Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Lines and Culture
2.3. Chemicals, Reagents and Plasmids
2.4. Cell Transfection and Luciferase Assay
2.5. MTS Assay
2.6. Western Blot Analysis and Antibody Information
2.7. Quantitative RT-PCR with Reverse Transcription
2.8. Enzyme-linked Immunosorbent Assay (ELISA)
2.9. Immunofluorescence Assay
2.10. JC-1 Staining
2.11. Monodansylcadaverine and Lysotracker Red Staining
2.12. Annexin V-FITC/PI Double Staining Assay
2.13. Preparation of RC-loaded Microemulsion
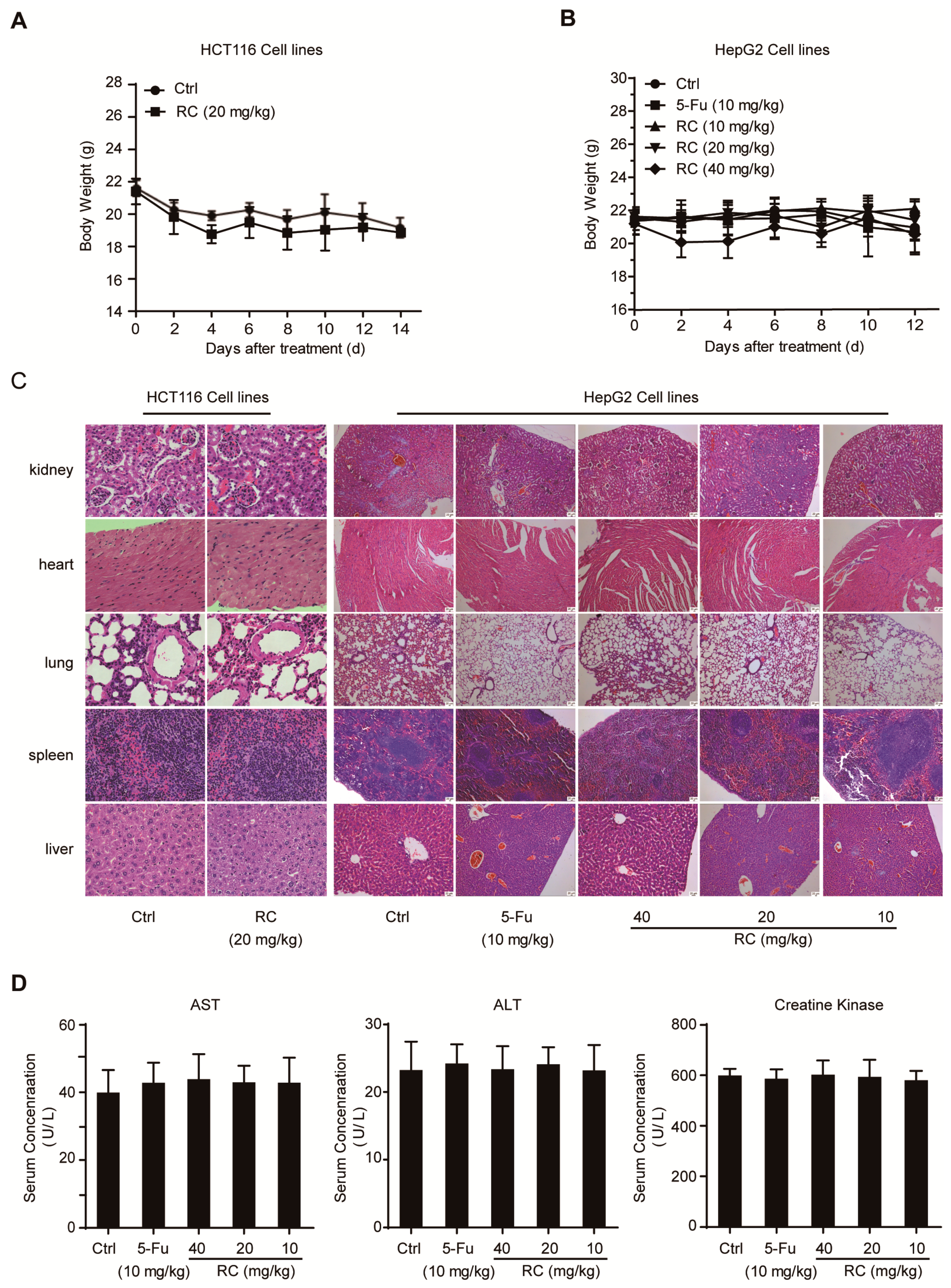
2.14. Tumor Xenograft in Nude Mice and Toxicity Assessment
2.15. Statistical Analysis
3. Results
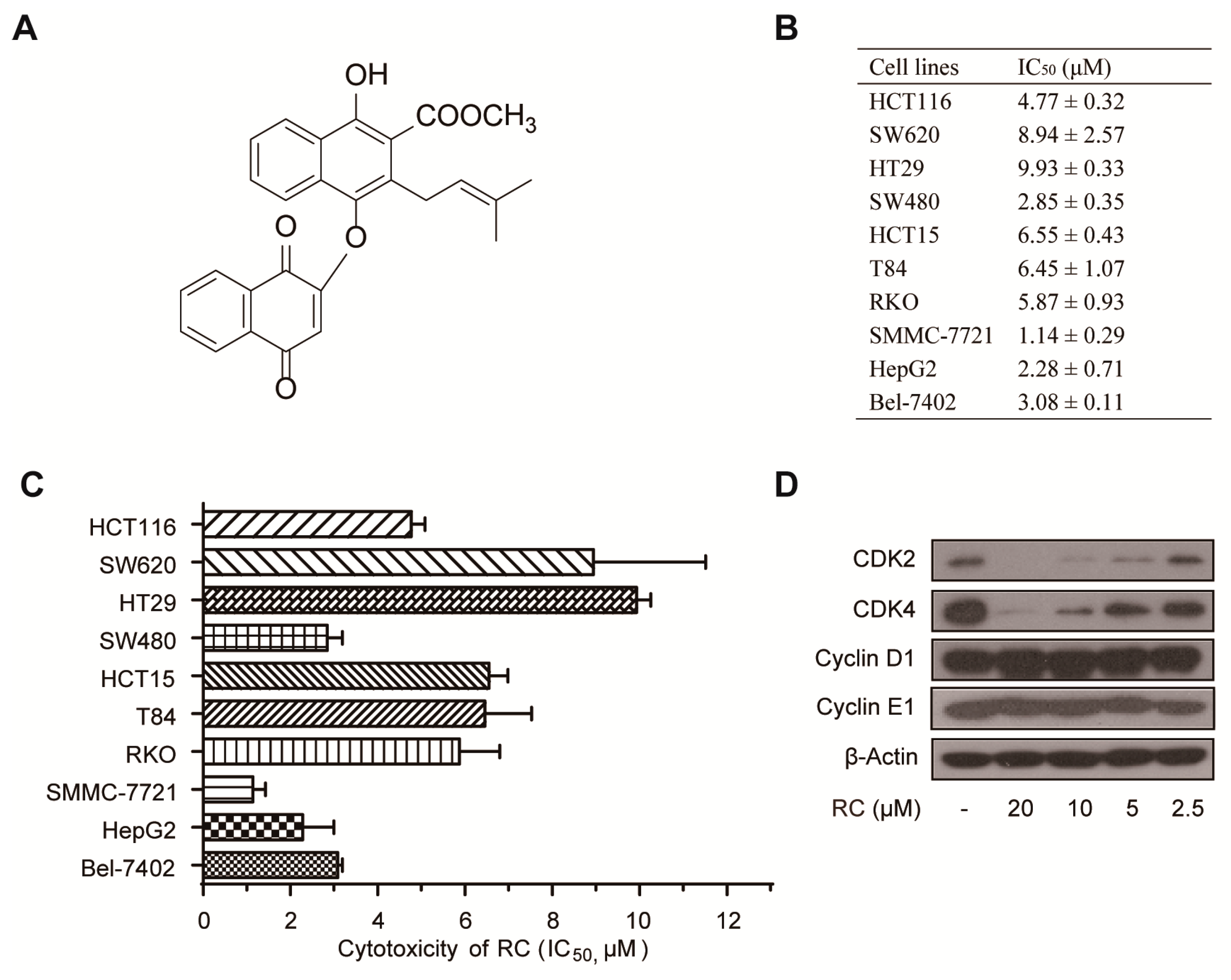
3.1. RC Inhibits the Growth of Cancer Cell Lines
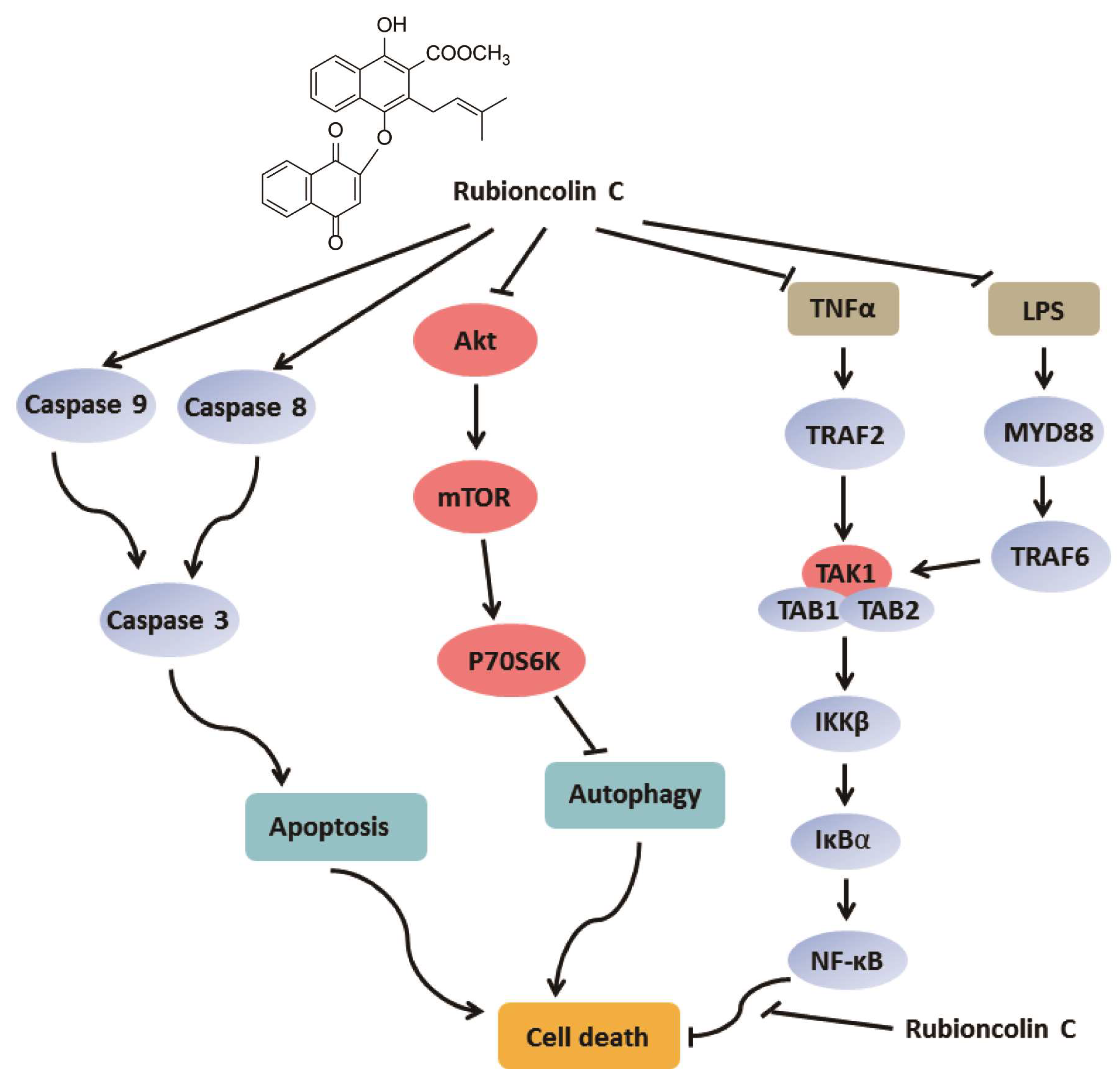
3.2. RC Induces Apoptosis in HCT116 and HepG2 cells
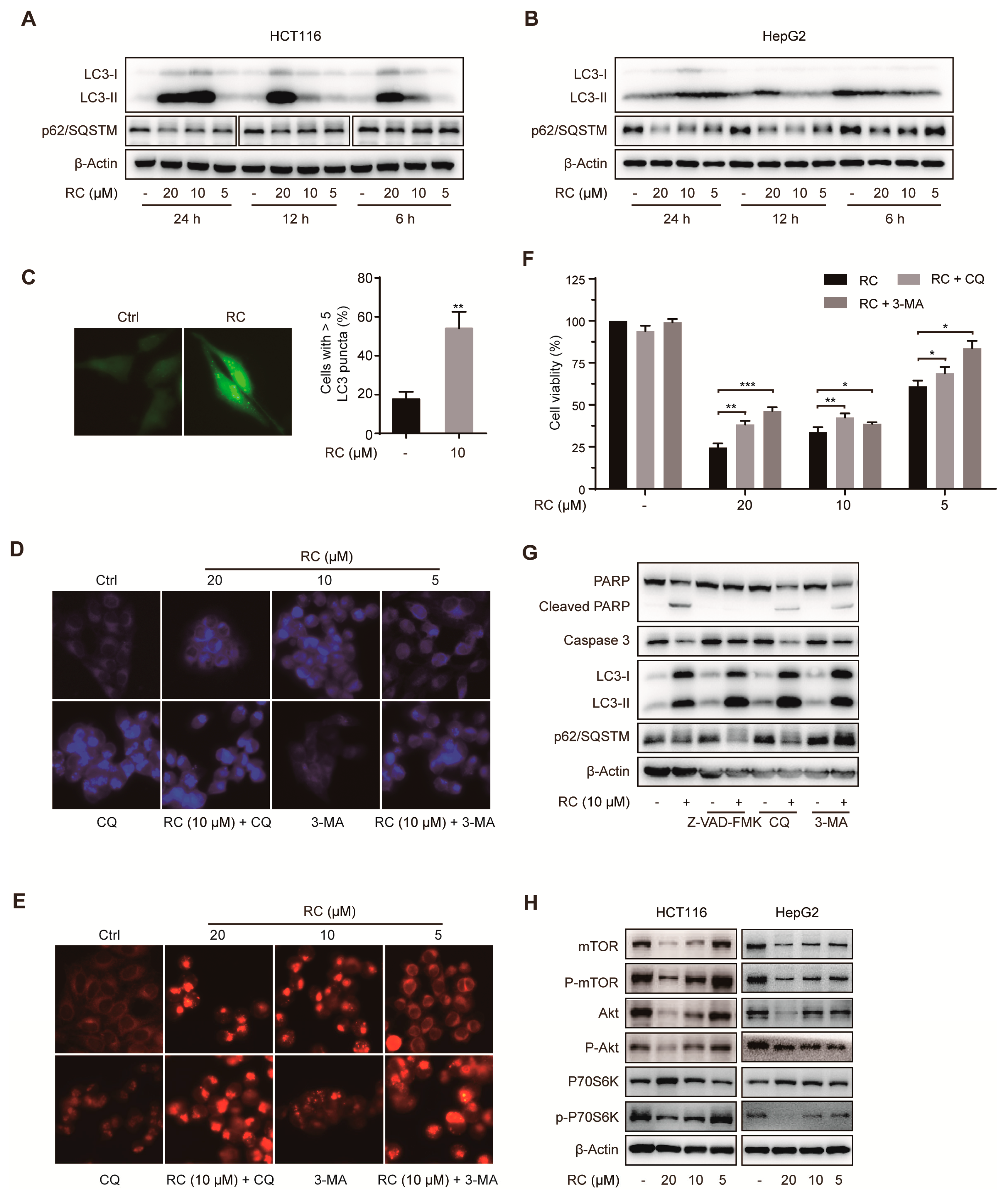
3.3. RC Induces Autophagic Cell Death Through Inhibiting Akt/mTOR/P70S6K Signaling Pathway in HCT116 and HepG2 cells
