Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health
Abstract
1. Introduction
2. Contemporary Theory behind Exercise-Induced Cardiovascular Benefit
2.1. Exercise-Induced Functional and Structural Changes
2.2. Acute Alterations in Cardiac Function during Exercise
2.3. Metabolic Flexibility
2.4. Chronic Adaptations in Heart and Vasculature
2.5. Cellular and Molecular Alternations Induced by Exercise
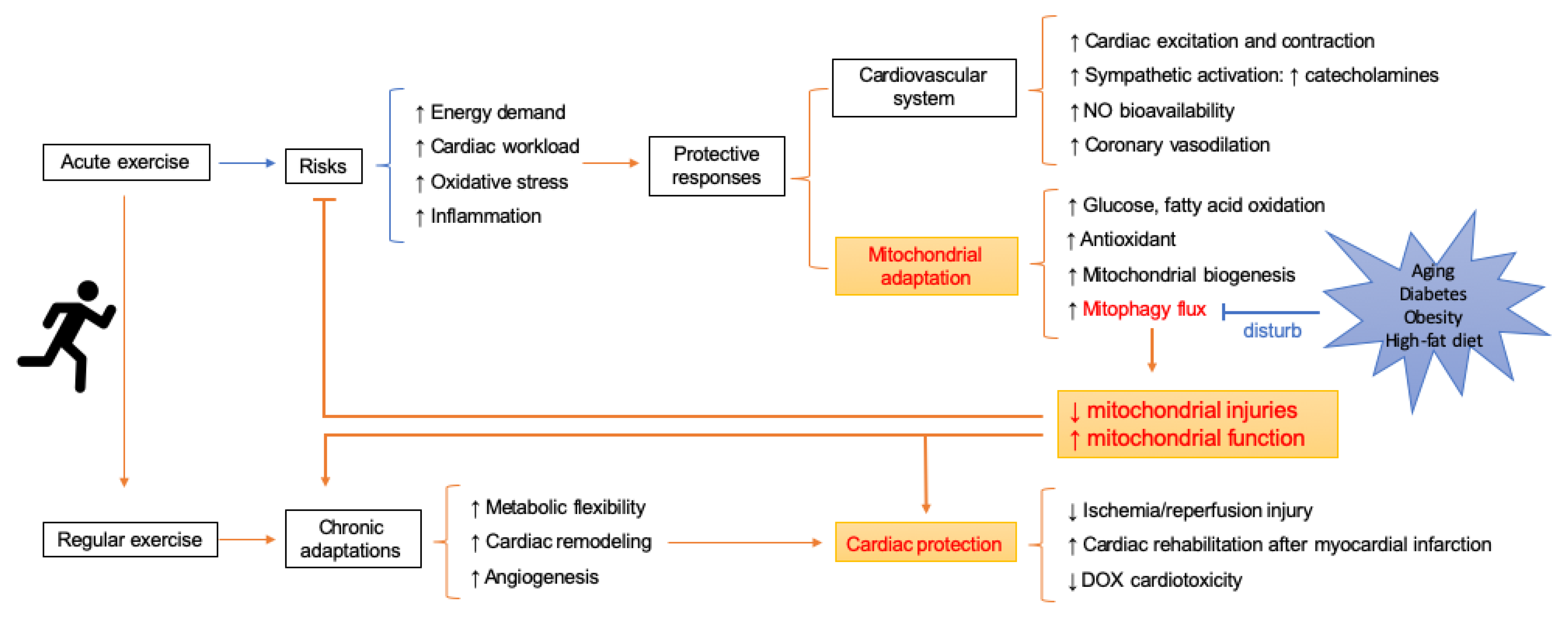
3. Risk of Exercise for Cardiovascular Function
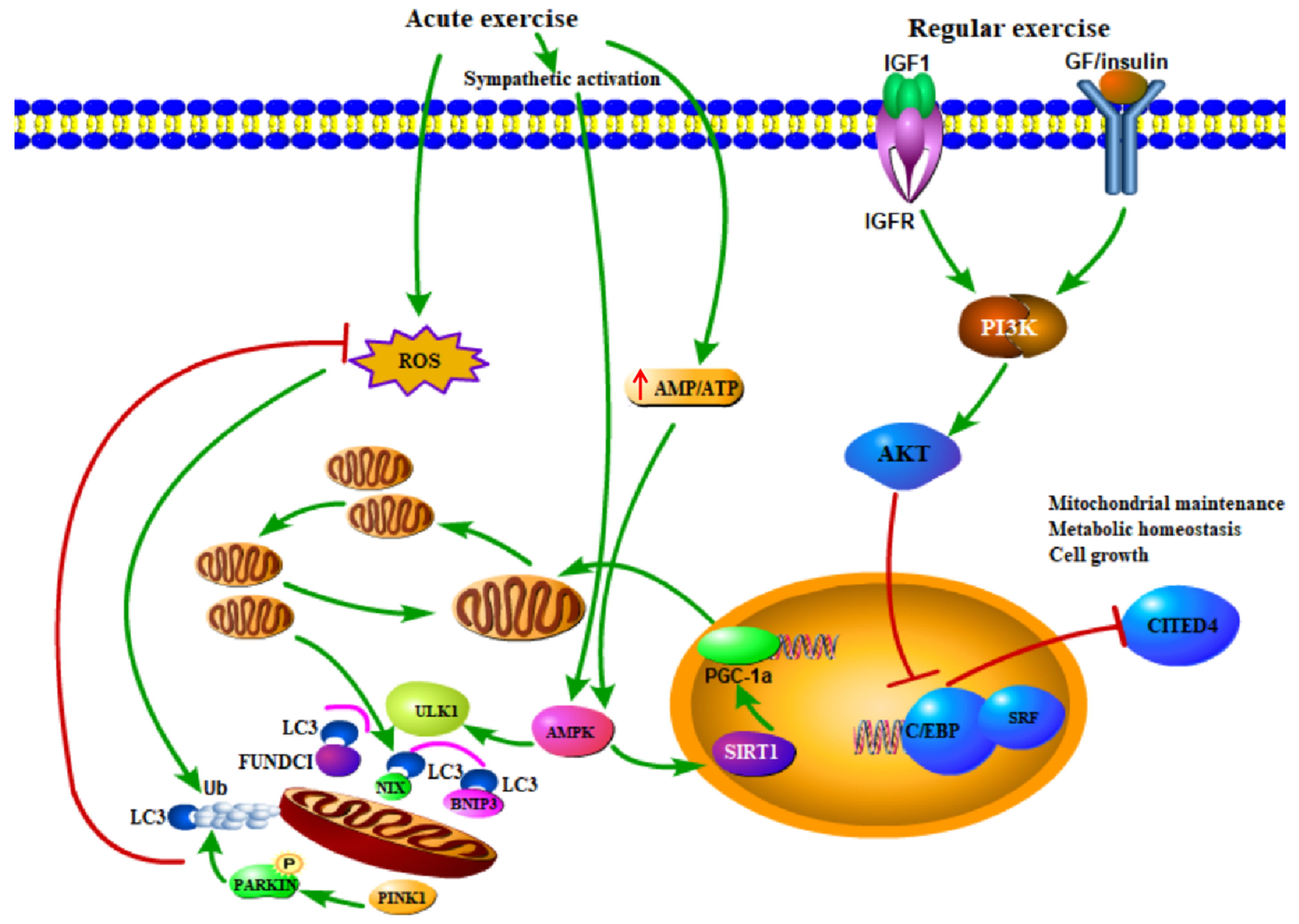
4. Mitophagy and Exercise
4.1. Exercise as a Treatment or Prevention to Diseases: The Role of Mitophagy
4.2. Mitophagy is Attenuated Due to Improved Mitochondrial Pool after Sustained Exercise Training
4.3. Compromised Mitophagy Response under Certain Pathological Conditions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; Patterson, N.L.; McMullen, J.R. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol. Rev. 2018, 98, 419–475. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, R. Children’s motor skill competence, physical activity, fitness, andhealthpromotion. J. Sport Health Sci. 2019, 8, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Brandt, N.; Gunnarsson, T.P.; Bangsbo, J.; Pilegaard, H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol. Rep. 2018, 6, e13651. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J. Obesity paradox in aging: From prevalence to pathophysiology. Prog. Cardiovasc. Dis. 2018, 61, 182–189. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical exercise inhibits inflammation and microglial activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef]
- Pinckard, K.; Baskin, K.K.; Stanford, K.I. Effects of exercise to improve cardiovascular health. Front. Cardiovasc. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Fernandez, A.B.; Thompson, P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol. Rev. 2016, 96, 99–125. [Google Scholar] [CrossRef]
- da Costa, B.G.G.; da Silva, K.S.; da Silva, J.A.; Minatto, G.; de Lima, L.R.A.; Petroski, E.L. Sociodemographic, biological, and psychosocial correlates of light- and moderate-to-vigorous-intensity physical activity during school time, recesses, and physical education classes. J. Sport Health Sci. 2019, 8, 177–182. [Google Scholar] [CrossRef]
- Martin-Garcia, M.; Alegre, L.M.; Garcia-Cuartero, B.; Bryant, E.J.; Gutin, B.; Ara, I. Effects of a 3-month vigorous physical activity intervention on eating behaviors and body composition in overweight and obese boys and girls. J. Sport Health Sci. 2019, 8, 170–176. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bellavere, F.; Cacciatori, V.; Bacchi, E.; Gemma, M.L.; Raimondo, D.; Negri, C.; Thomaseth, K.; Muggeo, M.; Bonora, E.; Moghetti, P. Effects of aerobic or resistance exercise training on cardiovascular autonomic function of subjects with type 2 diabetes: A pilot study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Candau, R.; Bernardi, H. Recent data on cellular component turnover: Focus on adaptations to physical exercise. Cells 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, J. Role of autophagy and regulatory mechanisms in alcoholic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2003–2009. [Google Scholar] [CrossRef]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef]
