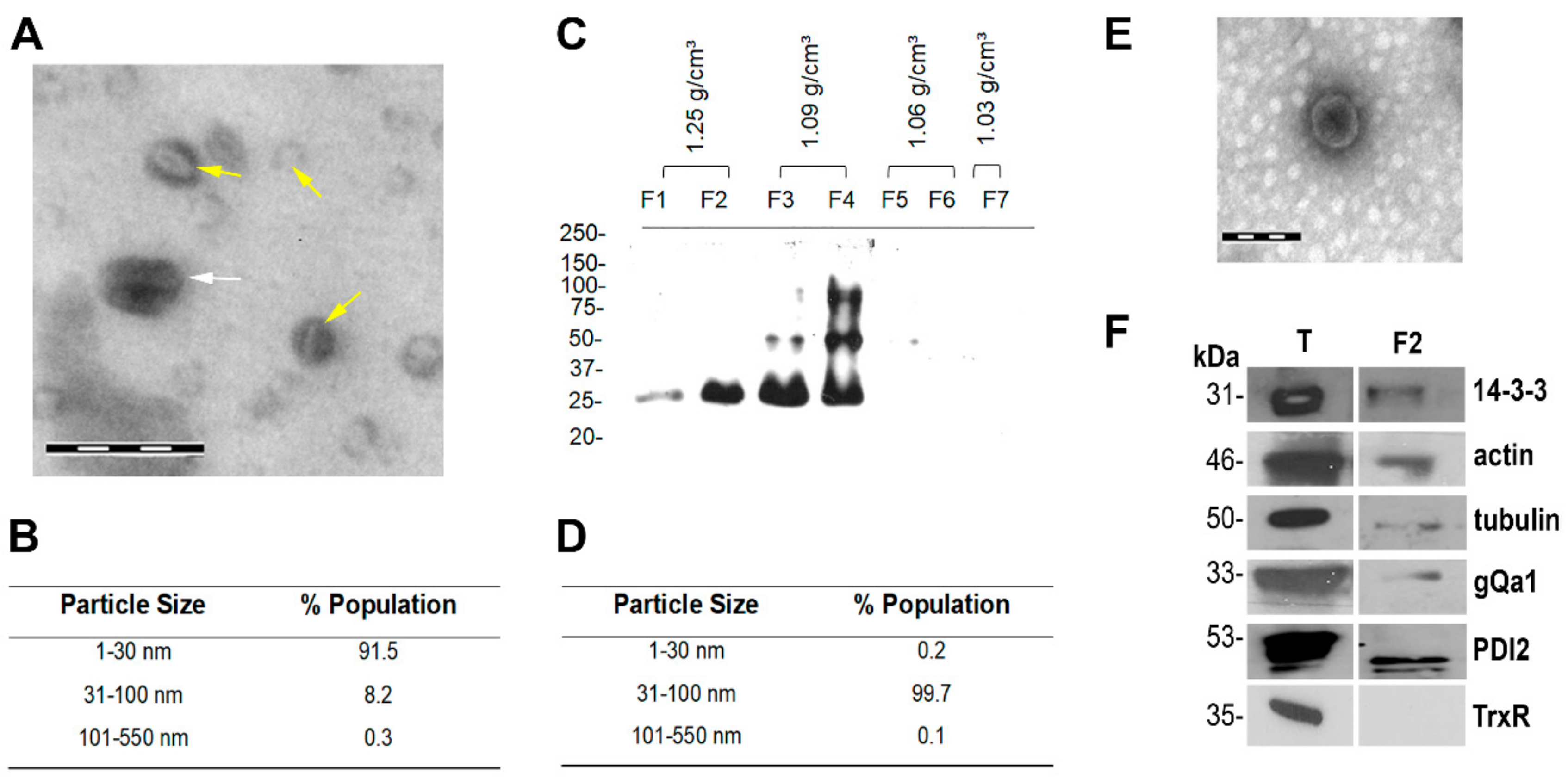
2.4. Exosome Enrichment
2.5. Negative Staining and Electron Microscopy
2.6. Dynamic Light Scattering (DLS)
2.7. Immunoblot Analysis
2.8. RT-qPCR
2.9. Immunofluorescence Assay (IFA)
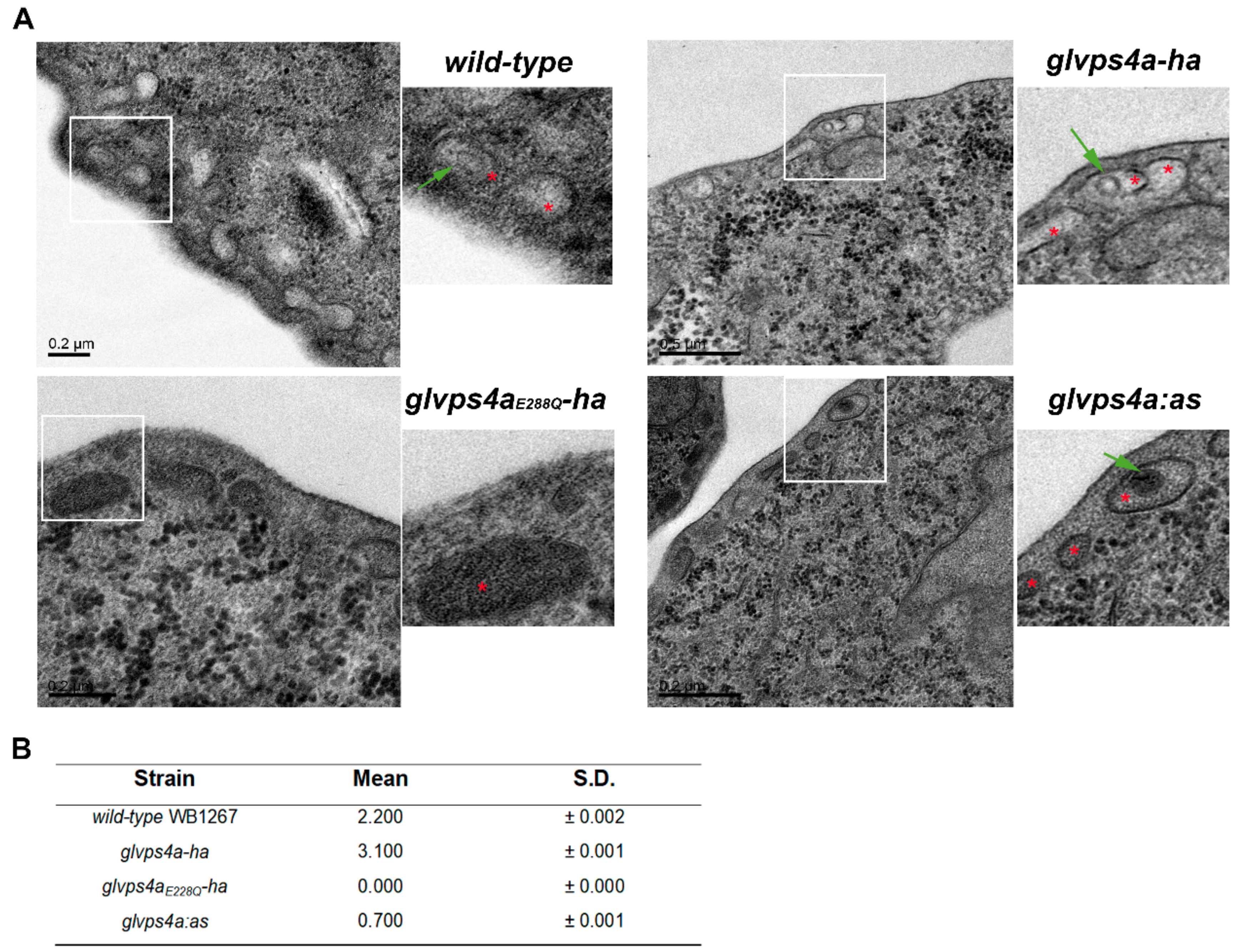
2.10. Transmission Electron Microscopy (TEM) of Giardia trophozoites
2.11. Labeling Membranes with Fluorescent Bodipy FL C5-Ceramide
3. Results
3.1. G. lamblia Secretes Exosome-Like Vesicles
3.2. The Giardial GlVPS4a Protein is Involved in ElV and ILV Biogenesis
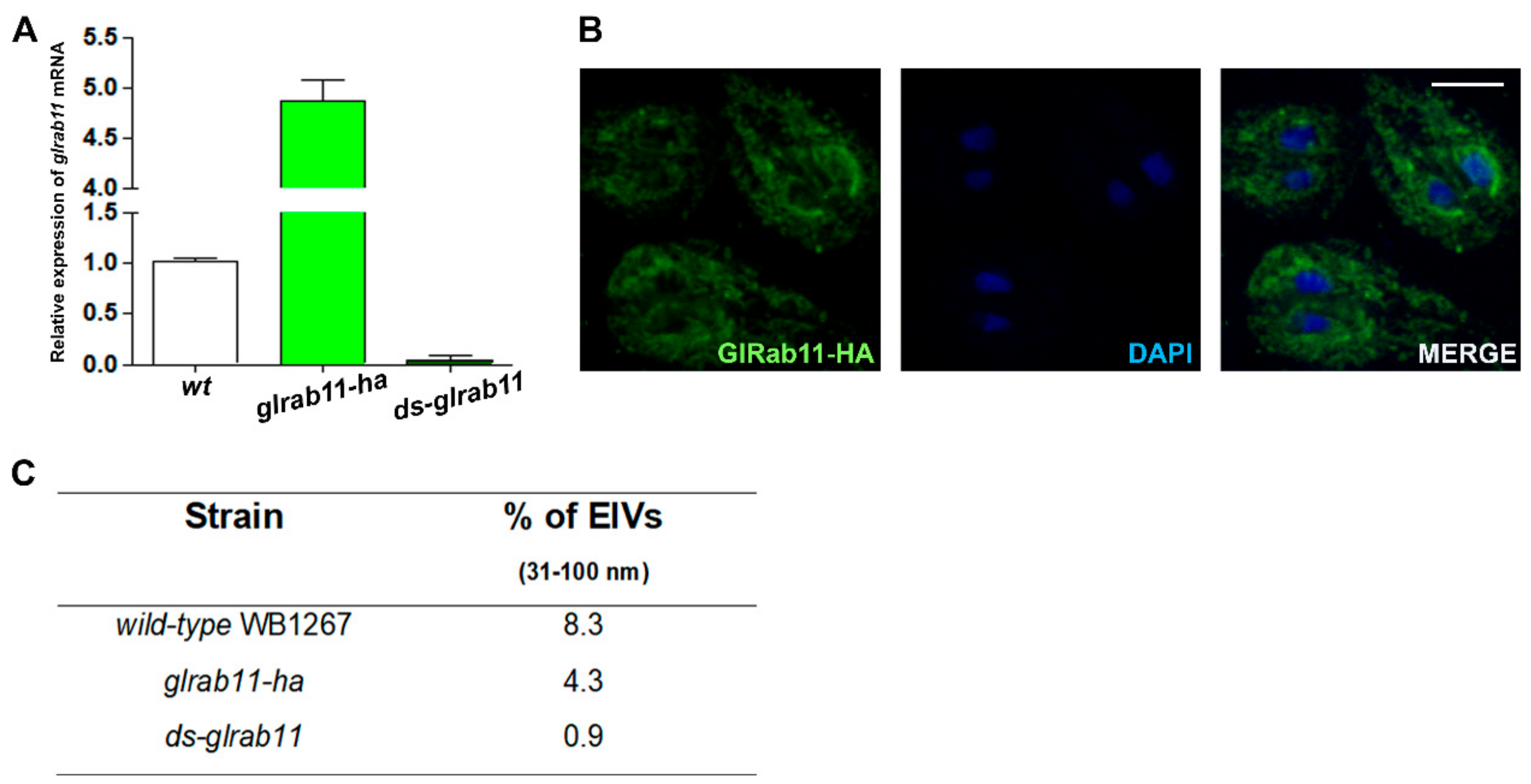
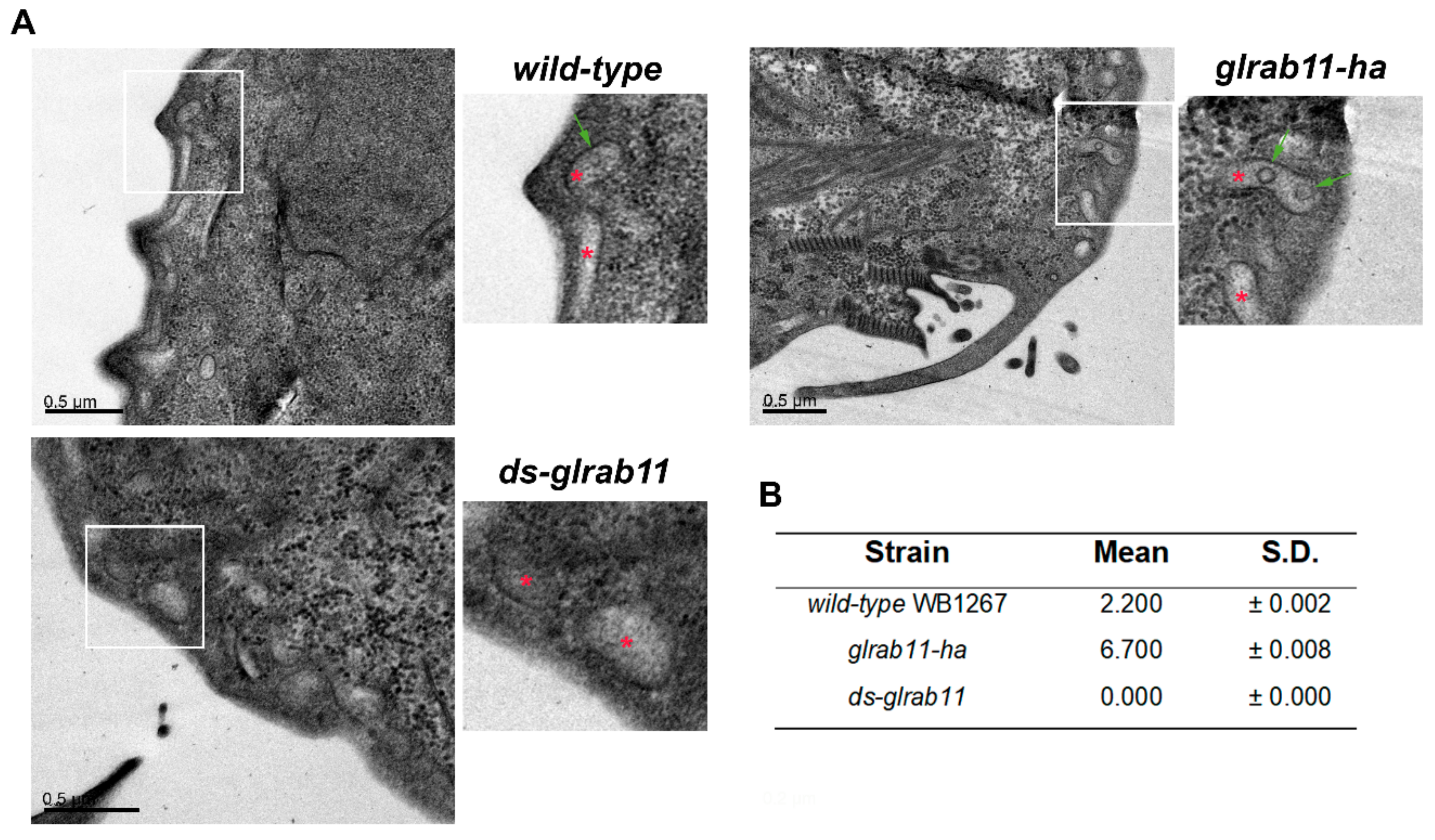
3.3. GlRab11 is Necessary for Exosome-Like Vesicle Production
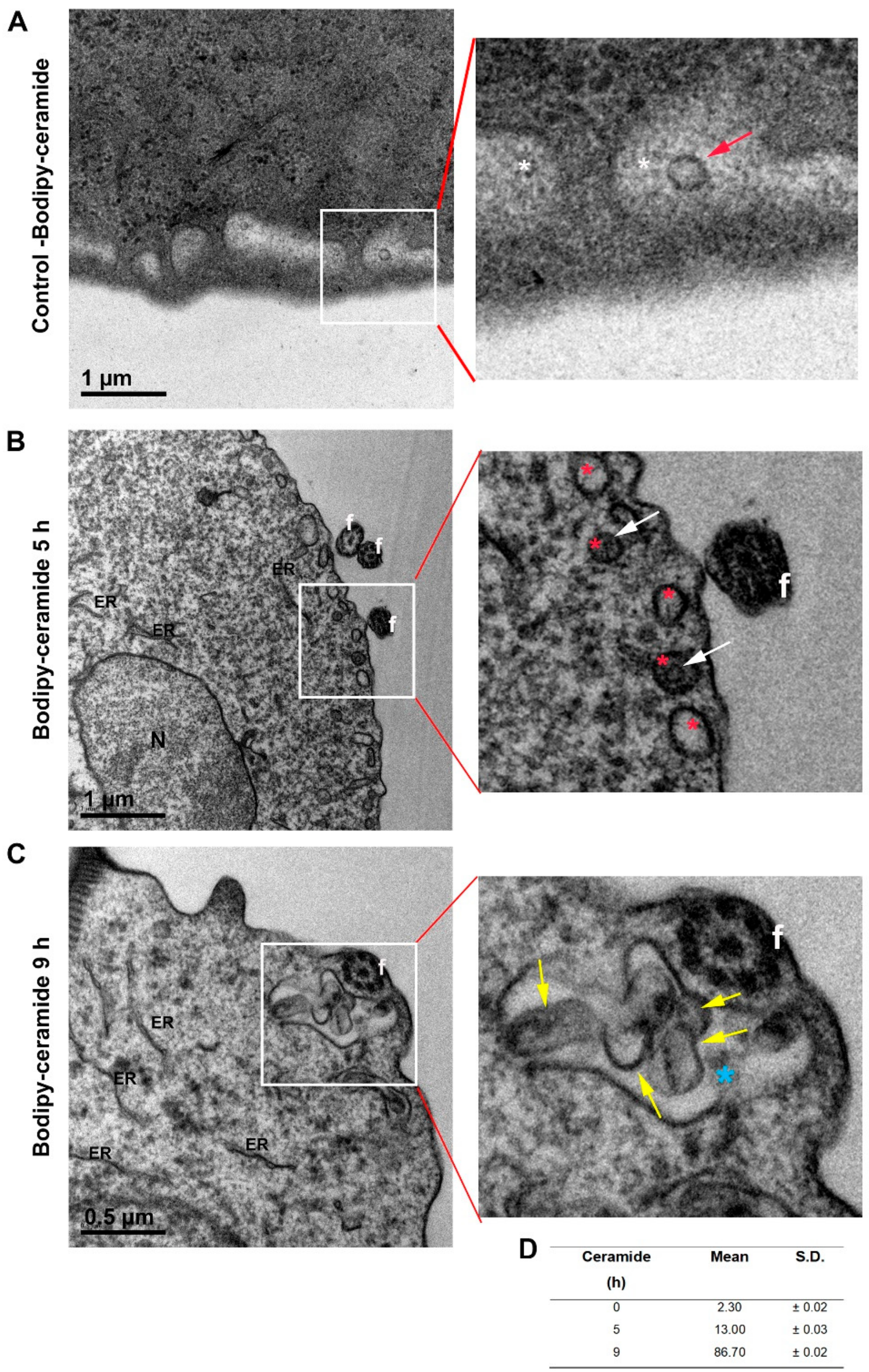
3.4. External Ceramide Triggers ILVs Budding Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, E.; Ma’ayeh, S.; Svard, S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dubourg, A.; Xia, D.; Winpenny, J.P.; Al Naimi, S.; Bouzid, M.; Sexton, D.W.; Wastling, J.M.; Hunter, P.R.; Tyler, K.M. Giardia secretome highlights secreted tenascins as a key component of pathogenesis. GigaScience 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma’ayeh, S.; Peirasmaki, D.; Lundstrom-Stadelmann, B.; Hellman, L.; Svard, S.G. Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines. Virulence 2018, 9, 879–894. [Google Scholar] [CrossRef]
- Evans-Osses, I.; Mojoli, A.; Monguio-Tortajada, M.; Marcilla, A.; Aran, V.; Amorim, M.; Inal, J.; Borras, F.E.; Ramirez, M.I. Microvesicles released from Giardia intestinalis disturb host-pathogen response in vitro. Eur. J. Cell Biol. 2017, 96, 131–142. [Google Scholar] [CrossRef]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Thery, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Stuffers, S.; Sem Wegner, C.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J.; Stenmark, H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008, 20, 4–11. [Google Scholar] [CrossRef]
- Williams, R.L.; Urbe, S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007, 8, 355–368. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Lanfredi-Rangel, A.; Attias, M.; de Carvalho, T.M.; Kattenbach, W.M.; De Souza, W. The peripheral vesicles of trophozoites of the primitive protozoan Giardia lamblia may correspond to early and late endosomes and to lysosomes. J. Struct. Biol. 1998, 123, 225–235. [Google Scholar] [CrossRef]
