mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Sample Collection
2.3. Amino Acid Detection
2.4. Protein Extraction
2.5. iTRAQ Proteome Analysis
2.6. Immunohistochemical Analysis
2.7. Western Blotting
2.8. Isolation and Culture of SCs
2.9. Lys Depletion and Supplementation
2.10. Cell Proliferation Assay
2.11. Flow Cytometry
2.12. Protein Synthesis
2.13. Immunofluorescence Staining
2.14. Rapamycin Inhibition
2.15. Statistical Analysis
3. Results
3.1. Skeletal Muscle Growth in Piglets Relies on Dietary Lys Supplementation
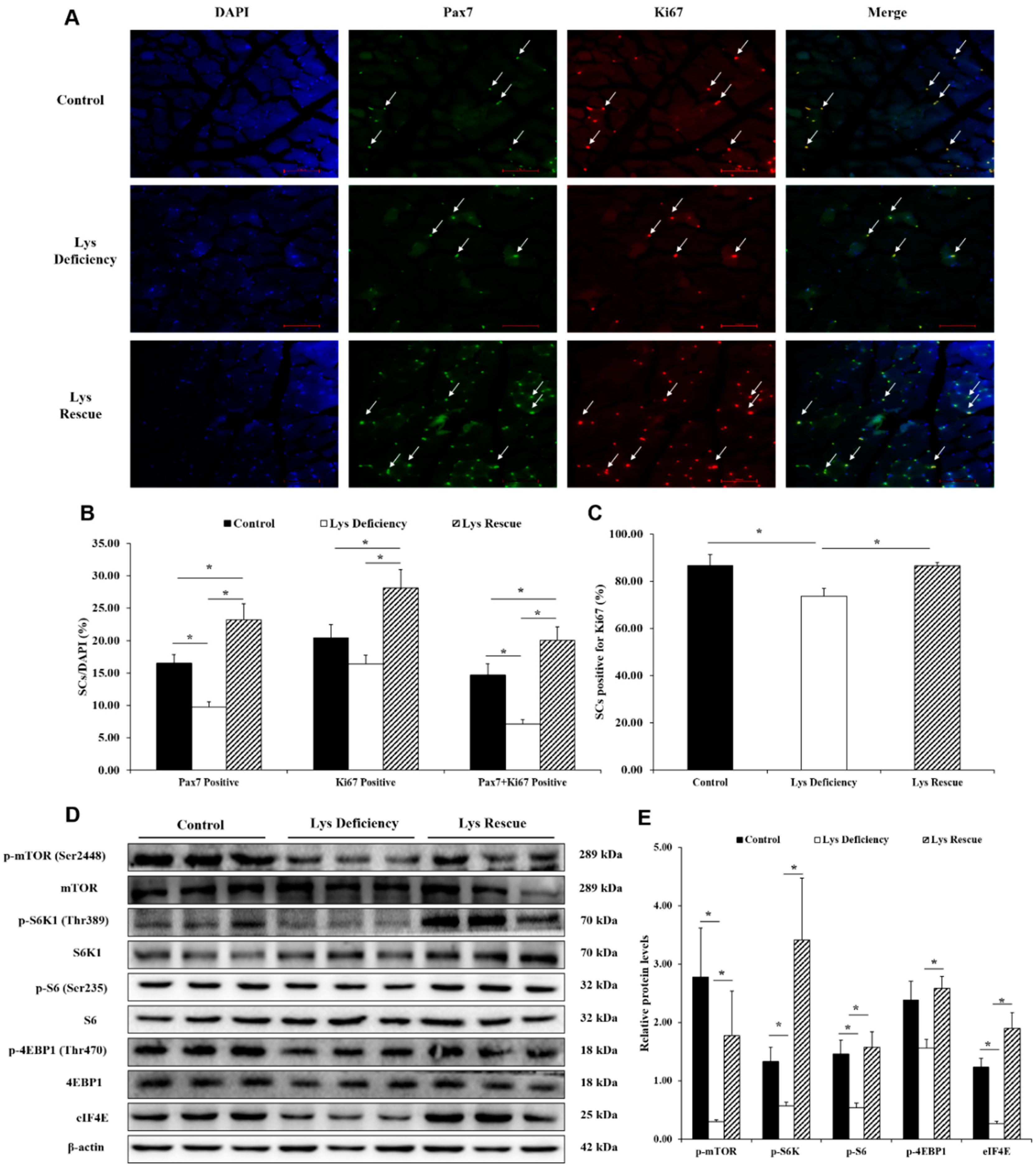
3.2. Lys-induced Skeletal Muscle Growth in Relation to SC Activation Level
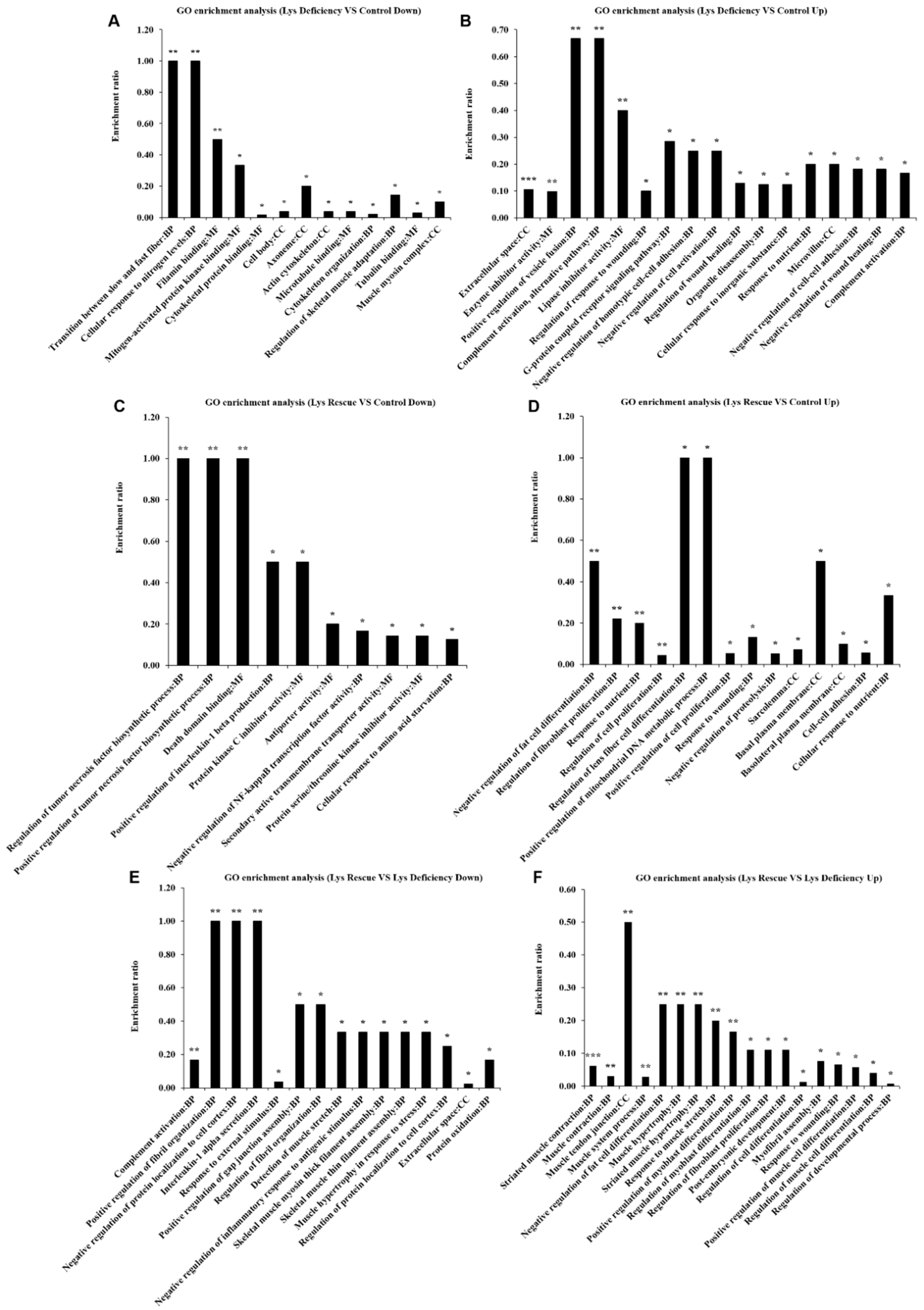
3.3. SCs and mTORC1 Activity Are Enhanced in Lys-induced Skeletal Muscle Growth
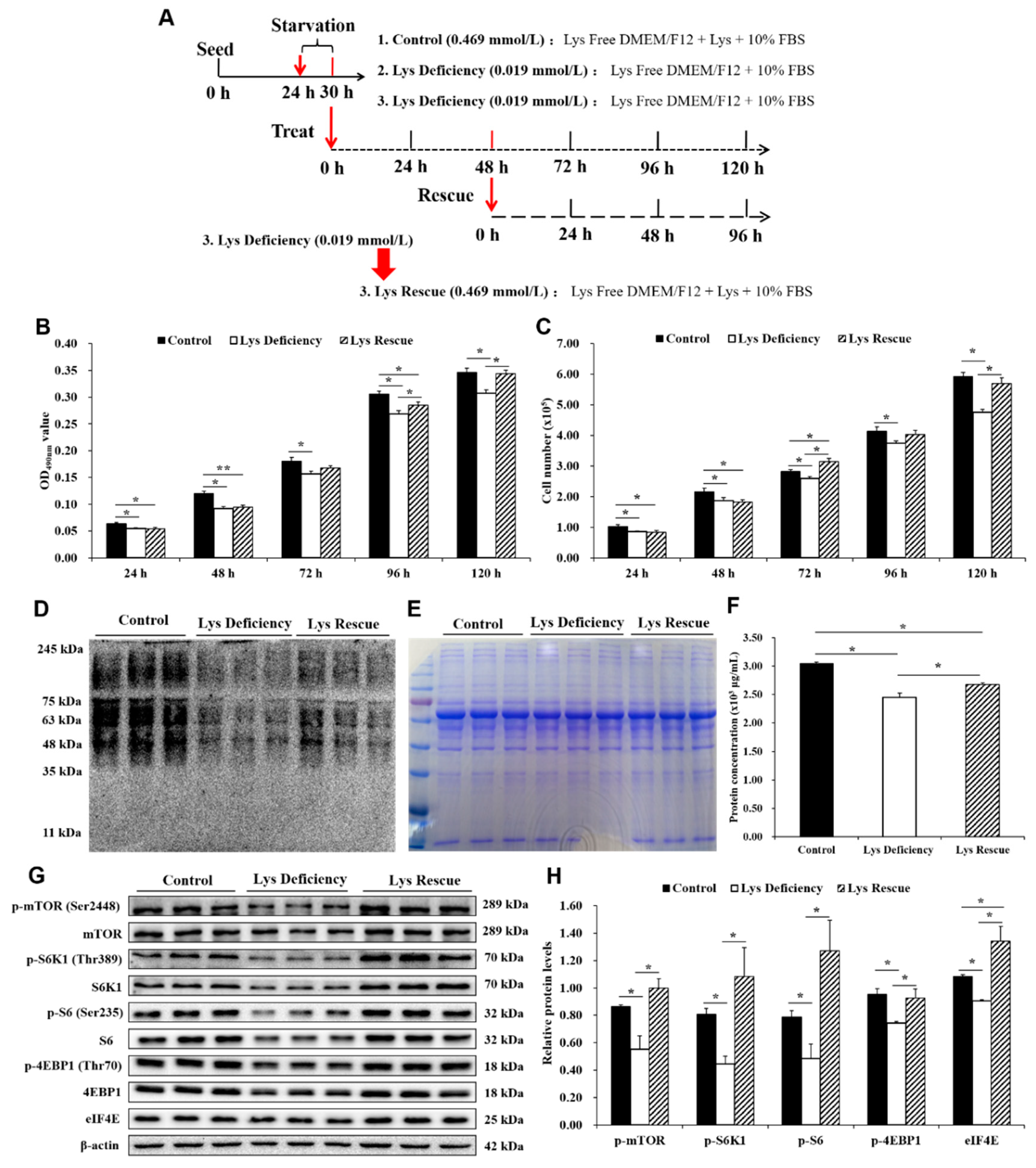
3.4. Cell Proliferation Was Rescued with Increased mTORC1 by Lys Re-supplementation.
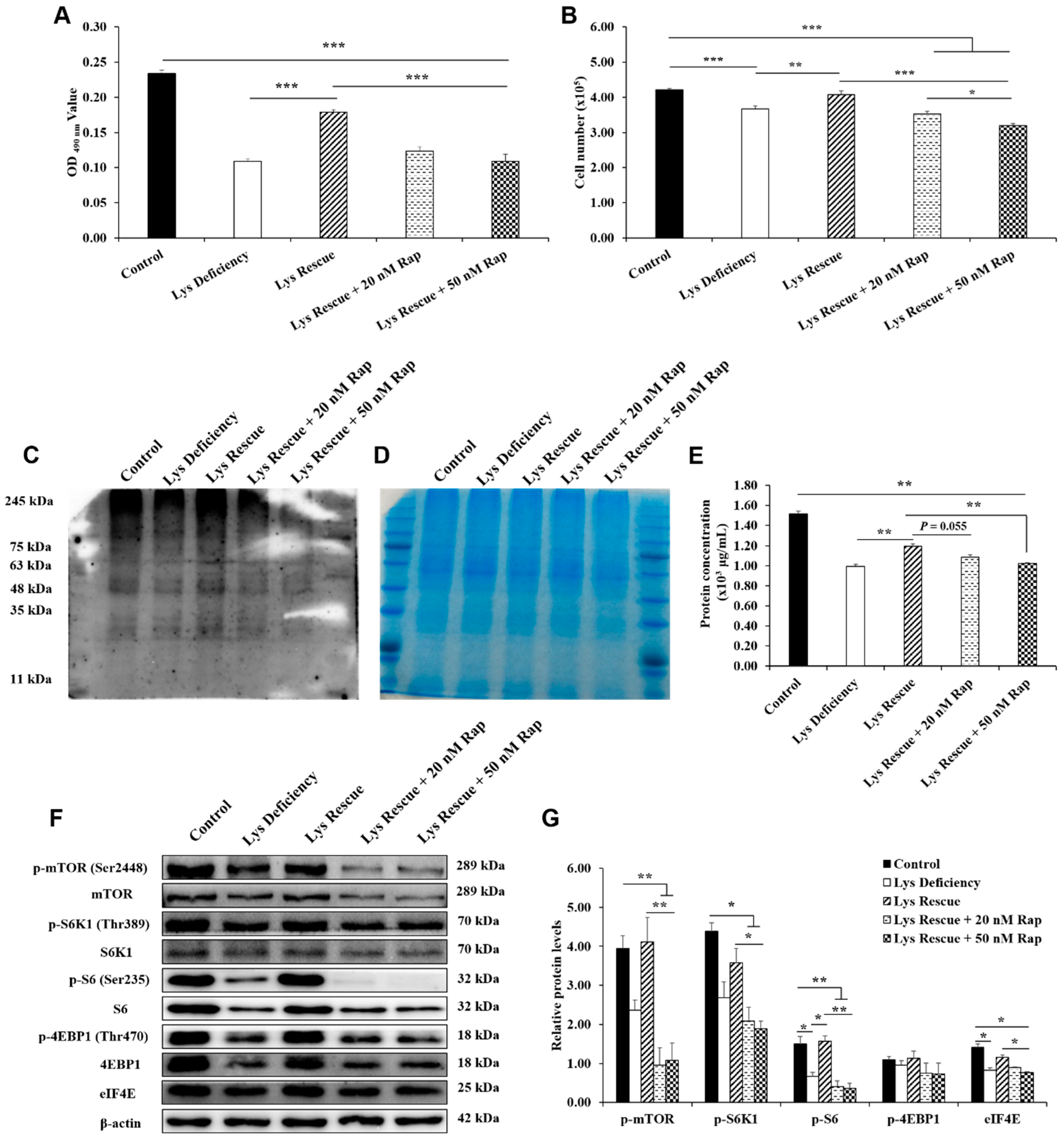
3.5. Lys Rescue of SC Proliferation and mTORC1 Pathway Activation Were Inhibited by Rapamycin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, P.F.; Zeng, Z.K.; Wang, D.; Xue, L.F.; Zhang, R.F.; Piao, X.S. Effects of the standardized ileal digestible lysine to metabolizable energy ratio on performance and carcass characteristics of growing-finishing pigs. J. Anim. Sci. Biotechno. 2012, 3, 9. [Google Scholar] [CrossRef]
- Zeng, P.L.; Yan, H.C.; Wang, X.Q.; Zhang, C.M.; Zhu, C.; Shu, G.; Jiang, Q.Y. Effects of dietary lysine levels on apparent nutrient digestibility and serum amino acid absorption mode in growing pigs. J. Anim. Sci. 2013, 26, 1003–1011. [Google Scholar] [CrossRef]