- Ren, J.; Sowers, J.R.; Zhang, Y. Metabolic stress, autophagy, and cardiovascular aging: From pathophysiology to therapeutics. Trends Endocrinol. Metab. 2018, 29, 699–711. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Dorn, G.W., 2nd. Evolving and expanding the roles of mitophagy as a homeostatic and pathogenic process. Physiol. Rev. 2019, 99, 853–892. [Google Scholar] [CrossRef]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, mitochondrial dynamics and homeostasis in cardiovascular aging. Oxid. Med. Cell. Longev. 2019, 2019, 9825061. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd. Parkin-dependent mitophagy in the heart. J. Mol. Cell Cardiol. 2016, 95, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.E.; Kubli, D.A.; Gustafsson, A.B. Autophagy and mitophagy in the myocardium: Therapeutic potential and concerns. Br. J. Pharm. 2014, 171, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.; Fernandez, A.F.; Kitsis, R.N.; Levine, B.; Sadoshima, J. Does autophagy mediate cardiac myocyte death during stress? Circ. Res. 2016, 119, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, K.Z.; Chu, C.T. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013, 9, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J. Targeting autophagy for the therapeutic application of histone deacetylase inhibitors in ischemia/reperfusion heart injury. Circulation 2014, 129, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J. Autophagy in aldh2-elicited cardioprotection against ischemic heart disease: Slayer or savior? Autophagy 2010, 6, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Balan, E.; Schwalm, C.; Naslain, D.; Nielens, H.; Francaux, M.; Deldicque, L. Regular endurance exercise promotes fission, mitophagy, and oxidative phosphorylation in human skeletal muscle independently of age. Front. Physiol. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisloff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef]
- Burtscher, M. Exercise limitations by the oxygen delivery and utilization systems in aging and disease: Coordinated adaptation and deadaptation of the lung-heart muscle axis - A mini-review. Gerontology 2013, 59, 289–296. [Google Scholar] [CrossRef]
- Olver, T.D.; Ferguson, B.S.; Laughlin, M.H. Molecular mechanisms for exercise training-induced changes in vascular structure and function: Skeletal muscle, cardiac muscle, and the brain. Prog. Mol. Biol. Transl. Sci. 2015, 135, 227–257. [Google Scholar] [PubMed]
- Hellsten, Y.; Nyberg, M. Cardiovascular adaptations to exercise training. Compr. Physiol. 2015, 6, 1–32. [Google Scholar] [PubMed]
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Culver, B.; Ren, J. Benefit and risk of exercise on myocardial function in diabetes. Pharm. Res. 2003, 48, 127–132. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Thompson, D.; Karpe, F.; Lafontan, M.; Frayn, K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol. Rev. 2012, 92, 157–191. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef]
- Hafstad, A.D.; Boardman, N.; Aasum, E. How exercise may amend metabolic disturbances in diabetic cardiomyopathy. Antioxid. Redox Signal. 2015, 22, 1587–1605. [Google Scholar] [CrossRef]
