The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cardiocirculatory Death Induction
2.3. Surgical Procedure of Liver Transplantation
2.4. Experimental Design
- Group 1. Sham (n = 12). Six Ob and six Ln Zucker rats were anesthetized and maintained for 45 min.
- Group 3. LT (n = 24, 12 transplantations). Six Ob and six Ln Zucker rats were anesthetized and maintained for 45 min. Then, steatotic and non-steatotic liver grafts were flushed, isolated, and stored in ice-cold UW solution for 6 h. Finally, they were implanted in 12 Ln Zucker rats [9].
- Group 4. CD+LT (n = 24, 12 transplantations). Six Ob and six Ln Zucker rats were anesthetized. After the induction of CD, the animals were maintained in situ in warm ischemia for 45 min. Then, steatotic and non-steatotic liver grafts were flushed, isolated, and stored in ice-cold UW solution for 6 h. Finally, they were implanted in 12 Ln Zucker rats [9,10,11].
- Group 5. CD+GLP1+LT (n = 24, 12 transplantations). As group 4, but donors were treated with GLP1 [glucagon-like peptide 1 (7–36) amide] infusion into the portal vein at a rate of 1 pMol × kg × min, 10 min before CD. Then, steatotic and non-steatotic liver grafts were flushed, isolated, and stored in ice-cold UW solution for 6 h. Finally, they were implanted in 12 Ln Zucker rats [13].