- Ishida, A.; Kyoya, T.; Nakashima, K.; Katsumata, M. Muscle protein metabolism during compensatory growth with changing dietary lysine levels from deficient to sufficient in growing rats. J. Nutr. Sci. Vitaminol. 2011, 57, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mastellar, S.L.; Coleman, R.J.; Urschel, K.L. Controlled trial of whole body protein synthesis and plasma amino acid concentrations in yearling horses fed graded amounts of lysine. Vet. J. 2016, 216, 93–100. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Amino acid intakes are associated with bone mineral density and prevalence of low bone mass in women: Evidence from discordant monozygotic twins. J. Bone Miner. Res. 2016, 31, 326–335. [Google Scholar] [CrossRef]
- Karnebeek, C.D.; Hartmann, H.; Jaggumantri, S.; Bok, L.A.; Cheng, B.; Connolly, M.; Coughlin, C.R.; Das, A.M.; Gospe, S.M.; Jakobs, C.; et al. Lysine restricted diet for pyridoxine-dependent epilepsy: First evidence and future trials. Mol. Genet. Metab. 2012, 107, 335–344. [Google Scholar] [CrossRef]
- Boy, N.; Haege, G.; Heringer, J.; Assmann, B.; Mühlhausen, C.; Ensenauer, R.; Maier, E.M.; Lücke, T.; Hoffmann, G.F.; Müller, E.; et al. Low lysine diet in glutaric aciduria type I--effect on anthropometric and biochemical follow-up parameters. J. Inherit. Metab. Dis. 2013, 36, 525–533. [Google Scholar] [CrossRef]
- Rodriguez, J.; Sanz, M.; Blanco, M.; Serrano, M.P.; Joy, M.; Latorre, M.A. The influence of dietary lysine restriction during the finishing period on growth performance and carcass, meat, and fat characteristics of barrows and gilts intended for dry-cured ham production. J. Anim. Sci. 2011, 89, 3651–3662. [Google Scholar] [CrossRef]
- Sato, T.; Ito, Y.; Nagasawa, T. Dietary L-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J. Agric. Food. Chem. 2015, 63, 8192–8198. [Google Scholar] [CrossRef]
