The Effect of Sertoli Cells on Xenotransplantation and Allotransplantation of Ventral Mesencephalic Tissue in a Rat Model of Parkinson’s Disease
Abstract
1. Introduction
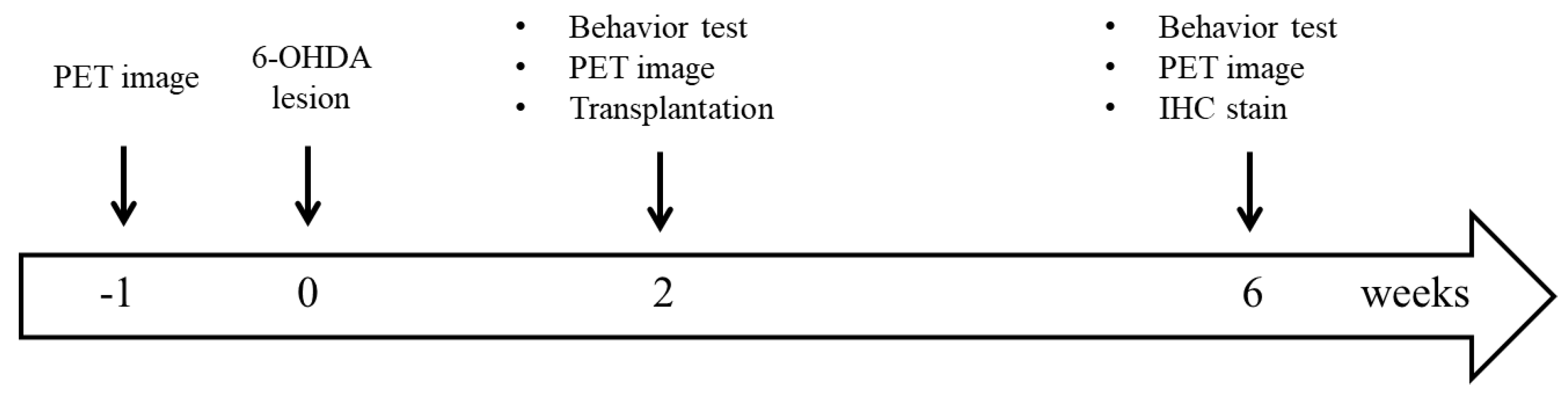
2. Material and Methods
2.1. Animals
2.2. Hemiparkinsonian Rat Model
2.3. Behavioral Test
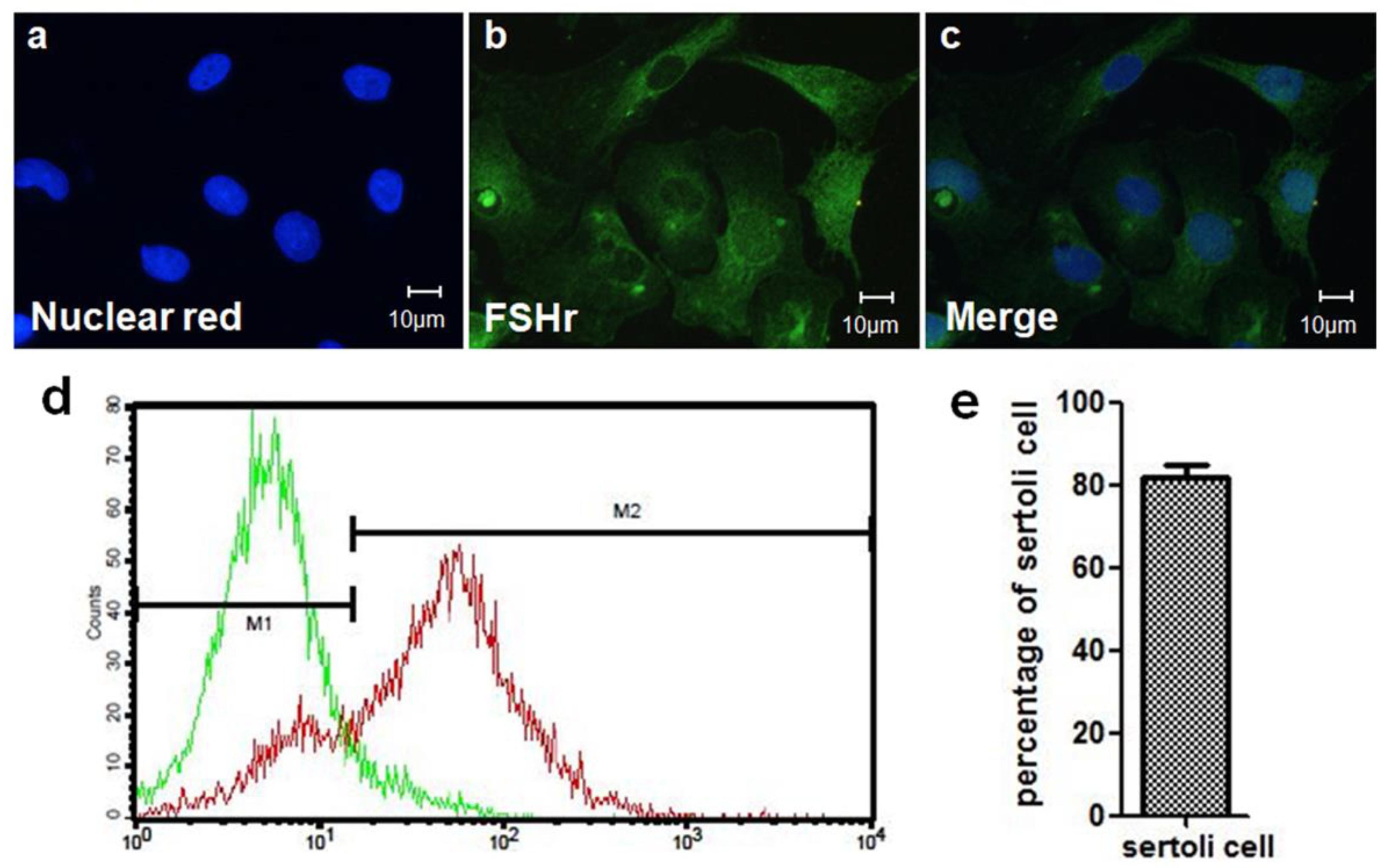
2.4. SC Isolation