2.5. Biochemical Assays
2.6. Western Blotting
2.7. Liver and Intestine Histology
2.8. Immunohistochemistry
2.9. Statistics
3. Results
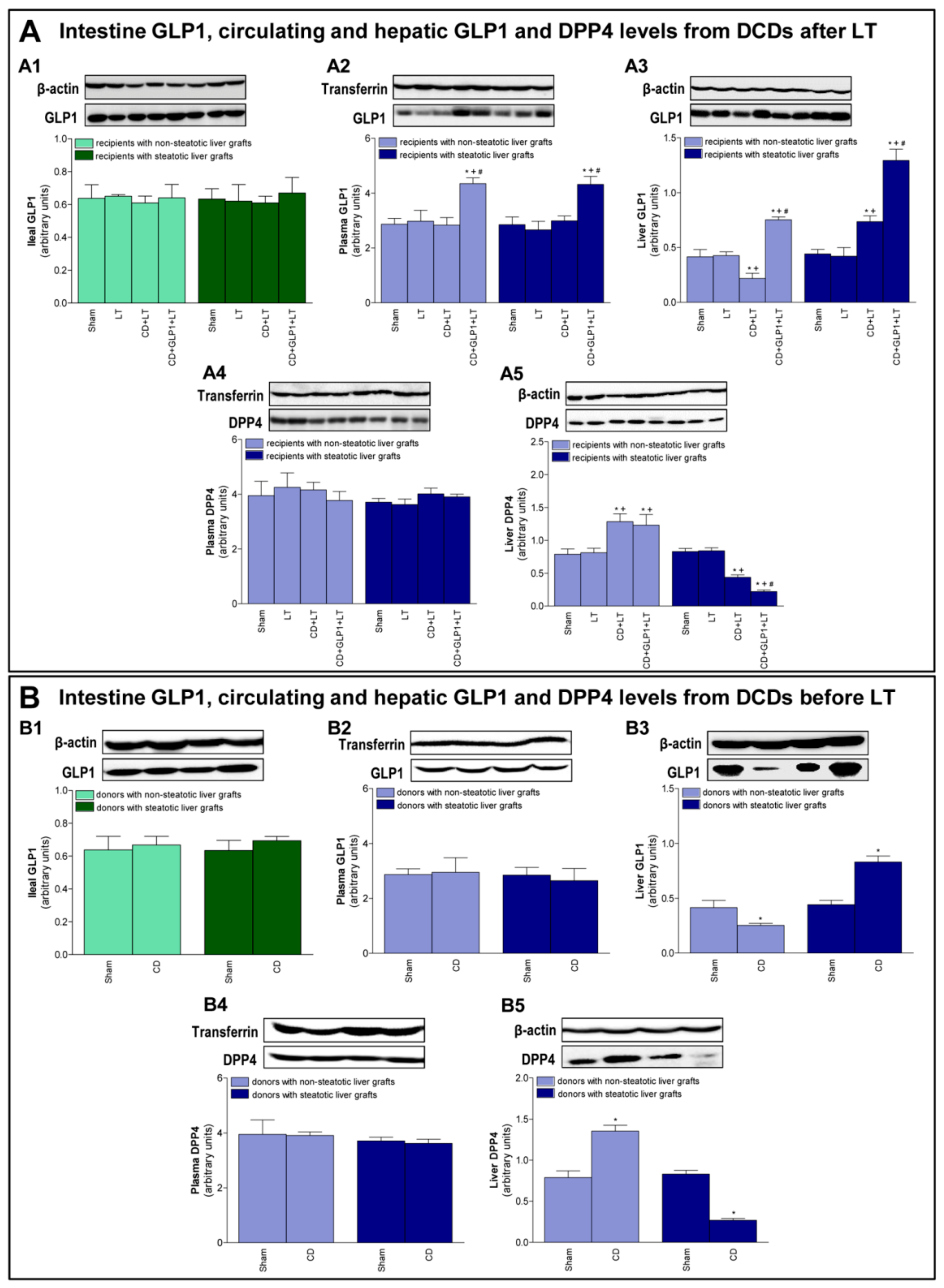
3.1. GLP1 and DPP4 in Recipients of Steatotic and Non-Steatotic Liver Grafts from DCDs
3.2. GLP1 and DPP4 Levels in Donors Before Procurement of Liver Grafts from DCDs
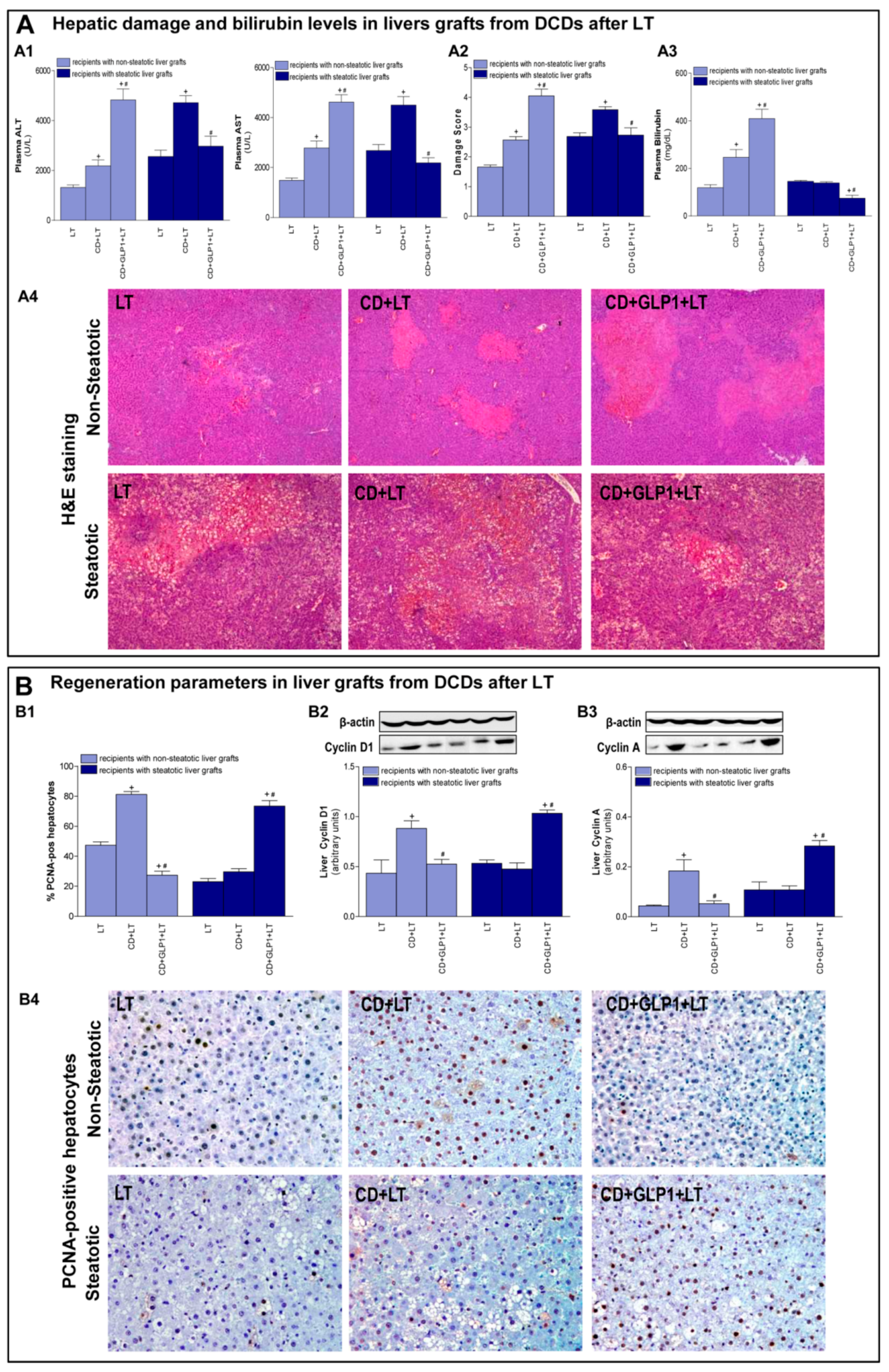
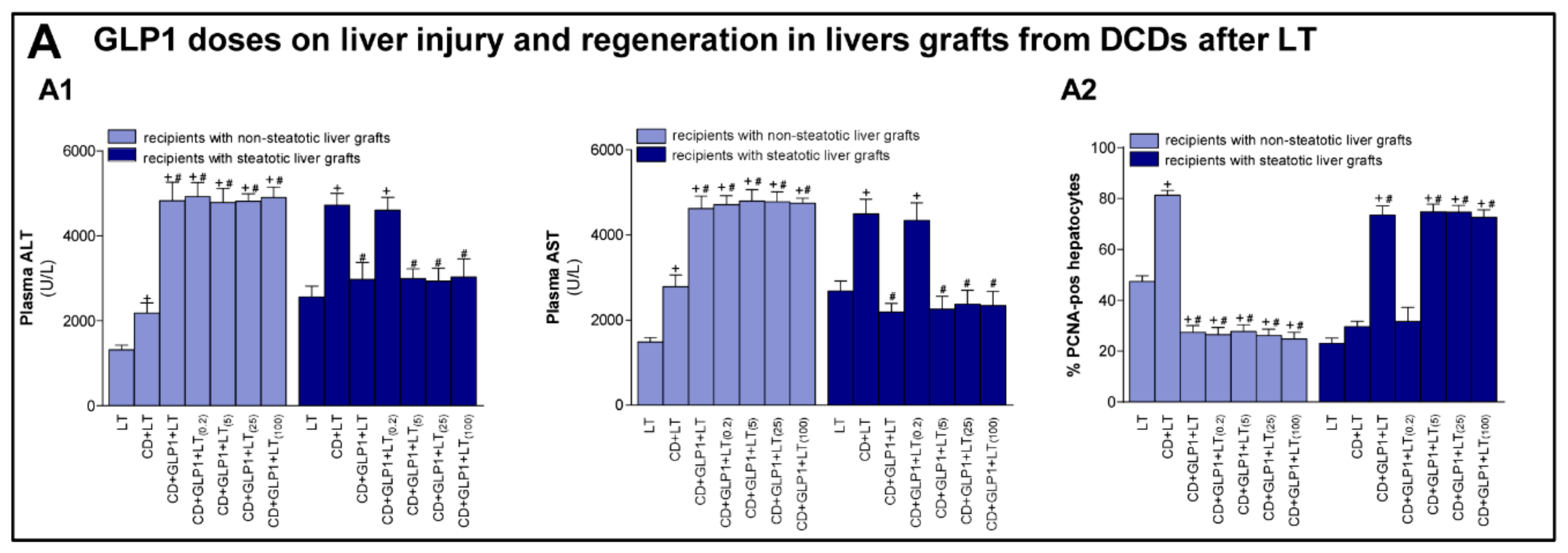
3.3. Relevance of GLP1 in Non-Steatotic and Steatotic LT from DCDs
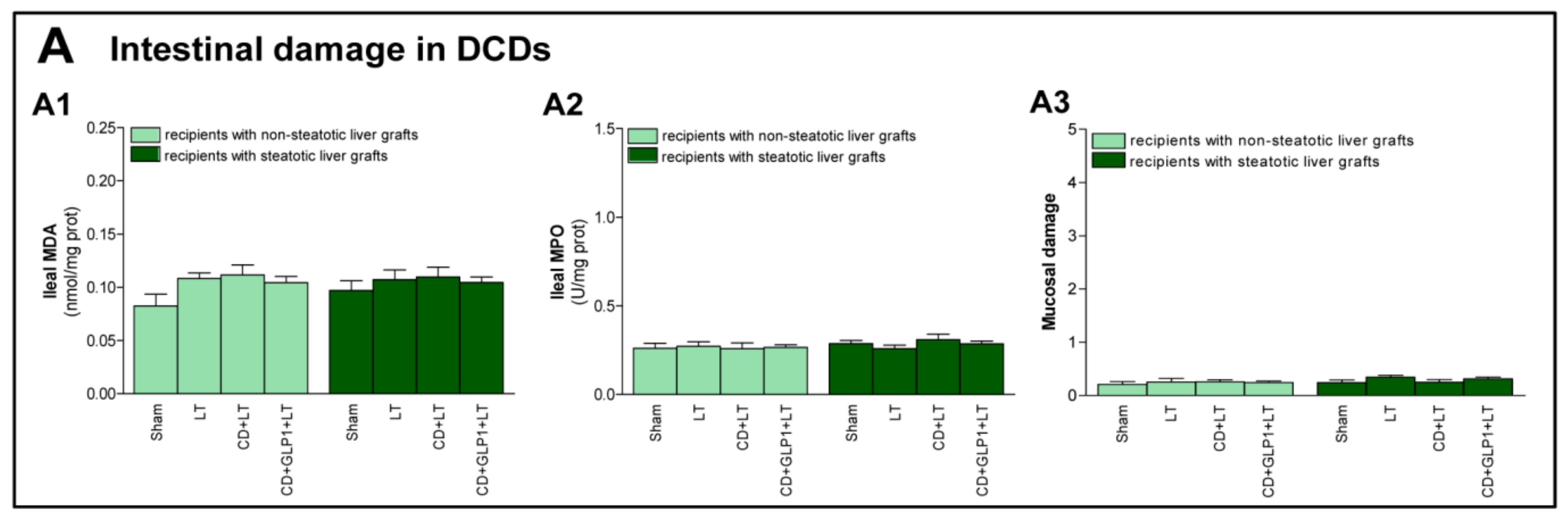
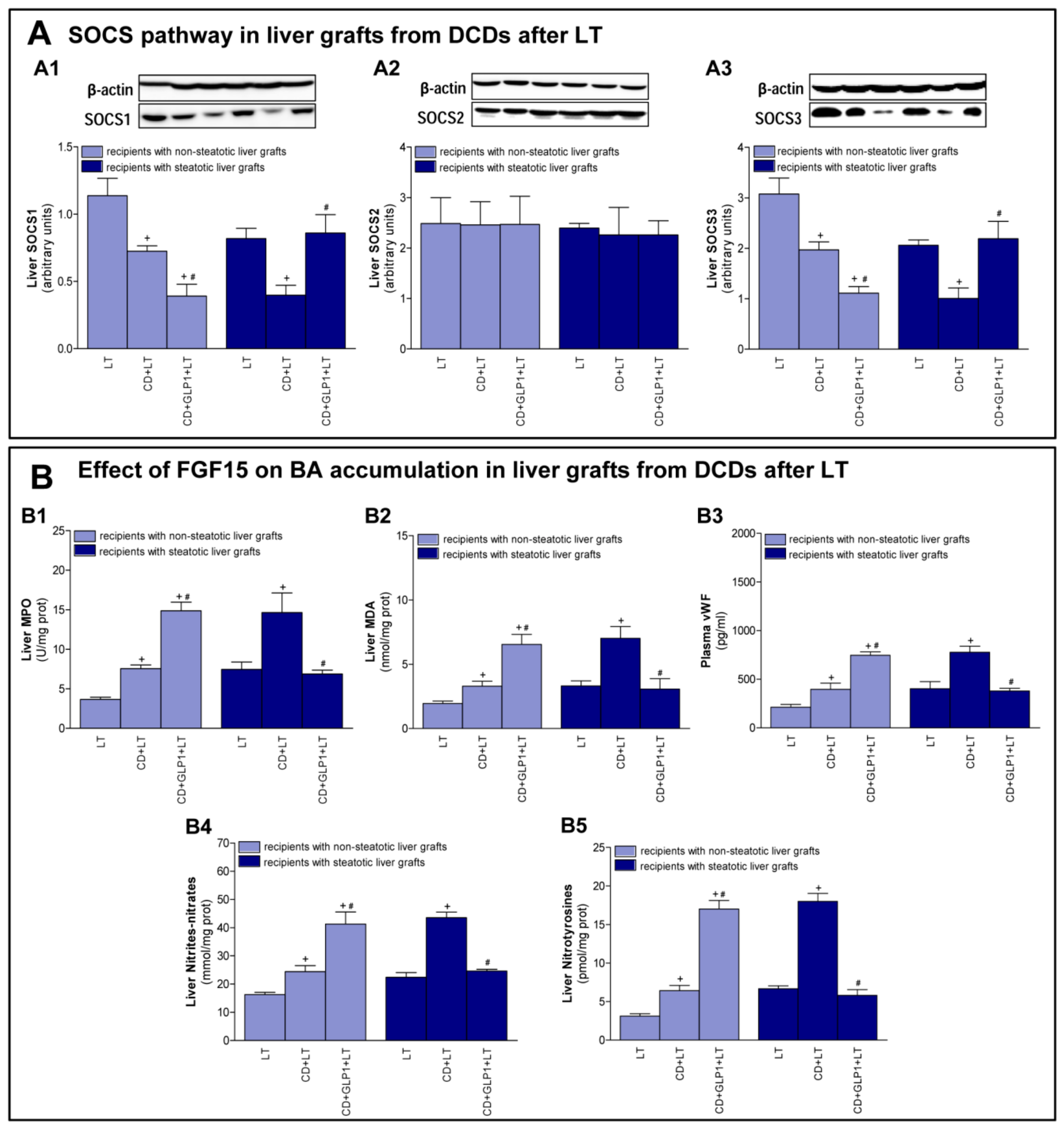
3.4. The Effect of GLP1 on Inflammatory Response in Non-Steatotic and Steatotic LT from DCDs
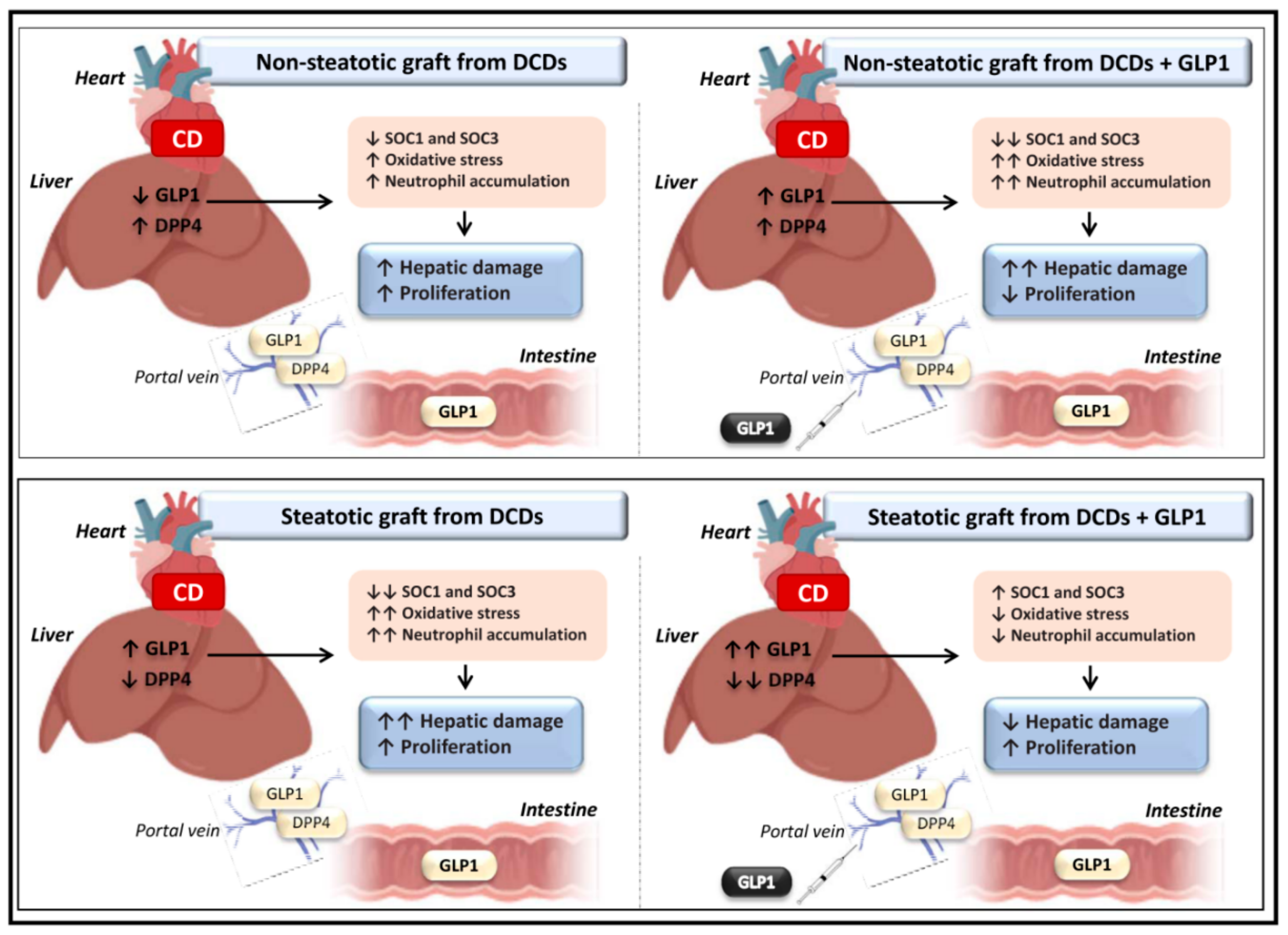
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Detry, O.; Donckier, V.; Lucidi, V.; Ysebaert, D.; Chapelle, T.; Lerut, J.; Ciccarelli, O.; Pirenne, J.; Monbaliu, D.; De Roover, A.; et al. Liver transplantation from donation after cardiac death donors: Initial Belgian experience 2003-2007. Transpl. Int. 2010, 23, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.G.; Brubaker, P.L. Glucagon-like peptide 1 secretion by the L-cell. Diabetes 2006, 55, 70–77. [Google Scholar] [CrossRef]