- Sato, T.; Ito, Y.; Nedachi, T.; Nagasawa, T. Lysine suppresses protein degradation through autophagic-lysosomal system in C2C12 myotubes. Mol. Cell Biochem. 2014, 391, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, K. Muscle memory and a new cellular model for muscle atrophy and Hypertrophy. J. Exp. Biol. 2016, 219, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Calhabeu, F.; Morgan, J.E.; Katagiri, T.; Amthor, H.; Zammit, P.S. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death. Differ. 2011, 18, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Yang, W.J.; Yang, Z.; Shu, G.; Wang, S.B.; Jiang, Q.Y.; Yuan, L.; Wu, T.S. The differential proliferative ability of satellite cells in Lantang and Landrace pigs. PLoS ONE 2012, 7, e32537. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, C.Q.; Chen, R.Q.; Jin, C.L.; Li, H.C.; Yan, H.C.; Wang, X.Q. Focal adhesion kinase and paxillin promote migration and adhesion to fibronectin by swine skeletal muscle satellite cells. Oncotarget 2016, 7, 30845–30854. [Google Scholar] [CrossRef]
- Pallafacchina, G.; Blaauw, B.; Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 12–18. [Google Scholar] [CrossRef]
- Jash, S.; Dhar, G.; Ghosh, U.; Adhya, S. Role of the mTORC1 complex in satellite cell activation by RNA-induced mitochondrial restoration: Dual control of cyclin D1 through microRNAs. Mol. Cell Biol. 2014, 34, 3594–3606. [Google Scholar] [CrossRef]
- Gao, C.Q.; Zhi, R.; Yang, Z.; Li, H.C.; Yan, H.C.; Wang, X.Q. Low dose of IGF-I increases cell size of skeletal muscle satellite cells via Akt/S6K signaling pathway. J. Cell Biochem. 2015, 116, 2637–2648. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, X.; Shan, T.; Jiang, Q.; Deng, C.; Zheng, R.; Kuang, S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 2015, 463, 102–108. [Google Scholar] [CrossRef]