2.5. Mesencephalic Tissue Preparation and Transplantation
2.6. Radiopharmaceuticals
2.7. Small Animal PET Imaging
2.8. IHC Staining
2.9. Statistical Analysis
3. Results
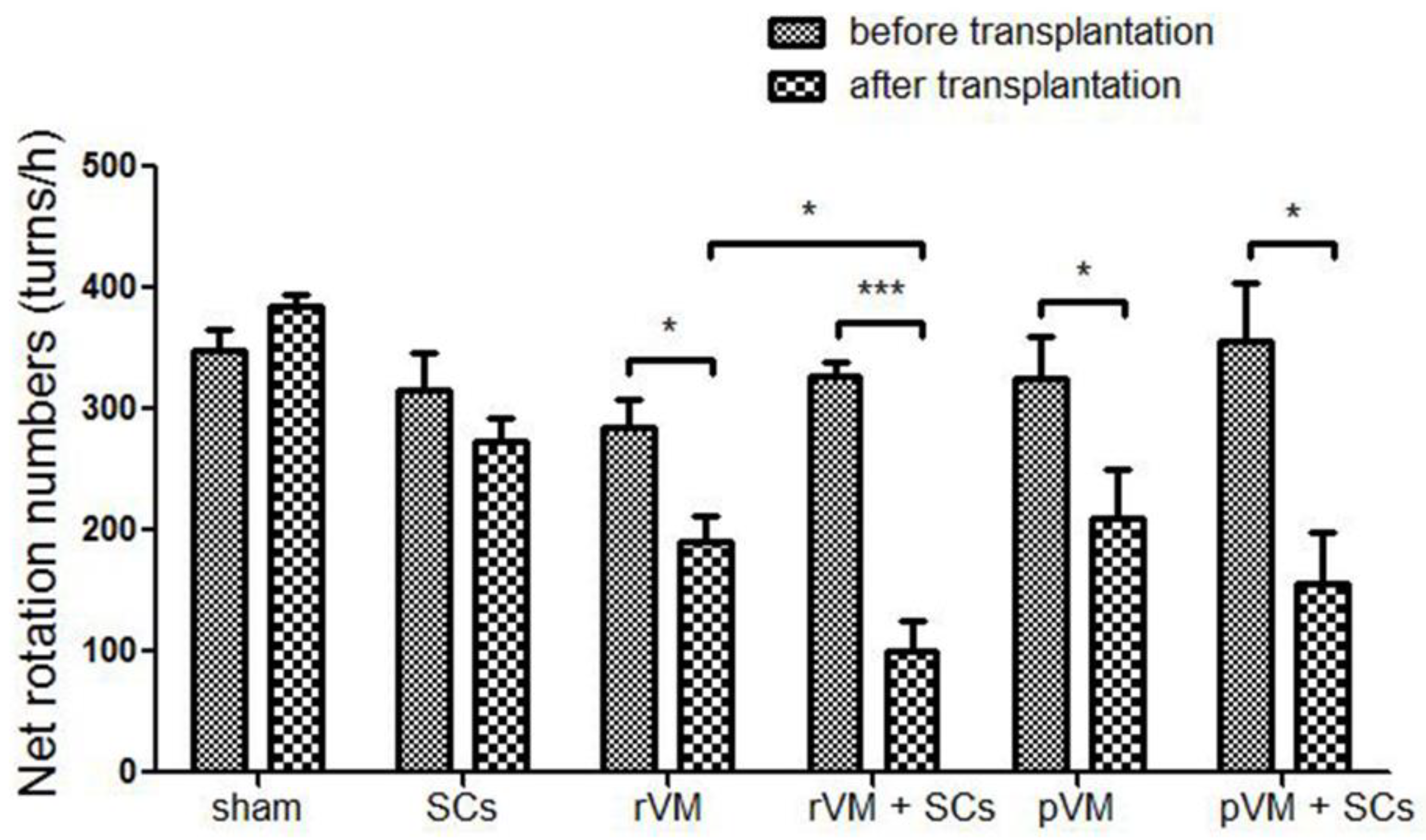