- Kattenbach, W.M.; Pimenta, P.F.; de Souza, W.; Pinto da Silva, P. Giardia duodenalis: A freeze-fracture, fracture-flip and cytochemistry study. Parasitol. Res. 1991, 77, 651–658. [Google Scholar] [CrossRef]
- Luchtel, D.L.; Lawrence, W.P.; DeWalle, F.B. Electron microscopy of Giardia lamblia cysts. Appl. Environ. Microbiol. 1980, 40, 821–832. [Google Scholar]
- Abodeely, M.; Dubois, K.N.; Hehl, A.; Stefanic, S.; Sajid, M.; Desouza, W.; Attias, M.; Engel, J.C.; Hsieh, I.; Fetter, R.D.; et al. A Contiguous Compartment Functions as Er and Endosome/Lysosome in Giardia Lamblia. Eukaryot. Cell 2009, 8, 1665–1676. [Google Scholar] [CrossRef]
- Rivero, M.R.; Jausoro, I.; Bisbal, M.; Feliziani, C.; Lanfredi-Rangel, A.; Touz, M.C. Receptor-mediated endocytosis and trafficking between endosomal-lysosomal vacuoles in Giardia lamblia. Parasitol. Res. 2013, 112, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.F.; Dacks, J.B.; Field, M.C. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic 2008, 9, 1698–1716. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Dutta, S.; Datta, S.P.; Sarkar, S. The minimal ESCRT machinery of Giardia lamblia has altered inter-subunit interactions within the ESCRT-II and ESCRT-III complexes. Eur. J. Cell Biol. 2018, 97, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Mandal, S.; Banerjee, S.; Ghosh, A.; Ganguly, S.; Sil, A.K.; Sarkar, S. Identification and characterization of a FYVE domain from the early diverging eukaryote Giardia lamblia. Curr. Microbiol. 2011, 62, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Shpak, M.; Duarte, T.T.; Mendez, T.L.; Maldonado, R.A.; Roychowdhury, S.; Rodrigues, M.L.; Das, S. Novel role of sphingolipid synthesis genes in regulating giardial encystation. Infect. Immun. 2008, 76, 2939–2949. [Google Scholar] [CrossRef]