- Dai, J.M.; Yu, M.X.; Shen, Z.Y.; Guo, C.Y.; Zhuang, S.Q.; Qiu, X.S. Leucine promotes proliferation and differentiation of primary preterm rat satellite cells in part through mTORC1 signaling pathway. Nutrients 2015, 7, 3387–3400. [Google Scholar] [CrossRef]
- Alway, S.E.; Pereira, S.L.; Edens, N.K.; Hao, Y.; Bennett, B.T. β-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp. Gerontol. 2013, 48, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Garcia, H.; Arce, N.; Cota, M.; Zijlstra, R.T.; Araiza, B.A.; Cervantes, M. Effect of L-lysine on expression of selected genes, serum concentration of amino acids, muscle growth and performance of growing pigs. J. Anim. Physiol. Anim. Nutr. 2015, 99, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.L.; Zhang, Z.M.; Ye, J.L.; Gao, C.Q.; Yan, H.C.; Li, H.C.; Yang, J.Z.; Wang, X.Q. Lysine-induced swine satellite cell migration is mediated by the FAK pathway. Food Funct. 2019, 10, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.L.; Gao, C.Q.; Li, X.G.; Jin, C.L.; Wang, D.; Shu, G.; Wang, W.C.; Kong, X.F.; Yao, K.; Yan, H.C.; et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Sui, W.G.; Gao, C.Q.; Yan, H.C.; Yin, Y.L.; Li, H.C.; Wang, X.Q. L-Glutamate deficiency can trigger proliferation inhibition via down regulation of the mTOR/S6K1 pathway in pig intestinal epithelial cells. J. Anim. Sci. 2016, 94, 1541–1549. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods. 2009, 6, 275–277. [Google Scholar] [CrossRef]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef]