3.1. SCs Enhanced the Effect of VM Allotransplantation on DA Functional Recovery in Hemiparkinsonian Rats
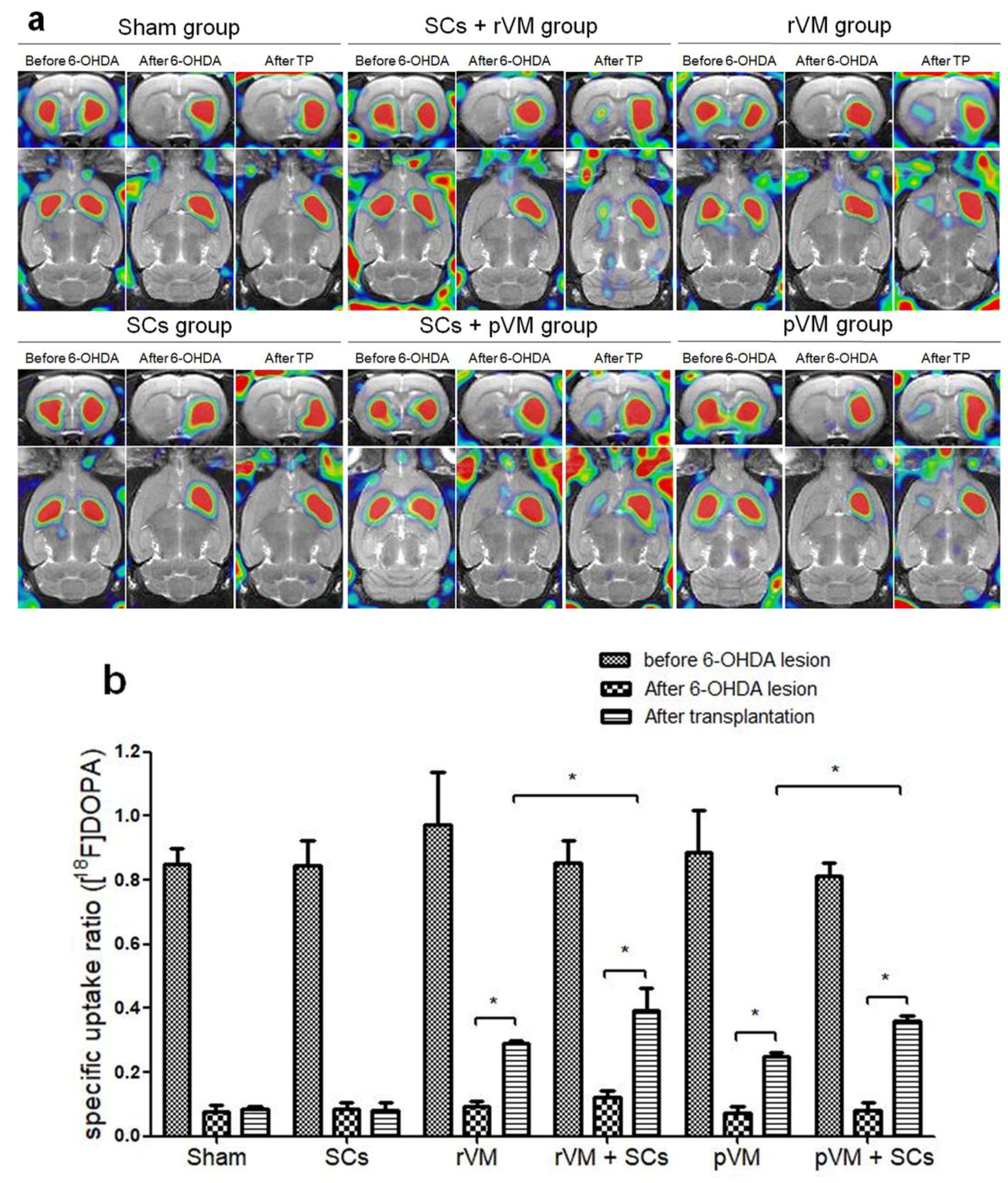
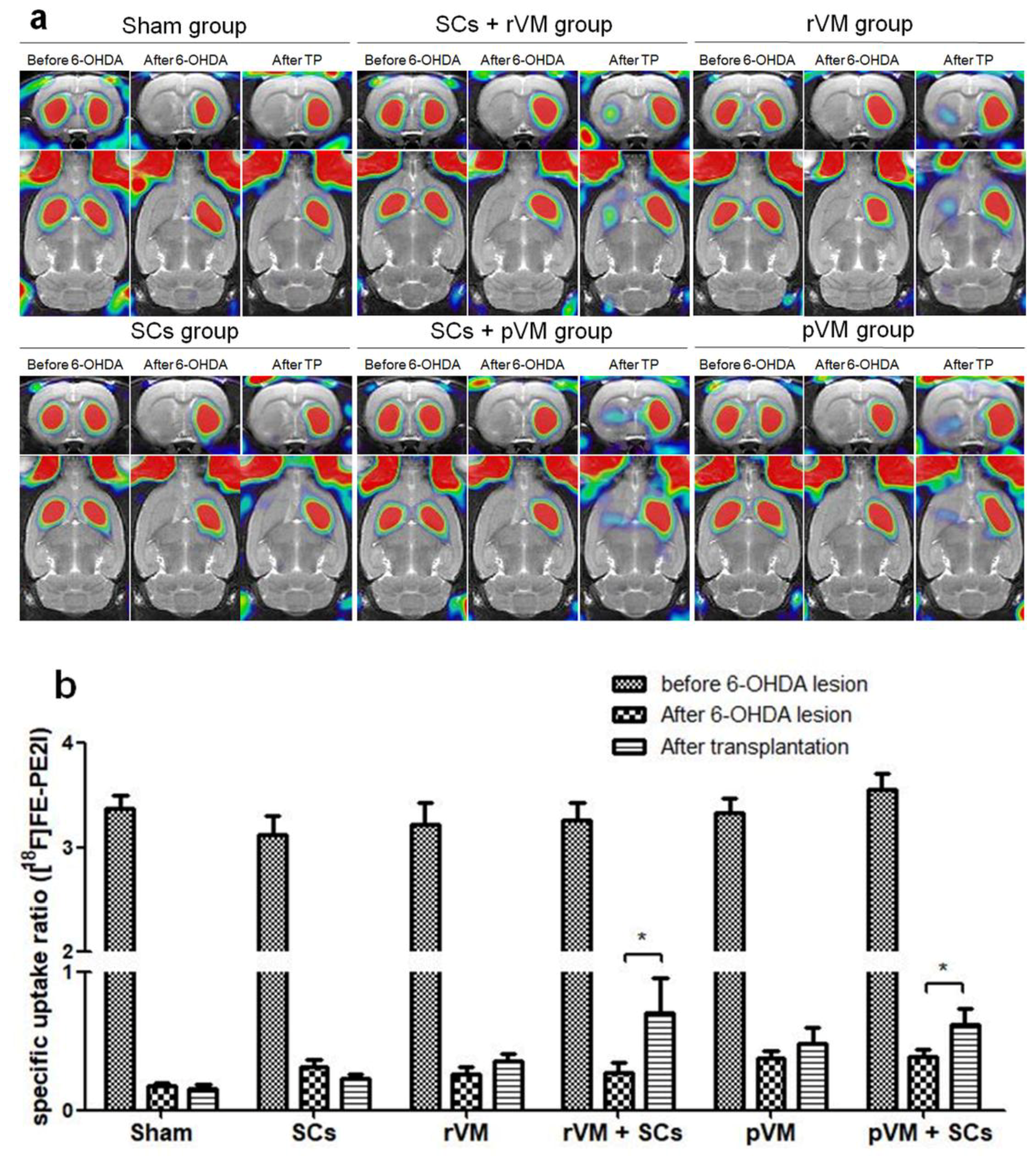