- Hernandez, Y.; Castillo, C.; Roychowdhury, S.; Hehl, A.; Aley, S.B.; Das, S. Clathrin-dependent pathways and the cytoskeleton network are involved in ceramide endocytosis by a parasitic protozoan, Giardia lamblia. Int. J. Parasitol. 2007, 37, 21–32. [Google Scholar] [CrossRef][Green Version]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Charrin, S.; le Naour, F.; Silvie, O.; Milhiet, P.E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [CrossRef]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome release of beta-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Tejera, E.; Rocha-Perugini, V.; Lopez-Martin, S.; Perez-Hernandez, D.; Bachir, A.I.; Horwitz, A.R.; Vazquez, J.; Sanchez-Madrid, F.; Yanez-Mo, M. CD81 regulates cell migration through its association with Rac GTPase. Mol. Biol. Cell 2013, 24, 261–273. [Google Scholar] [CrossRef]
- Petersen, S.H.; Odintsova, E.; Haigh, T.A.; Rickinson, A.B.; Taylor, G.S.; Berditchevski, F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur. J. Immunol. 2011, 41, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Hyenne, V.; Apaydin, A.; Rodriguez, D.; Spiegelhalter, C.; Hoff-Yoessle, S.; Diem, M.; Tak, S.; Lefebvre, O.; Schwab, Y.; Goetz, J.G.; et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 2015, 211, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Langford, T.D.; Silberman, J.D.; Weiland, M.E.; Svard, S.G.; McCaffery, J.M.; Sogin, M.L.; Gillin, F.D. Giardia lamblia: Identification and characterization of Rab and GDI proteins in a genome survey of the ER to Golgi endomembrane system. Exp. Parasitol. 2002, 101, 13–24. [Google Scholar] [CrossRef]
- Hardin, W.R.; Li, R.; Xu, J.; Shelton, A.M.; Alas, G.C.M.; Minin, V.N.; Paredez, A.R. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. Proc. Natl. Acad. Sci. USA 2017, 114, E5854–E5863. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.; Regos, A.; Li, Y.; Schraner, E.M.; Wild, P.; Muller, N.; Knopf, L.G.; Hehl, A.B. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J. Biol. Chem. 2003, 278, 24837–24848. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Romero, A.; Leon-Avila, G.; Wang, C.C.; Perez Rangel, A.; Camacho Nuez, M.; Garcia Tovar, C.; Ayala-Sumuano, J.T.; Luna-Arias, J.P.; Hernandez, J.M. Rab11 and actin cytoskeleton participate in Giardia lamblia encystation, guiding the specific vesicles to the cyst wall. PLoS Negl. Trop. Dis. 2010, 4, e697. [Google Scholar] [CrossRef]