- Abdelsameea, A.A.; Abbas, N.A.; Abdel Raouf, S.M. Liraglutide attenuates partial warm ischemia-reperfusion injury in rat livers. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 311–319. [Google Scholar] [CrossRef]
- Sharma, D.; Verma, S.; Vaidya, S.; Kalia, K.; Tiwari, V. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed. Pharmacother. 2018, 108, 952–962. [Google Scholar]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Chowdhury, H.H.; Velebit, J.; Radić, N.; Frančič, V.; Kreft, M.; Zorec, R. Hypoxia alters the expression of dipeptidyl peptidase 4 and induces developmental remodeling of human preadipocytes. J. Diabetes Res. 2016, 2016, 7481470. [Google Scholar] [CrossRef]
- Kim, Y.O.; Schuppan, D. When GLP-1 hits the liver: A novel approach for insulin resistance and NASH. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, 759–761. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.B.; Meroño, N.; Mendes-Braz, M.; Gracia-Sancho, J.; Martínez-Carreres, L.; Cornide-Petronio, M.E.; Casillas-Ramirez, A.; Rodés, J.; Peralta, C. The effect of brain death in rat steatotic and non-steatotic liver transplantation with previous ischemic preconditioning. J. Hepatol. 2015, 62, 83–91. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, G.D.; Wu, L.W.; Hu, R.D. Dynamical changing patterns of histological structure and ultrastructure of liver graft undergoing warm ischemia injury from non-heart-beating donor in rats. World J. Gastroenterol. 2006, 12, 4902–4905. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J. Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Calne, R.Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation 1979, 28, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Nakabayashi, H.; Uehara, K.; Nakagawa, A.; Uchida, K.; Koya, D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Bujaldon, E.; Cornide-Petronio, M.E.; Gulfo, J.; Rotondo, F.; Ávalos de León, C.; Negrete-Sánchez, E.; Gracia-Sancho, J.; Novials, A.; Jiménez-Castro, M.B.; Peralta Uroz, C. Relevance of VEGFA in rat livers subjected to partial hepatectomy under ischemia-reperfusion. J. Mol. Med. 2019, 97, 1299–1314. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Negrete-Sánchez, E.; Gulfo, J.; Avalos de León, C.G.; Casillas-Ramirez, A.; Cornide-Petronio, M.E.; Bujaldon, E.; Rotondo, F.; Gracia-Sancho, J.; Jiménez-Castro, M.B.; et al. EGF-GH axis in rat steatotic and non-steatotic liver transplantation from brain-dead donors. Transplantation 2019, 103, 1349–1359. [Google Scholar] [CrossRef]