- Gibb, A.A.; Hill, B.G. Metabolic coordination of physiological and pathological cardiac remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, glut4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake - Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Parry, T.L.; Starnes, J.W.; O’Neal, S.K.; Bain, J.R.; Muehlbauer, M.J.; Honcoop, A.; Ilaiwy, A.; Christopher, P.; Patterson, C.; Willis, M.S. Untargeted metabolomics analysis of ischemia-reperfusion-injured hearts ex vivo from sedentary and exercise-trained rats. Metabolomics 2018, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, A.F.; Wang, S.; Kandadi, M.R.; Chen, J.; Hua, Y.; Pei, Z.; Nair, S.; Ren, J. Cardiomyocyte-specific knockout of endothelin receptor a attenuates obesity cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Turdi, S.; Richmond, K.L.; Zhang, Y.; Ren, J. Aldh2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: Role of cam kinase ii, histone h3k9 methyltransferase suv39h, sirt1, and pgc-1alpha deacetylation. Int. J. Obes. 2018, 42, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular effects and benefits of exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Platt, C.; Houstis, N.; Rosenzweig, A. Using exercise to measure and modify cardiac function. Cell Metab. 2015, 21, 227–236. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Wang, C.; Redgrave, J.; Shafizadeh, M.; Majid, A.; Kilner, K.; Ali, A.N. Aerobic exercise interventions reduce blood pressure in patients after stroke or transient ischaemic attack: A systematic review and meta-analysis. Br. J. Sports Med. 2018, bjsports-2017. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Segers, V.F.M.; Zannad, F.; Brutsaert, D.L. The future of pleiotropic therapy in heart failure. Lessons from the benefits of exercise training on endothelial function. Eur. J. Heart Fail. 2017, 19, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Balligand, J.L. Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovasc. Res. 2016, 111, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, E.; Hamik, A.; Josephson, R.A. Cardiorespiratory fitness and atherosclerosis: Recent data and future directions. Curr. Atheroscler. Rep. 2016, 18, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roe, N.D.; Ren, J. Nitric oxide synthase uncoupling: A therapeutic target in cardiovascular diseases. Vasc. Pharm. 2012, 57, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.F.; Ren, J.; Miao, C.Y. Nitric oxide synthase gene therapy for cardiovascular disease. Jpn. J. Pharm. 2002, 89, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; La Salle, D.T.; Cerbie, J.; Cho, J.M.; Bledsoe, A.; Nelson, A.; Morgan, D.E.; Richardson, R.S.; Shiu, Y.T.; Boudina, S.; et al. Elevated arterial shear rate increases indexes of endothelial cell autophagy and nitric oxide synthase activation in humans. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H106–H112. [Google Scholar] [CrossRef]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef]
- Tian, H.; Chen, P.; Ren, J. Physical exercise, autophagy and cardiometabolic stress in aging. Aging 2019, 11, 5287–5288. [Google Scholar] [CrossRef]
- Mansueto, G.; Armani, A.; Viscomi, C.; D’Orsi, L.; De Cegli, R.; Polishchuk, E.V.; Lamperti, C.; Di Meo, I.; Romanello, V.; Marchet, S.; et al. Transcription factor eb controls metabolic flexibility during exercise. Cell Metab. 2017, 25, 182–196. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, I.; Jang, Y.; Song, W.; Cosio-Lima, L.M.; Roltsch, M.H. Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J. Physiol. Sci. 2017, 67, 639–654. [Google Scholar] [CrossRef]