- Savina, A.; Vidal, M.; Colombo, M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002, 115, 2505–2515. [Google Scholar]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Wehman, A.M.; Poggioli, C.; Schweinsberg, P.; Grant, B.D.; Nance, J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr. Biol. 2011, 21, 1951–1959. [Google Scholar] [CrossRef]
- Corrigan, L.; Redhai, S.; Leiblich, A.; Fan, S.J.; Perera, S.M.; Patel, R.; Gandy, C.; Wainwright, S.M.; Morris, J.F.; Hamdy, F.; et al. BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J. Cell Biol. 2014, 206, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Beckett, K.; Monier, S.; Palmer, L.; Alexandre, C.; Green, H.; Bonneil, E.; Raposo, G.; Thibault, P.; Le Borgne, R.; Vincent, J.P. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 2013, 14, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Touz, M.C.; Nores, M.J.; Slavin, I.; Carmona, C.; Conrad, J.T.; Mowatt, M.R.; Nash, T.E.; Coronel, C.E.; Lujan, H.D. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 2002, 277, 8474–8481. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.R.; Kulakova, L.; Touz, M.C. Long double-stranded RNA produces specific gene downregulation in Giardia lamblia. J. Parasitol. 2010, 96, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Touz, M.C.; Kulakova, L.; Nash, T.E. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol. Biol. Cell 2004, 15, 3053–3060. [Google Scholar] [CrossRef]
- Nash, T.E.; Aggarwal, A.; Adam, R.D.; Conrad, J.T.; Merritt, J.W., Jr. Antigenic variation in Giardia lamblia. J. Immunol. 1988, 141, 636–641. [Google Scholar]
- Gillin, F.D.; Diamond, L.S. Entamoeba histolytica and Giardia lamblia: Effects of cysteine and oxygen tension on trophozoite attachment to glass and survival in culture media. Exp. Parasitol. 1981, 52, 9–17. [Google Scholar] [CrossRef]
- Singer, S.M.; Yee, J.; Nash, T.E. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol. Biochem. Parasitol. 1998, 92, 59–69. [Google Scholar] [CrossRef]
- Chiou, N.-T.; Ansel, K.M. Improved exosome isolation by sucrose gradient fractionation of ultracentrifuged crude exosome pellets. Protoc. Exch. 2016, 10. [Google Scholar] [CrossRef]