- Peralta, C.; Rull, R.; Rimola, A.; Deulofeu, R.; Rosellò-Catafau, J.; Gelpí, E.; Rodés, J. Endogenous nitric oxide and exogenous nitric oxide supplementation in hepatic ischemia-reperfusion injury in the rat. Transplantation 2001, 71, 529–536. [Google Scholar] [CrossRef]
- Chiu, C.J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Monbaliu, D.; Pirenne, J.; Talbot, D. Liver transplantation using donation after cardiac death donors. J. Hepatol. 2012, 56, 474–485. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Badry, A.M.E.; Hassan, A.E.A.; Redwan, A.A.; Vivarelli, M. Review on liver steatosis and its impact on liver transplantation. J. Liver Res. Disord Ther. 2017, 3, 100–110. [Google Scholar] [CrossRef]
- McCormack, L.; Dutkowski, P.; El-Badry, A.M.; Clavien, P.A. Liver transplantation using fatty livers: Always feasible? J. Hepatol. 2011, 54, 1055–1062. [Google Scholar] [CrossRef]
- Jin, T.; Weng, J. Hepatic functions of GLP-1 and its based drugs: Current disputes and perspectives. Am. J. Physiol. Endocrinol. Metab. 2016, 311, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.M.; March, J.E.; Kemp, P.A.; Bennett, T.; Baker, D.J. Possible involvement of GLP-1(9-36) in the regional haemodynamic effects of GLP-1(7-36) in conscious rats. Br. J. Pharmacol. 