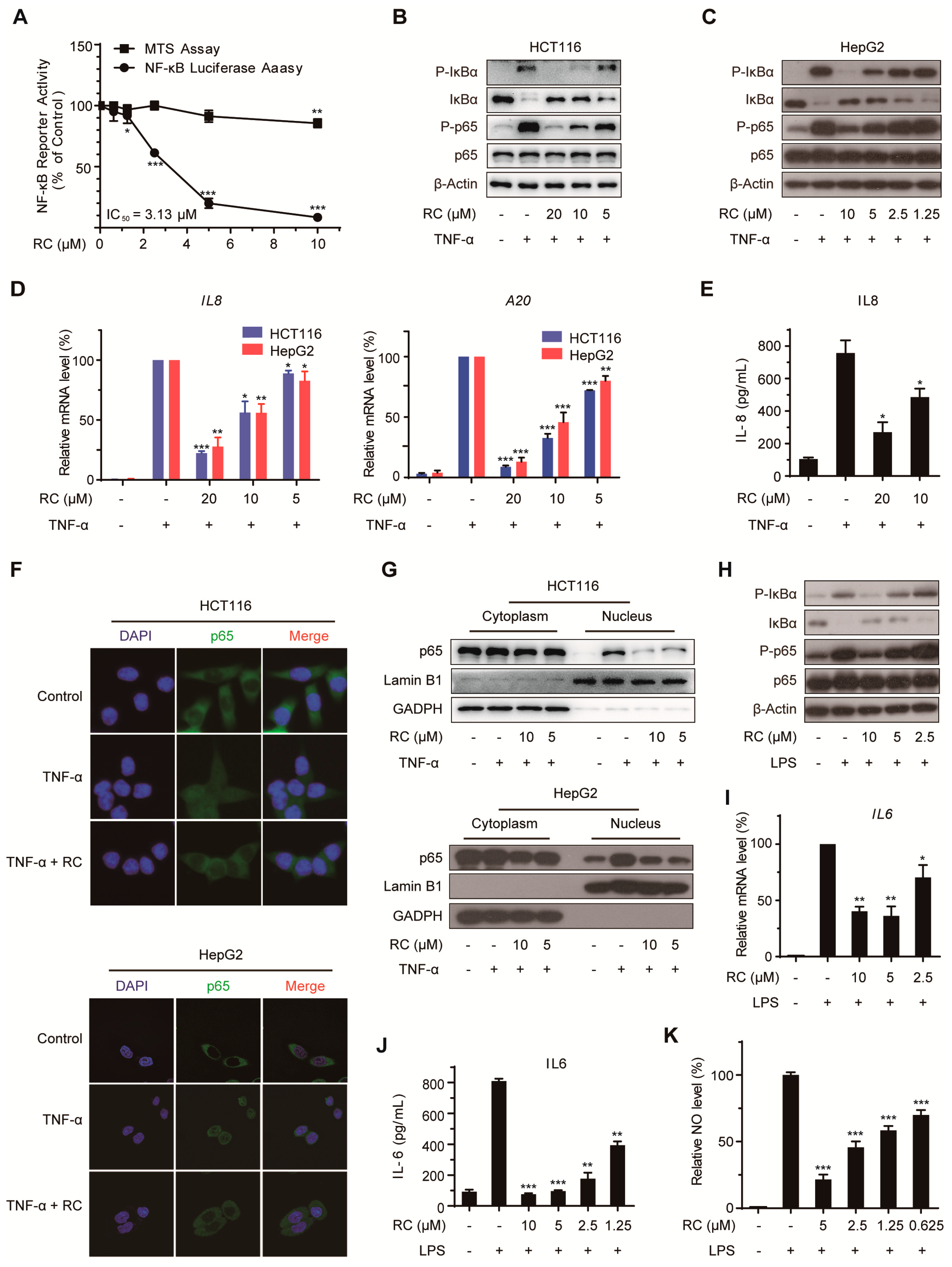
3.4. RC Inhibits the Activation of NF-κB Signaling Pathway In Vitro and In Vivo
3.5. RC Represses NF-κB Activation Upstream of the p65 Protein and Contributes to Cell Death
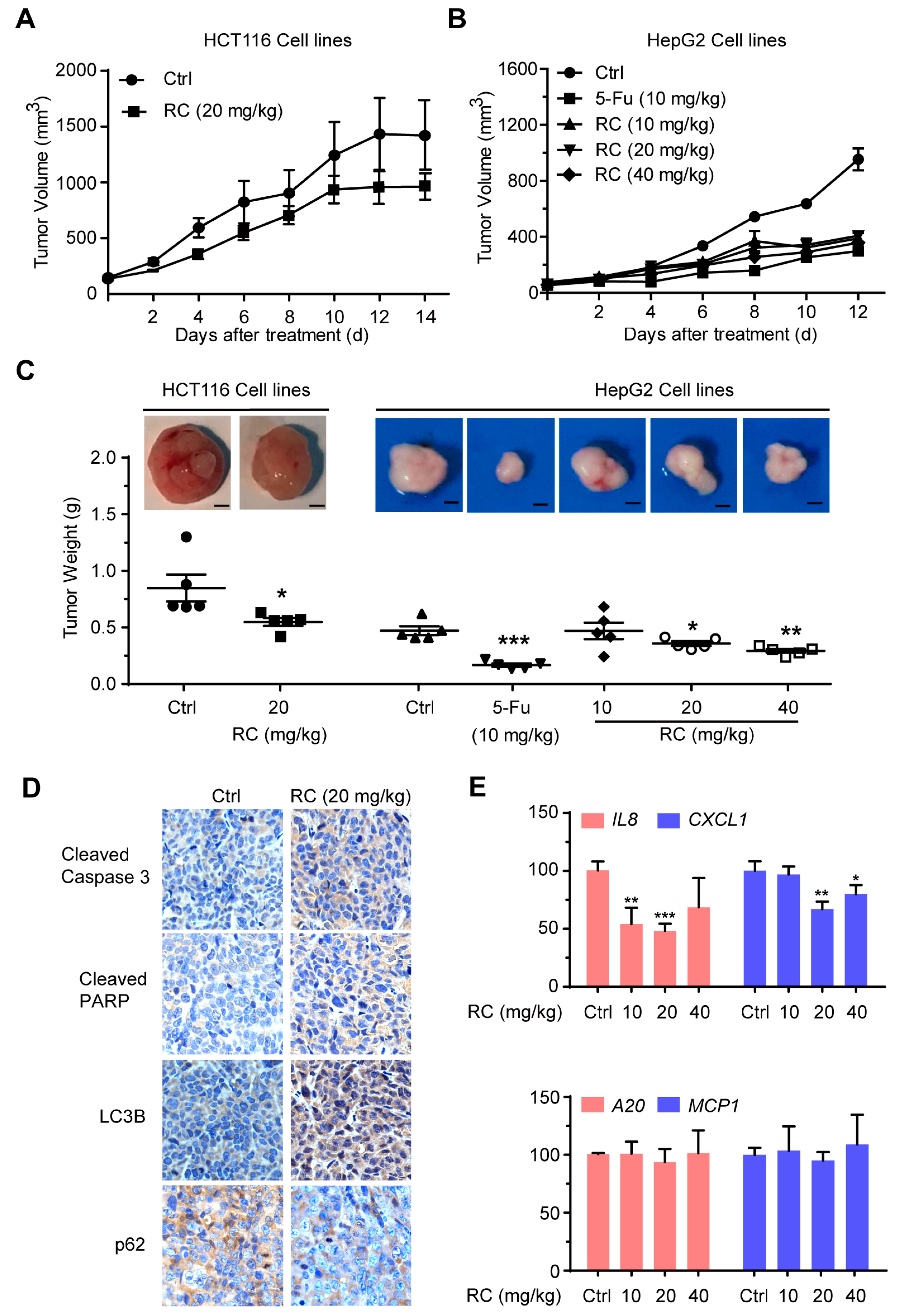
3.6. RC Inhibits Tumor Growth and Induces Apoptosis and Autophagy with Inhibition of NF-κB In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonistst. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Micco, S.D.; Spatafora, C.; Cardullo, N.; Riccio, R.; Fischer, K.; Pergola, C.; Koeberle, A.; Werz, O.; Chalal, M.; Fasseur, D.V.; et al. 2,3-Dihydrobenzofuran privileged structures as new bioinspired lead compounds for the design of mPGES-1 inhibitors. Bioorg. Med. Chem. Lett. 2016, 24, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.H. Naturally Occuring Quinones IV, Recent Advances, 4th ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Wang, Z.; Zhao, S.M.; Hu, Y.Y.; Feng, L.; Zhao, L.M.; Di, Y.T.; Tan, N.H. Rubipodanones A-D, naphthohydroquinone dimers from the roots and rhizomes of Rubia podantha. Phytochemistry 2018, 145, 153–160. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, Y.S.; Xu, W.Y.; Du, L.; Zeng, G.Z.; Zhang, Y.M.; Su, J.; Li, Y.; Tan, N.H. Rubiyunnanins A and B, two novel cyclic hexapeptides from Rubia yunnanensis. Tetrahedron Lett. 2010, 51, 6810–6813. [Google Scholar] [CrossRef]
- Fan, J.T.; Su, J.; Peng, Y.M.; Li, Y.; Li, J.; Zhou, Y.B.; Zeng, G.Z.; Yan, H.; Tan, N.H. Rubiyunnanins C-H, cytotoxic cyclic hexapeptides from Rubia yunnanensis inhibiting nitric oxide production and NF-κB activation. Bioorg. Med. Chem. 2010, 18, 8226–8234. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, S.M.; Wang, Z.; Zeng, G.Z.; Huang, M.B.; Tan, N.H. Rubicordins A-C, new cyclopeptides from Rubia cordifolia with cytotoxicity and inhibiting NF-κB signaling pathway. Tetrahedron 2015, 71, 9673–9678. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.M.; Zhao, L.M.; Chen, X.Q.; Zeng, G.Z.; Tan, N.H. Rubipodanin A, the first natural N-desmonomethyl Rubiaceae-type cyclopeptide from Rubia podantha, indicating an important role of the N9-methyl group in the conformation and bioactivity. PloS One 2015, 10, e0144950. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Feng, L.; Wang, J.; Zhang, X.J.; Wang, Z.; Tan, N.H. Rubipodanin B, a new cytotoxic cyclopeptide from Rubia podantha. Chem. Biodivers. 2019, 16, e1800438. [Google Scholar]
- Qiao, Y.F.; Takeya, K.; Itokawa, H.; Iitaka, Y. Three novel naphthohydroquinone dimers from Rubia oncotricha. Chem. Pharm. Bull. 1990, 38, 2896–2898. [Google Scholar] [CrossRef]
- Itokawa, H.; Ibraheim, Z.Z.; Qiao, Y.F.; Takeya, K. Anthraquinone, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 1993, 41, 1869–1872. [Google Scholar] [CrossRef]
- Hassanean, H.A.; Ibraheim, Z.Z.; Takeya, K.; Itorawa, H. Further quinoidal derivatives from Rubia cordifolia L. Pharmazie 2000, 55, 317–319. [Google Scholar]