- Touz, M.C.; Conrad, J.T.; Nash, T.E. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Mol. Microbiol. 2005, 58, 999–1011. [Google Scholar] [CrossRef]
- Lalle, M.; Salzano, A.M.; Crescenzi, M.; Pozio, E. The Giardia duodenalis 14-3-3 protein is post-translationally modified by phosphorylation and polyglycylation of the C-terminal tail. J. Biol. Chem. 2006, 281, 5137–5148. [Google Scholar] [CrossRef]
- Camerini, S.; Bocedi, A.; Cecchetti, S.; Casella, M.; Carbo, M.; Morea, V.; Pozio, E.; Ricci, G.; Lalle, M. Proteomic and functional analyses reveal pleiotropic action of the anti-tumoral compound NBDHEX in Giardia duodenalis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 147–158. [Google Scholar] [CrossRef]
- Paredez, A.R.; Assaf, Z.J.; Sept, D.; Timofejeva, L.; Dawson, S.C.; Wang, C.J.; Cande, W.Z. An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 6151–6156. [Google Scholar] [CrossRef]
- Feliziani, C.; Zamponi, N.; Gottig, N.; Ropolo, A.S.; Lanfredi-Rangel, A.; Touz, M.C. The giardial ENTH protein participates in lysosomal protein trafficking and endocytosis. Biochim. Biophys. Acta 2015, 1853, 646–659. [Google Scholar] [CrossRef]
- Feliziani, C.; Merino, M.C.; Rivero, M.R.; Hellman, U.; Pistoresi-Palencia, M.C.; Ropolo, A.S. Immunodominant proteins alpha-1 giardin and beta-giardin are expressed in both assemblages A and B of Giardia lamblia. BMC Microbiol. 2011, 11, 233. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Zamponi, N.; Zamponi, E.; Mayol, G.F.; Lanfredi-Rangel, A.; Svard, S.G.; Touz, M.C. The ER is the sorting core facility in the Golgi-lacking protozoan Giardia lamblia. Traffic 2017. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Kaplan, A.; Bueno, M.; Fournier, A.E. Extracellular functions of 14-3-3 adaptor proteins. Cell. Signal. 2017, 31, 26–30. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Arora, V.; Kottarath, S.K.; Dinda, A.K.; Vaishnav, P.K.; Nayak, B.; Mohanty, S. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 180. [Google Scholar] [CrossRef]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Touz, M.C.; Gottig, N.; Nash, T.E.; Lujan, H.D. Identification and characterization of a novel secretory granule calcium-binding protein from the early branching eukaryote Giardia lamblia. J. Biol. Chem. 2002, 277, 50557–50563. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.V.; Quiroga, R.; Gottig, N.; Nakanishi, H.; Nash, T.E.; Neiman, A.; Lujan, H.D. Characterization of SNAREs determines the absence of a typical Golgi apparatus in the ancient eukaryote Giardia lamblia. J. Biol. Chem. 2008, 283, 35996–36010. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.A.; Noiva, R.; Mehta, K.; McCaffery, J.M.; Aley, S.B.; Svard, S.G.; Nystul, T.G.; Reiner, D.S.; Silberman, J.D.; Gillin, F.D. Novel protein-disulfide isomerases from the early-diverging protist Giardia lamblia. J. Biol. Chem. 1999, 274, 29805–29811. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. Embo J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Jackson, C.E.; Scruggs, B.S.; Schaffer, J.E.; Hanson, P.I. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys. J. 2017, 113, 1342–1352. [Google Scholar] [CrossRef]
- Lin, Y.; Kimpler, L.A.; Naismith, T.V.; Lauer, J.M.; Hanson, P.I. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J. Biol. Chem. 2005, 280, 12799–12809. [Google Scholar] [CrossRef]
- Taylor, G.M.; Hanson, P.I.; Kielian, M. Ubiquitin depletion and dominant-negative VPS4 inhibit rhabdovirus budding without affecting alphavirus budding. J. Virol. 2007, 81, 13631–13639. [Google Scholar] [CrossRef]
- Vale, R.D. AAA proteins. Lords of the ring. J. Cell Biol. 2000, 150, F13–F19. [Google Scholar] [CrossRef]
- Takahashi, S.; Kubo, K.; Waguri, S.; Yabashi, A.; Shin, H.W.; Katoh, Y.; Nakayama, K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J. Cell Sci. 2012, 125, 4049–4057. [Google Scholar] [CrossRef]
- Holopainen, J.M.; Angelova, M.I.; Kinnunen, P.K. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys. J. 2000, 78, 830–838. [Google Scholar] [CrossRef]
- Zha, X.; Pierini, L.M.; Leopold, P.L.; Skiba, P.J.; Tabas, I.; Maxfield, F.R. Sphingomyelinase treatment induces ATP-independent endocytosis. J. Cell Biol. 1998, 140, 39–47. [Google Scholar] [CrossRef]
- Li, R.; Blanchette-Mackie, E.J.; Ladisch, S. Induction of endocytic vesicles by exogenous C(6)-ceramide. J. Biol. Chem. 1999, 274, 21121–21127. [Google Scholar] [CrossRef]