- Guan, Y.; Drake, J.C.; Yan, Z. Exercise-induced mitophagy in skeletal muscle and heart. Exerc. Sport Sci. Rev. 2019, 47, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, Y.; Tan, Y.; Zhang, Z.; Hou, Z.; Gao, C.; Feng, P.; Zhang, X.; Yi, W.; Gao, F. Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Delaney, N.F.; Sharma, R.; Tadvalkar, L.; Clish, C.B.; Haller, R.G.; Mootha, V.K. Metabolic profiles of exercise in patients with mcardle disease or mitochondrial myopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 8402–8407. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Koh, J.H.; Hancock, C.R.; Terada, S.; Higashida, K.; Holloszy, J.O.; Han, D.H. Pparbeta is essential for maintaining normal levels of pgc-1alpha and mitochondria and for the increase in muscle mitochondria induced by exercise. Cell Metab. 2017, 25, 1176–1185. [Google Scholar] [CrossRef]
- Dethlefsen, M.M.; Kristensen, C.M.; Tondering, A.S.; Lassen, S.B.; Ringholm, S.; Pilegaard, H. Impact of liver pgc-1alpha on exercise and exercise training-induced regulation of hepatic autophagy and mitophagy in mice on hff. Physiol. Rep. 2018, 6, e13731. [Google Scholar] [CrossRef]
- Vainshtein, A.; Tryon, L.D.; Pauly, M.; Hood, D.A. Role of pgc-1alpha during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef]
- Kang, C.; Ji, L.L. Pgc-1alpha overexpression via local transfection attenuates mitophagy pathway in muscle disuse atrophy. Free Radic. Biol. Med. 2016, 93, 32–40. [Google Scholar] [CrossRef]
- Palmer, K.K.; Chinn, K.M.; Robinson, L.E. The effect of the champ intervention on fundamental motor skills and outdoor physical activity in preschoolers. J. Sport Health Sci. 2019, 8, 98–105. [Google Scholar] [CrossRef]
- Wilson, M.G.; Ellison, G.M.; Cable, N.T. Basic science behind the cardiovascular benefits of exercise. Br. J. Sports Med. 2016, 50, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Eijsvogels, T.M.; Thompson, P.D. Exercise is medicine: At any dose? JAMA 2015, 314, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Pate, R.R.; Lavie, C.J.; Sui, X.; Church, T.S.; Blair, S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 2014, 64, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.E.; Green, J.; Reeves, G.K.; Beral, V.; Cairns, B.J.; Million Women Study Collaborators. Response to letter regarding article “Frequent physical activity may not reduce vascular disease risk as much as moderate activity: Large prospective study of women in the united kingdom”. Circulation 2015, 132, e225. [Google Scholar] [CrossRef][Green Version]
- Schnohr, P.; O’Keefe, J.H.; Marott, J.L.; Lange, P.; Jensen, G.B. Dose of jogging and long-term mortality: The copenhagen city heart study. J. Am. Coll. Cardiol. 2015, 65, 411–419. [Google Scholar] [CrossRef]
- Sharman, J.E.; La Gerche, A.; Coombes, J.S. Exercise and cardiovascular risk in patients with hypertension. Am. J. Hypertens 2015, 28, 147–158. [Google Scholar] [CrossRef]
- Li, J.; Siegrist, J. Physical activity and risk of cardiovascular disease--A meta-analysis of prospective cohort studies. Int. J. Environ. Res. Public Health 2012, 9, 391–407. [Google Scholar] [CrossRef]
- Roberts, W.O.; Schwartz, R.S.; Garberich, R.F.; Carlson, S.; Knickelbine, T.; Schwartz, J.G.; Peichel, G.; Lesser, J.R.; Wickstrom, K.; Harris, K.M. Fifty men, 3510 marathons, cardiac risk factors, and coronary artery calcium scores. Med. Sci. Sports Exerc. 2017, 49, 2369–2373. [Google Scholar] [CrossRef]