- Ibraheim, Z.Z.; Gouda, Y.G. Minor constituents from Rubia Cordifolia L. root. Bull. Pharm. Sci. 2010, 33, 225–233. [Google Scholar]
- Zhao, S.M.; Wang, Z.; Zeng, G.Z.; Song, W.W.; Chen, X.Q.; Li, X.N.; Tan, N.H. New cytotoxic naphthohydroquinone dimers from Rubia alata. Org. Lett. 2014, 16, 5576–5579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.M.; Wang, Z.; Chen, X.Q.; Huang, M.B.; Tan, N.H. (±)-Rubioncolin D, a pair of enantiomeric naphthohydroquinone dimers from Rubia oncotricha. Tetrahedron Lett. 2017, 58, 3041–3043. [Google Scholar] [CrossRef]
- Suyama, Y.; Higashino, Y.; Tanaka, N.; Tatano, Y.; Yagi, H.; Kawazoe, K.; Murakami, K.; Li, S.L.; Sun, H.D.; Kashiwada, Y. Stereochemical assignments of rubiaquinones A-C, naphthoquinone derivatives from Rubia yunnanensis. Tetrahedron Lett. 2017, 58, 4568–4571. [Google Scholar] [CrossRef]
- Lumb, J.P.; Trauner, D. Biomimetic synthesis and structure elucidation of rubicordifolin, a cytotoxic natural product from Rubia cordifolia. J. Am. Chem. Soc. 2005, 127, 2870–2871. [Google Scholar] [CrossRef]