- Pagano, R.E.; Sepanski, M.A.; Martin, O.C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: Interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J. Cell Biol. 1989, 109, 2067–2079. [Google Scholar] [CrossRef]
- Pagano, R.E.; Martin, O.C.; Kang, H.C.; Haugland, R.P. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: Accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J. Cell Biol. 1991, 113, 1267–1279. [Google Scholar] [CrossRef]
- Laulagnier, K.; Vincent-Schneider, H.; Hamdi, S.; Subra, C.; Lankar, D.; Record, M. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol. Dis. 2005, 35, 116–121. [Google Scholar] [CrossRef]
- Ma’ayeh, S.Y.; Liu, J.; Peirasmaki, D.; Hornaeus, K.; Bergstrom Lind, S.; Grabherr, M.; Bergquist, J.; Svard, S.G. Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells. PLoS Negl. Trop. Dis. 2017, 11, e0006120. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Field, M.C.; Dacks, J.B. First and last ancestors: Reconstructing evolution of the endomembrane system with ESCRTs, vesicle coat proteins, and nuclear pore complexes. Curr. Opin. Cell Biol. 2009, 21, 4–13. [Google Scholar] [CrossRef]
- Yang, M.; Coppens, I.; Wormsley, S.; Baevova, P.; Hoppe, H.C.; Joiner, K.A. The Plasmodium falciparum Vps4 homolog mediates multivesicular body formation. J. Cell Sci. 2004, 117, 3831–3838. [Google Scholar] [CrossRef]
- Allen, C.L.; Liao, D.; Chung, W.L.; Field, M.C. Dileucine signal-dependent and AP-1-independent targeting of a lysosomal glycoprotein in Trypanosoma brucei. Mol. Biochem. Parasitol. 2007, 156, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Midlej, V.; de Souza, W.; Benchimol, M. The peripheral vesicles gather multivesicular bodies with different behavior during the Giardia intestinalis life cycle. J. Struct. Biol. 2019, 207, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jae, N.; McEwan, D.G.; Manavski, Y.; Boon, R.A.; Dimmeler, S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015, 589, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Grant, B.D.; Harada, A.; Sato, K. Rab11 is required for synchronous secretion of chondroitin proteoglycans after fertilization in Caenorhabditis elegans. J. Cell Sci. 2008, 121, 3177–3186. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Gronborg, M.; Mobius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Chen, D.; Wandinger-Ness, A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell 1998, 9, 3241–3257. [Google Scholar] [CrossRef]