- McMillan, J.D.; Jennings, E.W.; Mohagheghi, A.; Zuccarello, M. Comparative performance of precommercial cellulases hydrolyzing pretreated corn stover. Biotechnol. Biofuels. 2011, 4, 29. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. Springerplus 2015, 4, 147. [Google Scholar] [CrossRef]
- Egner, I.M.; Bruusgaard, J.C.; Gundersen, K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 2016, 143, 2898–2906. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.S.; Kwon, D.H.; Bong, J.J.; Jeong, J.Y.; Nam, Y.S.; Lee, M.S.; Liu, X.; Baik, M. Severe dietary lysine restriction affects growth and body composition and hepatic gene expression for nitrogen metabolism in growing rats. J. Anim. Physiol. Anim. Nutr. 2014, 98, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Guo, J.; Jin, Z.; Yoon, S.Y.; Choi, J.Y.; Wang, M.H.; Piao, X.S.; Kim, B.W.; Chae, B.J. Lysine restriction and realimentation affected growth, blood profiles and expression of genes related to protein and fat metabolism in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2009, 93, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J. Nutr. Sci. Vitaminol. 2013, 59, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Popha, S.; Mo, P.E.; Vieira, S.L. Satellite cell mitotic activity of broilers fed differing levels of lysine. Int. J. Poult. Sci. 2004, 3, 758–763. [Google Scholar]
- Snijders, T.; Verdijk, L.B.; Smeets, J.S.J.; McKay, B.R.; Senden, J.M.G.; Hartgens, F.; Parise, G.; Greenhaff, P.; van Loon, L.J.C. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age 2014, 36, 9699. [Google Scholar] [CrossRef] [PubMed]