- Lumb, J.P.; Choong, K.C.; Trauner, D. ortho-Quinone methides from para-quinones: Total synthesis of rubioncolin B. J. Am. Chem. Soc. 2008, 130, 9230–9231. [Google Scholar] [CrossRef]
- Yang, H.; Feng, J.; Li, Y.; Tang, Y.F. Biomimetic syntheses of rubialatins A, B and related congeners. Org. Lett. 2015, 17, 1441–1444. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.Q.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, S.M.; Song, L.H.; Pu, Y.Z.; Wang, Q.; Zeng, G.Z.; Liu, X.; Bai, M.; Li, S.; Gao, F.B.; et al. Natural cyclopeptide RA-V inhibits the NF-κB signaling pathway by targeting TAK1. Cell Death Dis. 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Fan, J.T.; Kuang, B.; Zeng, G.Z.; Zhao, S.M.; Ji, C.J.; Zhang, Y.M.; Tan, N.H. Biologically active arborinane-type triterpenoids and anthraquinones from Rubia yunnanensis. J. Nat. Prod. 2011, 74, 2069–2080. [Google Scholar] [CrossRef]
- Zhao, S.M.; Kuang, B.; Zeng, G.Z.; Wang, Z.; Wang, J.; Chen, X.Q.; Tan, N.H. Nematicidal quinone derivatives from three Rubia plants. Tetrahedron 2018, 74, 2115–2120. [Google Scholar] [CrossRef]
- Zeng, G.Z.; Fan, J.T.; Xu, J.J.; Li, Y.; Tan, N.H. Apoptosis induction and G2/M arrest of 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone from Rubia yunnanensis in human cervical cancer Hela cells. Die Pharm. 2013, 68, 293–299. [Google Scholar]
- Zeng, G.Z.; Wang, Z.; Zhao, L.M.; Fan, J.T.; Tan, N.H. NF-κB and JNK mediated apoptosis and G0/G1 arrest of HeLa cells induced by rubiarbonol G, an arborinane-type triterpenoid from Rubia yunnanensis. J. Ethnopharmacol. 2018, 220, 220–227. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.M.; Zeng, G.Z.; Tan, N.H. Chemical constituents from roots and rhizomes of Rubia oncotricha and their cytotoxic activities. China J. Chin. Mater. Med. 2018, 43, 4462–4468. [Google Scholar]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nature Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Baldwin, A.S. NF-kappaB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Mayo, M.W.; Baldwin, A.S. The transcription factor NF-kappaB: Control of oncogenesis and cancer therapy resistance. Biochim. Biophys. Acta 2000, 1470, M55–M62. [Google Scholar] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, L.; Wang, J.; Song, L.; Tan, N.; Wang, Z. Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells. Cells 2019, 8, 1593. https://doi.org/10.3390/cells8121593
Wang J, Li L, Wang J, Song L, Tan N, Wang Z. Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells. Cells. 2019; 8(12):1593. https://doi.org/10.3390/cells8121593
Chicago/Turabian StyleWang, Jia, Ling Li, Jing Wang, Lihua Song, Ninghua Tan, and Zhe Wang. 2019. "Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells" Cells 8, no. 12: 1593. https://doi.org/10.3390/cells8121593
APA StyleWang, J., Li, L., Wang, J., Song, L., Tan, N., & Wang, Z. (2019). Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells. Cells, 8(12), 1593. https://doi.org/10.3390/cells8121593