- Miras, S.L.; Merino, M.C.; Gottig, N.; Ropolo, A.S.; Touz, M.C. The giardial VPS35 retromer subunit is necessary for multimeric complex assembly and interaction with the vacuolar protein sorting receptor. Biochim. Biophys. Acta 2013, 1833, 2628–2638. [Google Scholar] [CrossRef][Green Version]
- Rivero, M.R.; Miras, S.L.; Feliziani, C.; Zamponi, N.; Quiroga, R.; Hayes, S.F.; Ropolo, A.S.; Touz, M.C. Vacuolar protein sorting receptor in Giardia lamblia. PLoS ONE 2012, 7, e43712. [Google Scholar] [CrossRef]
- Midlej, V.; Benchimol, M. Giardia lamblia behavior during encystment: How morphological changes in shape occur. Parasitol. Int. 2009, 58, 72–80. [Google Scholar] [CrossRef]
- Faso, C.; Hehl, A.B. Membrane trafficking and organelle biogenesis in Giardia lamblia: Use it or lose it. Int. J. Parasitol. 2011, 41, 471–480. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano, S.; Musso, J.; Feliziani, C.; Zamponi, N.; Frontera, L.S.; Ropolo, A.S.; Lanfredi-Rangel, A.; Lalle, M.; Touz, M.C. Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk. Cells 2019, 8, 1600. https://doi.org/10.3390/cells8121600
Moyano S, Musso J, Feliziani C, Zamponi N, Frontera LS, Ropolo AS, Lanfredi-Rangel A, Lalle M, Touz MC. Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk. Cells. 2019; 8(12):1600. https://doi.org/10.3390/cells8121600
Chicago/Turabian StyleMoyano, Sofía, Juliana Musso, Constanza Feliziani, Nahuel Zamponi, Lorena Soledad Frontera, Andrea Silvana Ropolo, Adriana Lanfredi-Rangel, Marco Lalle, and María C. Touz. 2019. "Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk" Cells 8, no. 12: 1600. https://doi.org/10.3390/cells8121600
APA StyleMoyano, S., Musso, J., Feliziani, C., Zamponi, N., Frontera, L. S., Ropolo, A. S., Lanfredi-Rangel, A., Lalle, M., & Touz, M. C. (2019). Exosome Biogenesis in the Protozoa Parasite Giardia lamblia: A Model of Reduced Interorganellar Crosstalk. Cells, 8(12), 1600. https://doi.org/10.3390/cells8121600