- Laddu, D.R.; Rana, J.S.; Murillo, R.; Sorel, M.E.; Quesenberry, C.P., Jr.; Allen, N.B.; Gabriel, K.P.; Carnethon, M.R.; Liu, K.; Reis, J.P.; et al. 25-year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: The coronary artery risk development in young adults (cardia) study. Mayo Clin. Proc. 2017, 92, 1660–1670. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Rehsia, N.S.; Dhalla, N.S. Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp. Clin. Cardiol. 2010, 15, E86–E95. [Google Scholar] [PubMed]
- Flachskampf, F.A.; Biering-Sorensen, T.; Solomon, S.D.; Duvernoy, O.; Bjerner, T.; Smiseth, O.A. Cardiac imaging to evaluate left ventricular diastolic function. JACC Cardiovasc. Imaging 2015, 8, 1071–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, W.; Ren, J. Stress signaling in paraquat-induced target organ toxicity. ROS 2016, 1, 131–140. [Google Scholar] [CrossRef]
- Kalia, R.; Wang, R.Y.; Yusuf, A.; Thomas, P.V.; Agard, D.A.; Shaw, J.M.; Frost, A. Structural basis of mitochondrial receptor binding and constriction by drp1. Nature 2018, 558, 401–405. [Google Scholar] [CrossRef]
- Saito, T.; Sadoshima, J. Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart. Circ. Res. 2015, 116, 1477–1490. [Google Scholar] [CrossRef]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The ubiquitin kinase pink1 recruits autophagy receptors to induce mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Anton, Z.; Landajuela, A.; Hervas, J.H.; Montes, L.R.; Hernandez-Tiedra, S.; Velasco, G.; Goni, F.M.; Alonso, A. Human atg8-cardiolipin interactions in mitophagy: Specific properties of lc3b, gabarapl2 and gabarap. Autophagy 2016, 12, 2386–2403. [Google Scholar] [CrossRef]
- Liu, L.; Sakakibara, K.; Chen, Q.; Okamoto, K. Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res. 2014, 24, 787–795. [Google Scholar] [CrossRef]
- Gyongyosi, A.; Terraneo, L.; Bianciardi, P.; Tosaki, A.; Lekli, I.; Samaja, M. The impact of moderate chronic hypoxia and hyperoxia on the level of apoptotic and autophagic proteins in myocardial tissue. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Chen, C.C.W.; Erlich, A.T.; Hood, D.A. Role of parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet. Muscle 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.C.; Laker, R.C.; Wilson, R.J.; Zhang, M.; Yan, Z. Exercise-induced mitophagy in skeletal muscle occurs in the absence of stabilization of pink1 on mitochondria. Cell Cycle 2019, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Miao, W.; Ma, J.; Xv, Z.; Bo, H.; Li, J.; Zhang, Y.; Ji, L.L. Acute exercise-induced mitochondrial stress triggers an inflammatory response in the myocardium via nlrp3 inflammasome activation with mitophagy. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Xia, Z.; Bai, S.; Zhang, H.E.; Gu, B.; Wang, R. Downhill running acutely elicits mitophagy in rat soleus muscle. Med. Sci. Sports Exerc. 2019, 51, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Mueller, R.; Li, Y.; Ruan, T.; Kunz, D.; Goodrich, R.; Mills, T.; Deeter, L.; Sargsyan, A.; Anandh Babu, P.V.; et al. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can. J. Physiol Pharm. 2014, 92, 605–612. [Google Scholar] [CrossRef]
- Ju, J.S.; Jeon, S.I.; Park, J.Y.; Lee, J.Y.; Lee, S.C.; Cho, K.J.; Jeong, J.M. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J. Physiol. Sci. 2016, 66, 417–430. [Google Scholar] [CrossRef]
- Moradi, F.; Imani, A.R.; Faghihi, M. Effects of regular exercise plus food restriction on left ventricular pathological remodeling in heart failureinduced rats. Bratisl. Lek. Listy. 2019, 120, 243–248. [Google Scholar]