- Mackey, A.L.; Andersen, L.L.; Frandsen, U.; Sjogaard, G. Strength training increases the size of the satellite cell pool in type I and II fibres of chronically painful trapezius muscle in females. J. Physiol. 2011, 589, 5503–5515. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Verdijk, L.B.; Beelen, M.; McKay, B.R.; Parise, G.; Kadi, F.; van Loon, L.J.C. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp. Physiol. 2012, 97, 762–773. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.L. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef]
- Yao, K.; Yin, Y.L.; Chu, W.Y.; Liu, Z.Q.; Deng, D.; Li, T.J.; Huang, R.L.; Zhang, J.S.; Tan, B.; Wang, W.C.; et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 2008, 138, 867–872. [Google Scholar] [CrossRef] [PubMed]

| Item | Control | Lys Deficiency | Lys Rescue | p-Value |
|---|---|---|---|---|
| Initial Weight (kg) | 12.02 ± 0.29 | 11.32 ± 0.44 | 11.34 ± 0.35 | 0.336 |
| Final Weight (kg) | 17.95 ± 0.49 a | 15.99 ± 0.30 b | 17.44 ± 0.46 a | 0.018 |
| Longissimus Dorsi Muscle | 2.00 ± 0.06 ab | 1.86 ± 0.07 b | 2.19 ± 0.06 a | 0.018 |
| Psoas Major Muscle | 0.34 ± 0.01 a | 0.28 ± 0.01 b | 0.30 ± 0.01 b | 0.014 |
| Forequarters muscles | ||||
| Infraspinatus Muscle | 0.24 ± 0.02 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.221 |
| Supraspinatus Muscle | 0.41 ± 0.02 | 0.40 ± 0.01 | 0.42 ± 0.02 | 0.538 |
| Subclavius Muscle | 0.23 ± 0.02 | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.227 |
| Latissimus Dorsi Muscle | 0.26 ± 0.03 | 0.26 ± 0.04 | 0.27 ± 0.03 | 0.925 |
| Long Head of Triceps of Brachii Muscle | 0.63 ± 0.02 | 0.63 ± 0.01 | 0.67 ± 0.02 | 0.201 |
| Lateral Head of Triceps of Brachii Muscle | 0.17 ± 0.01 a | 0.14 ± 0.004 b | 0.18 ± 0.01 a | 0.003 |
| Extensor Carpi Radialis Muscle | 0.13 ± 0.003 a | 0.12 ± 0.002 b | 0.13 ± 0.003 a | 0.011 |