2010, 161, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Kim, J.G.; Drucker, D.J. Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes 2004, 53, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.; Brown, S.B.; Bhashyam, S.; Parikh, P.; Bolukoglu, H.; Shannon, R.P. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ. Heart Fail. 2008, 1, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Freyse, E.J.; Berg, S.; Kohnert, K.D.; Heinke, P.; Salzsieder, E. DPP-4 inhibition increases GIP and decreases GLP-1 incretin effects during intravenous glucose tolerance test in Wistar rats. Biol. Chem. 2011, 392, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Cywes, R.; Greig, P.D.; Sanabria, J.R.; Clavien, P.A.; Levy, G.A.; Harvey, P.R.; Strasberg, S.M. Effect of intraportal glucose infusion on hepatic glycogen content and degradation, and outcome of liver transplantation. Ann. Surg. 1992, 216, 235–247. [Google Scholar] [CrossRef]
- Kristo, I.; Wilflingseder, J.; Kainz, A.; Marschalek, J.; Wekerle, T.; Mühlbacher, F.; Oberbauer, R.; Bodingbauer, M. Effect of intraportal infusion of tacrolimus on ischaemic reperfusion injury in orthotopic liver transplantation: A randomized controlled trial. Transpl. Int. 2011, 24, 912–919. [Google Scholar] [CrossRef]