- Koo, J.H.; Kang, E.B.; Cho, J.Y. Resistance exercise improves mitochondrial quality control in a rat model of sporadic inclusion body myositis. Gerontology 2019, 65, 240–252. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R. Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol. Life Sci. 2016, 73, 775–795. [Google Scholar] [CrossRef]
- Barres, R.; Zierath, J.R. The role of diet and exercise in the transgenerational epigenetic landscape of t2dm. Nat. Rev. Endocrinol. 2016, 12, 441–451. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, E457–E471. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Lifestyle effects on hematopoiesis and atherosclerosis. Circ. Res. 2015, 116, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the american heart association. Circulation 2017, 136, E1–E23. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, K2139. [Google Scholar] [CrossRef] [PubMed]
- Pagnotti, G.M.; Styner, M.; Uzer, G.; Patel, V.S.; Wright, L.E.; Ness, K.K.; Guise, T.A.; Rubin, J.; Rubin, C.T. Combating osteoporosis and obesity with exercise: Leveraging cell mechanosensitivity. Nat. Rev. Endocrinol. 2019, 15, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, T.; Tolvanen, S.; Heinonen, A.; Kujala, U.M. Exercise therapy for functional capacity in chronic diseases: An overview of meta-analyses of randomised controlled trials. Br. J. Sports Med. 2017, 51, 1459–1465. [Google Scholar] [CrossRef]
- Goncalves, I.O.; Passos, E.; Diogo, C.V.; Rocha-Rodrigues, S.; Santos-Alves, E.; Oliveira, P.J.; Ascensao, A.; Magalhaes, J. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl. Physiol. Nutr. Metab. 2016, 41, 298–306. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Lee, D.E.; Brown, J.L.; Brown, L.A.; Perry, R.A., Jr.; Greene, E.S.; Carvallo Chaigneau, F.R.; Washington, T.A.; Greene, N.P. Moderate physical activity promotes basal hepatic autophagy in diet-induced obese mice. Appl. Physiol. Nutr. Metab. 2017, 42, 148–156. [Google Scholar] [CrossRef]
- Tarpey, M.D.; Davy, K.P.; McMillan, R.P.; Bowser, S.M.; Halliday, T.M.; Boutagy, N.E.; Davy, B.M.; Frisard, M.I.; Hulver, M.W. Skeletal muscle autophagy and mitophagy in endurance-trained runners before and after a high-fat meal. Mol. Metab. 2017, 6, 1597–1609. [Google Scholar] [CrossRef]
- Carter, H.N.; Kim, Y.; Erlich, A.T.; Zarrin-Khat, D.; Hood, D.A. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 2018, 596, 3567–3584. [Google Scholar] [CrossRef]
- Kim, Y.; Triolo, M.; Erlich, A.T.; Hood, D.A. Regulation of autophagic and mitophagic flux during chronic contractile activity-induced muscle adaptations. Pflug. Arch. 2019, 471, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Pan, S.S.; Wang, J.Y.; Lu, J. Changes in autophagy levels in rat myocardium during exercise preconditioning-initiated cardioprotective effects. Int. Heart J. 2019, 60, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Estebanez, B.; Moreira, O.C.; Almar, M.; de Paz, J.A.; Gonzalez-Gallego, J.; Cuevas, M.J. Effects of a resistance-training programme on endoplasmic reticulum unfolded protein response and mitochondrial functions in pbmcs from elderly subjects. Eur. J. Sport Sci. 2019, 19, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Pan, S.S. Parkin mediates mitophagy to participate in cardioprotection induced by late exercise preconditioning but bnip3 does not. J. Cardiovasc. Pharm. 