| Extensor Muscle of Second- and Third-Digits | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.684 |
| Lateral Digital Extensor Muscle | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.441 |
| Total Forequarters Muscles | 2.15 ± 0.07 | 2.02 ± 0.03 | 2.15 ± 0.03 | 0.111 |
| Hindquarters muscles | ||||
| Middle Gluteus Medius Muscle | 0.54 ± 0.02 | 0.48 ± 0.02 | 0.55 ± 0.03 | 0.102 |
| Superficial Gluteal Muscle | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.457 |
| Biceps Femoris Muscle | 1.15 ± 0.01 a | 1.04 ± 0.04 b | 1.21 ± 0.02 a | 0.008 |
| Semimembranosus Muscle | 1.36 ± 0.06 ab | 1.23 ± 0.05 b | 1.46 ± 0.03 a | 0.013 |
| Semitendinosus Muscle | 0.39 ± 0.02 ab | 0.36 ± 0.01 b | 0.41 ± 0.01 a | 0.043 |
| Tensor fascia Lata Muscle | 0.18 ± 0.02 | 0.17 ± 0.01 | 0.20 ± 0.01 | 0.313 |
| Cranial Tibial Muscle | 0.04 ± 0.003 a | 0.03 ± 0.001 b | 0.04 ± 0.002 a | 0.045 |
| Long Peroneal Muscle | 0.04 ± 0.003 a | 0.03 ± 0.001 b | 0.03 ± 0.002 b | 0.035 |
| Peroneus Tertius Muscle | 0.08 ± 0.003 | 0.08 ± 0.002 | 0.08 ± 0.002 | 0.191 |
| Gemelli Muscle | 0.27 ± 0.01 a | 0.23 ± 0.01 b | 0.25 ± 0.01 ab | 0.018 |
| Soleus Muscle | 0.23 ± 0.005 a | 0.19 ± 0.01 b | 0.23 ± 0.01 a | 0.020 |
| Lateral Head of Gastrocnemius Muscle | 0.37 ± 0.005 a | 0.31 ± 0.01 b | 0.36 ± 0.02 a | 0.027 |
| Adductor Muscle | 0.18 ± 0.02 | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.301 |
| Total Hindquarters Muscles | 4.98 ± 0.12 a | 4.49 ± 0.12 b | 5.17 ± 0.11 a | 0.004 |
| Item | Control | Lys Deficiency | Lys Rescue | p-Value |
|---|---|---|---|---|
| Aspartate | 0.11 ± 0.003 ab | 0.10 ± 0.006 b | 0.12 ± 0.003 a | 0.038 |
| Threonine | 0.06 ± 0.003 a | 0.05 ± 0.003 b | 0.06 ± 0.002 a | 0.015 |