3.2. SCs Enhanced the Functional Result of DA Allografts and Xenografts in the Striatum of Hemiparkinsonian Rats as Determined by [18F]DOPA Coupled with Small Animal PET
3.3. SCs Enhance Maturation of DA Allografts and Xenografts in the Striatum of Hemiparkinsonian Rats as Determined by [18F]FE-PE2I Coupled with Small Animal PET
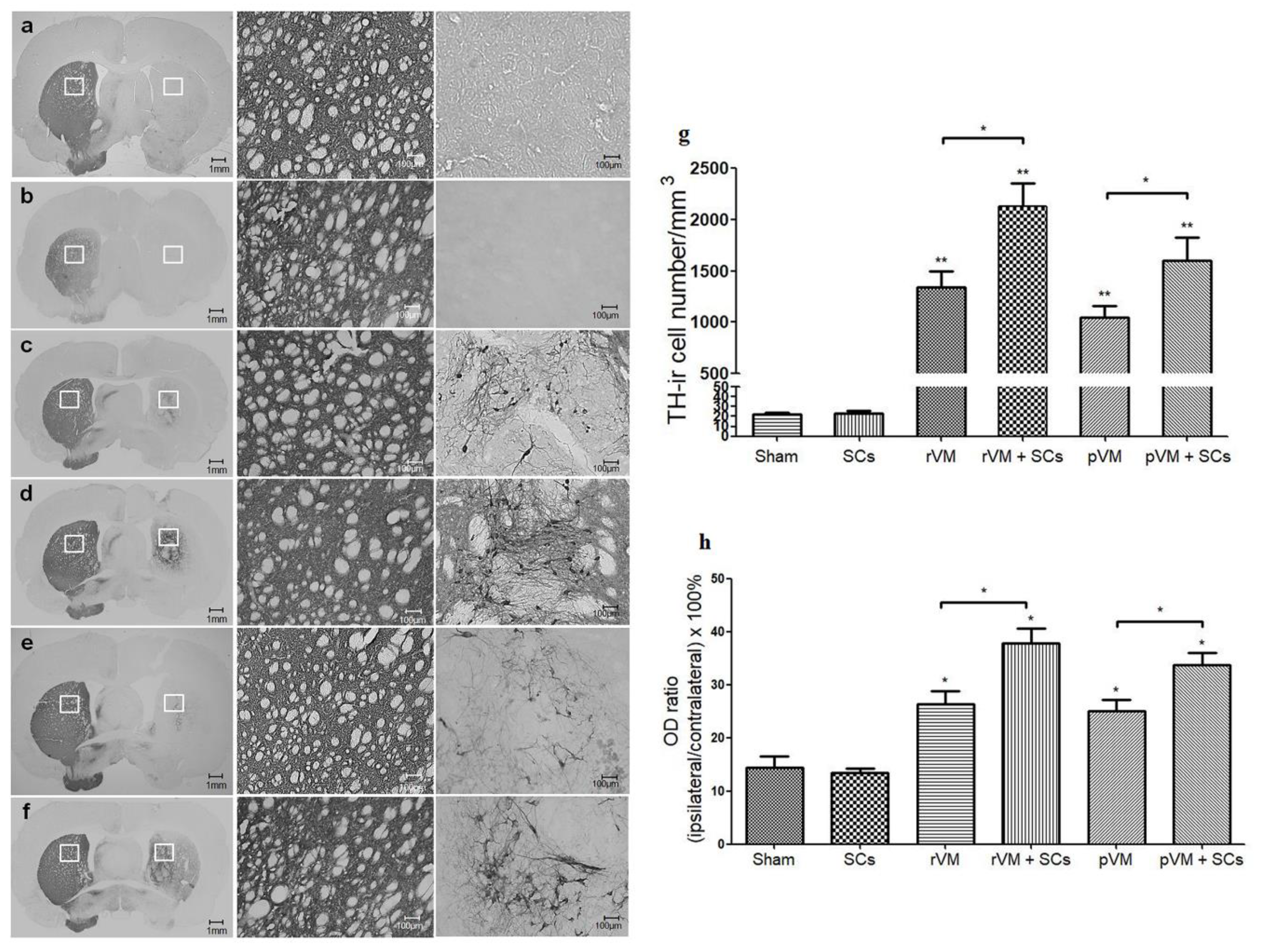