2018, 71, 303–316. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, S.S.; Wan, D.F.; Lu, J.; Huang, Y. H2o2 signaling-triggered pi3k mediates mitochondrial protection to participate in early cardioprotection by exercise preconditioning. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Dun, Y.; Liu, S.; Zhang, W.; Xie, M.; Qiu, L. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Hinkley, J.M.; Morton, A.B.; Ichinoseki-Sekine, N.; Huertas, A.M.; Smuder, A.J. Exercise training prevents doxorubicin-induced mitochondrial dysfunction of the liver. Med. Sci. Sports Exerc. 2019, 51, 1106–1115. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Torrella, J.R.; Oliveira, P.J.; Magalhaes, J.; Ascensao, A. Exercise and doxorubicin treatment modulate cardiac mitochondrial quality control signaling. Cardiovasc. Toxicol. 2018, 18, 43–55. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, I.; Jang, Y.; Cosio-Lima, L.; Barrington, P. Endurance exercise attenuates doxorubicin-induced cardiotoxicity. Med. Sci. Sports Exerc. 2019. [Google Scholar] [CrossRef]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2293–2302. [Google Scholar] [CrossRef]
- Lekli, I.; Haines, D.D.; Balla, G.; Tosaki, A. Autophagy: An adaptive physiological countermeasure to cellular senescence and ischaemia/reperfusion-associated cardiac arrhythmias. J. Cell Mol. Med. 2017, 21, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Czegledi, A.; Tosaki, A.; Gyongyosi, A.; Zilinyi, R.; Tosaki, A.; Lekli, I. Electrically-induced ventricular fibrillation alters cardiovascular function and expression of apoptotic and autophagic proteins in rat hearts. Int. J. Mol. Sci. 2019, 20, 1628. [Google Scholar] [CrossRef] [PubMed]
- Gyongyosi, A.; Zilinyi, R.; Czegledi, A.; Tosaki, A.; Tosaki, A.; Lekli, I. The role of autophagy and death pathways in dose-dependent isoproterenolinduced cardiotoxicity. Curr. Pharm. Des. 2019, 25, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends Pharm. Sci. 2018, 39, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Hentila, J.; Ahtiainen, J.P.; Paulsen, G.; Raastad, T.; Hakkinen, K.; Mero, A.A.; Hulmi, J.J. Autophagy is induced by resistance exercise in young men, but unfolded protein response is induced regardless of age. Acta Physiol. 2018, 224, e13069. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.R.; Ryan, Z.C.; Pfaffenbach, K.T.; Dasari, S.; Parvizi, M.; Lalia, A.Z.; Lanza, I.R. Attenuated activation of the unfolded protein response following exercise in skeletal muscle of older adults. Aging 2019, 11, 7587–7604. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Hart, N.; Taylor, A.W.; Goto, S.; Ngo, J.K.; Davies, K.J.; Radak, Z. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R127–R134. [Google Scholar] [CrossRef]
- Brinkmann, C.; Przyklenk, A.; Metten, A.; Schiffer, T.; Bloch, W.; Brixius, K.; Gehlert, S. Influence of endurance training on skeletal muscle mitophagy regulatory proteins in type 2 diabetic men. Endocr. Res. 2017, 42, 325–330. [Google Scholar] [CrossRef]
- Dethlefsen, M.M.; Halling, J.F.; Moller, H.D.; Plomgaard, P.; Regenberg, B.; Ringholm, S.; Pilegaard, H. Regulation of apoptosis and autophagy in mouse and human skeletal muscle with aging and lifelong exercise training. Exp. Gerontol. 2018, 111, 141–153. [Google Scholar] [CrossRef]