| Serine | 0.05 ± 0.000 a | 0.04 ± 0.003 b | 0.05 ± 0.002 a | 0.002 |
| Glutamate | 0.21 ± 0.003 a | 0.18 ± 0.012 b | 0.21 ± 0.006 a | 0.024 |
| Glycine | 0.07 ± 0.003 | 0.06 ± 0.000 | 0.07 ± 0.006 | 0.423 |
| Alanine | 0.07 ± 0.003 | 0.07 ± 0.006 | 0.08 ± 0.003 | 0.671 |
| Valine | 0.06 ± 0.003 ab | 0.06 ± 0.003 b | 0.07 ± 0.002 a | 0.017 |
| Isoleucine | 0.07 ± 0.003 ab | 0.06 ± 0.003 b | 0.07 ± 0.002 a | 0.025 |
| Leucine | 0.12 ± 0.003 ab | 0.11 ± 0.009 b | 0.13 ± 0.004 a | 0.059 |
| Tyrosine | 0.05 ± 0.000 | 0.05 ± 0.003 | 0.05 ± 0.003 | 0.354 |
| Phenylalanine | 0.07 ± 0.000 ab | 0.06 ± 0.006 b | 0.07 ± 0.003 a | 0.103 |
| Lysine | 0.11 ± 0.003 a | 0.09 ± 0.006 b | 0.11 ± 0.003 a | 0.006 |
| Histidine | 0.05 ± 0.003 | 0.04 ± 0.003 | 0.05 ± 0.003 | 0.547 |
| Arginine | 0.09 ± 0.000 a | 0.08 ± 0.006 b | 0.10 ± 0.002 a | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.-l.; Ye, J.-l.; Yang, J.; Gao, C.-q.; Yan, H.-c.; Li, H.-c.; Wang, X.-q. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells 2019, 8, 1549. https://doi.org/10.3390/cells8121549
Jin C-l, Ye J-l, Yang J, Gao C-q, Yan H-c, Li H-c, Wang X-q. mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells. 2019; 8(12):1549. https://doi.org/10.3390/cells8121549
Chicago/Turabian StyleJin, Cheng-long, Jin-ling Ye, Jinzeng Yang, Chun-qi Gao, Hui-chao Yan, Hai-chang Li, and Xiu-qi Wang. 2019. "mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth" Cells 8, no. 12: 1549. https://doi.org/10.3390/cells8121549
APA StyleJin, C.-l., Ye, J.-l., Yang, J., Gao, C.-q., Yan, H.-c., Li, H.-c., & Wang, X.-q. (2019). mTORC1 Mediates Lysine-Induced Satellite Cell Activation to Promote Skeletal Muscle Growth. Cells, 8(12), 1549. https://doi.org/10.3390/cells8121549