- Inagaki, H.; Kurokawa, T.; Nonami, T.; Miwa, T.; Nakao, A.; Takagi, H. The effect of intraportal administration of prostaglandin E1 on liver blood flow and liver function. Hepatogastroenterology 1999, 46, 2909–2913. [Google Scholar]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.W.; Rhee, E.J.; Lee, W.Y. GLP-1 Receptor agonist and non-alcoholic fatty liver disease. Diabetes Metab J. 2012, 36, 262–267. [Google Scholar] [CrossRef]
- Kim, N.H.; Yu, T.; Lee, D.H. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed. Res. Int. 2014, 2014, 368703. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005, 108, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Lambeir, A.M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Nauck, M.A.; Meier, J.; Hucking, K.; Holst, J.J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 2000, 85, 3575–3581. [Google Scholar] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucosedependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1 (7–36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef]
- Duncan, S.A.; Baganizi, D.R.; Sahu, R.; Singh, S.R.; Dennis, V.A. SOCS proteins as regulators of inflammatory responses induced by bacterial infections: A review. Front. Microbiol. 2017, 8, 2431. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Cesaratto, L.; Vascotto, C.; Calligaris, S.; Tell, G. The importance of redox state in liver damage. Ann. Hepatol. 2004, 3, 86–92. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avalos-de León, C.G.; Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Casillas-Ramírez, A.; Peralta, C. The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors. Cells 2019, 8, 1599. https://doi.org/10.3390/cells8121599
Avalos-de León CG, Jiménez-Castro MB, Cornide-Petronio ME, Casillas-Ramírez A, Peralta C. The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors. Cells. 2019; 8(12):1599. https://doi.org/10.3390/cells8121599
Chicago/Turabian StyleAvalos-de León, Cindy G., Mónica B. Jiménez-Castro, María Eugenia Cornide-Petronio, Araní Casillas-Ramírez, and Carmen Peralta. 2019. "The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors" Cells 8, no. 12: 1599. https://doi.org/10.3390/cells8121599
APA StyleAvalos-de León, C. G., Jiménez-Castro, M. B., Cornide-Petronio, M. E., Casillas-Ramírez, A., & Peralta, C. (2019). The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors. Cells, 8(12), 1599. https://doi.org/10.3390/cells8121599