- Ogborn, D.I.; McKay, B.R.; Crane, J.D.; Safdar, A.; Akhtar, M.; Parise, G.; Tarnopolsky, M.A. Effects of age and unaccustomed resistance exercise on mitochondrial transcript and protein abundance in skeletal muscle of men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R734–R741. [Google Scholar] [CrossRef]
- Ren, J. Influence of gender on oxidative stress, lipid peroxidation, protein damage and apoptosis in hearts and brains from spontaneously hypertensive rats. Clin. Exp. Pharm. Physiol. 2007, 34, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, G.; Duan, J.; Chen, A.F.; Ren, J. Influence of gender on intrinsic contractile properties of isolated ventricular myocytes from calmodulin-induced diabetic transgenic mice. Endocr. Res. 2003, 29, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Cunningham, T.; Hardin, K.M.; Liu, E.; Malhotra, R.; Nayor, M.; Lewis, G.D.; Ho, J.E. Sex differences in cardiometabolic traits and determinants of exercise capacity in heart failure with preserved ejection fraction. JAMA Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gheller, B.J.; Riddle, E.S.; Lem, M.R.; Thalacker-Mercer, A.E. Understanding age-related changes in skeletal muscle metabolism: Differences between females and males. Annu. Rev. Nutr. 2016, 36, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.; Deldicque, L.; Francaux, M. Lack of activation of mitophagy during endurance exercise in human. Med. Sci. Sports Exerc. 2017, 49, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Opichka, M.; Shute, R.; Marshall, K.; Slivka, D. Effects of exercise in a cold environment on gene expression for mitochondrial biogenesis and mitophagy. Cryobiology 2019, 90, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sowers, J.R.; Ren, J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018, 14, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Whaley-Connell, A.T.; Sowers, J.R.; Ren, J. Autophagy as an emerging target in cardiorenal metabolic disease: From pathophysiology to management. Pharmacol. Ther. 2018, 191, 1–22. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Ren, J. Autophagic regulation of lipid homeostasis in cardiometabolic syndrome. Front. Cardiovasc. Med. 2018, 5, 38. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y. Editorial: New therapetic approaches in the management of ischemia reperfusion injury and cardiometabolic diseases: Opportunities and challenges. Curr. Drug Targets 2017, 18, 1687–1688. [Google Scholar] [CrossRef]
- Claes, J.; Buys, R.; Budts, W.; Smart, N.; Cornelissen, V.A. Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Haire-Joshu, D.; Tabak, R. Preventing obesity across generations: Evidence for early life intervention. Annu. Rev. Public Health 2016, 37, 253–271. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.N.; Tian, H.; Chen, P.; Wang, D.; Ren, J.; Zhang, Y. Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells 2019, 8, 1436. https://doi.org/10.3390/cells8111436
Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y. Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells. 2019; 8(11):1436. https://doi.org/10.3390/cells8111436
Chicago/Turabian StyleWu, Ne N., Haili Tian, Peijie Chen, Dan Wang, Jun Ren, and Yingmei Zhang. 2019. "Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health" Cells 8, no. 11: 1436. https://doi.org/10.3390/cells8111436
APA StyleWu, N. N., Tian, H., Chen, P., Wang, D., Ren, J., & Zhang, Y. (2019). Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells, 8(11), 1436. https://doi.org/10.3390/cells8111436