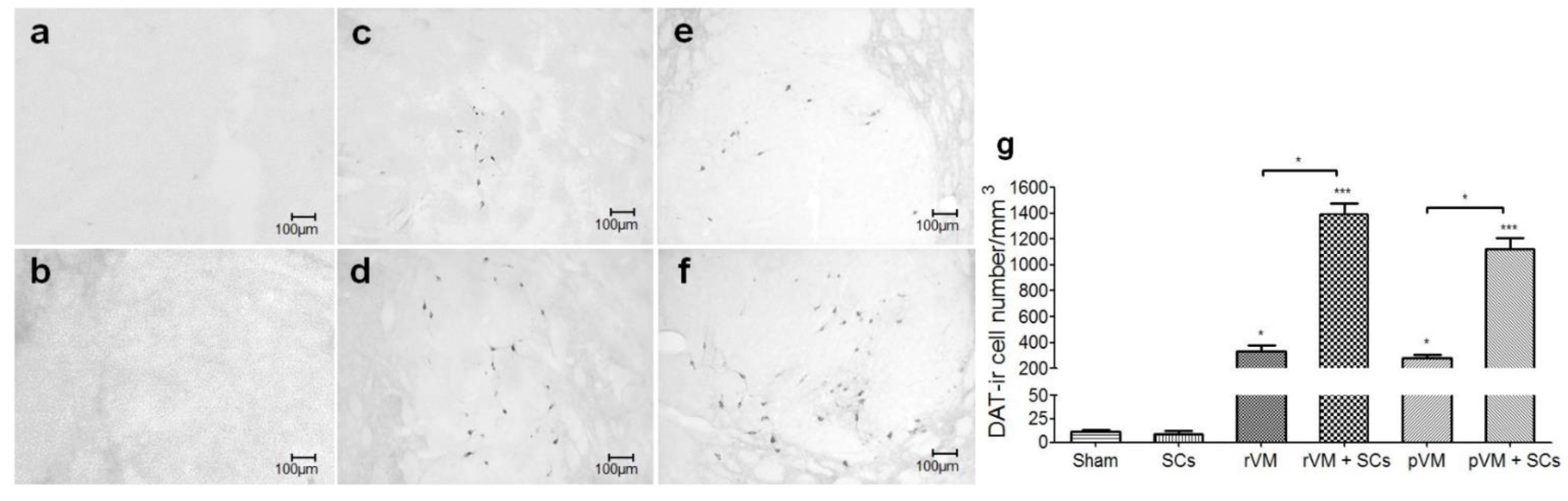
3.4. SCs Enhance the Survival of DA Cells in Allo- and Xeno-Transplantation
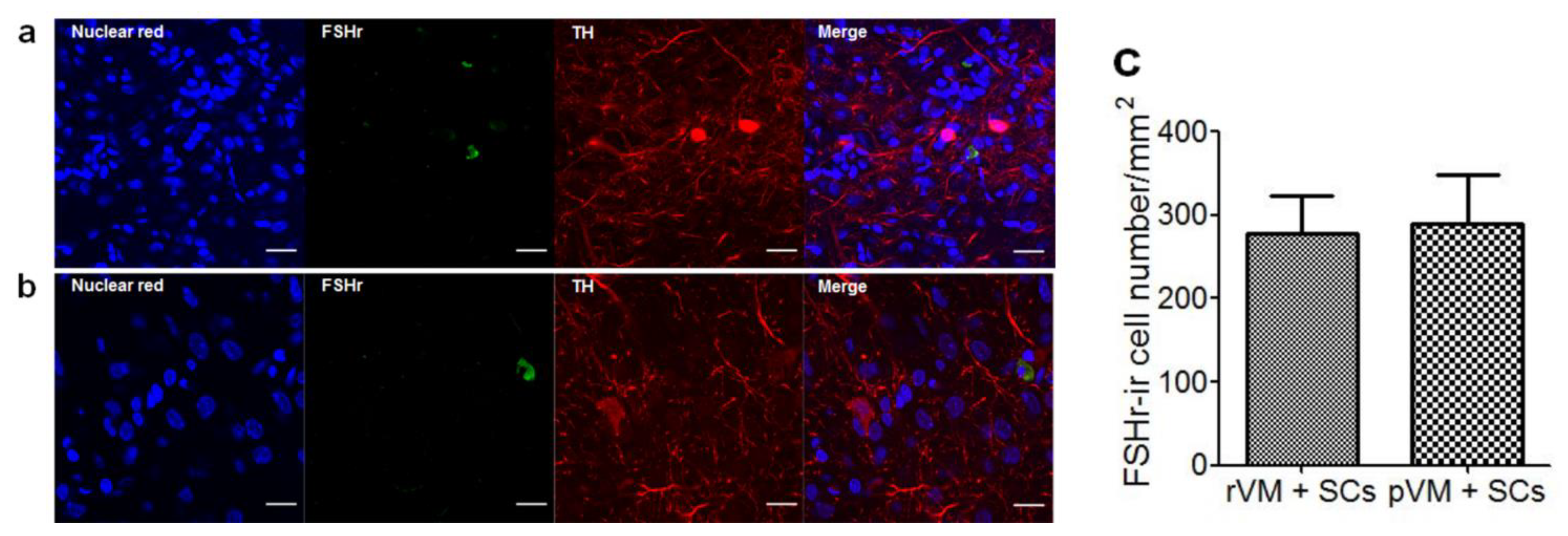
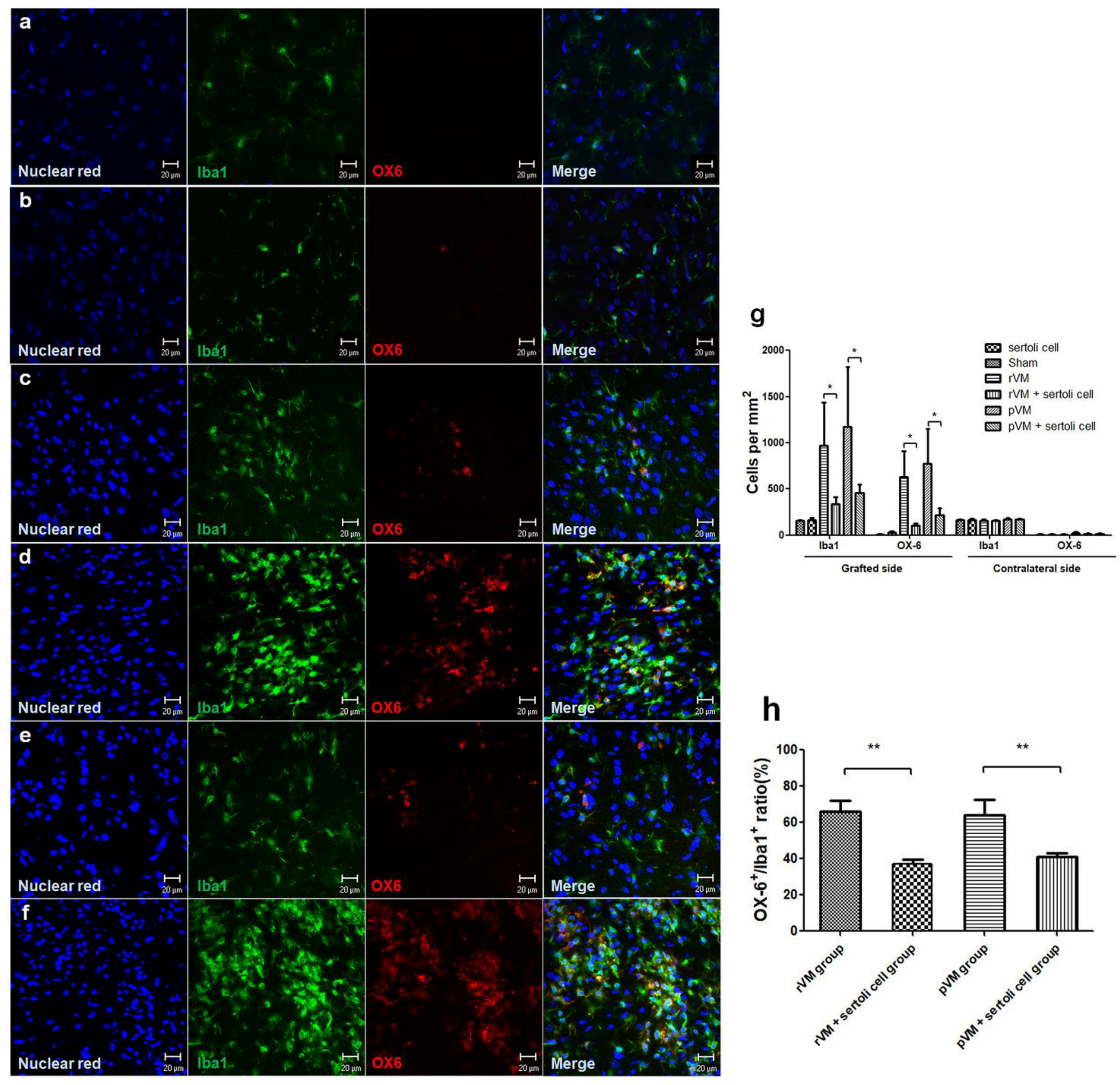
3.5. SCs Enhance DA Cell Maturation in Both Allo- and Xeno-Transplantation
3.6. SCs Attenuate the Immune Response of Microglia in Grafted Striatum after Allo- and Xeno-Transplantation
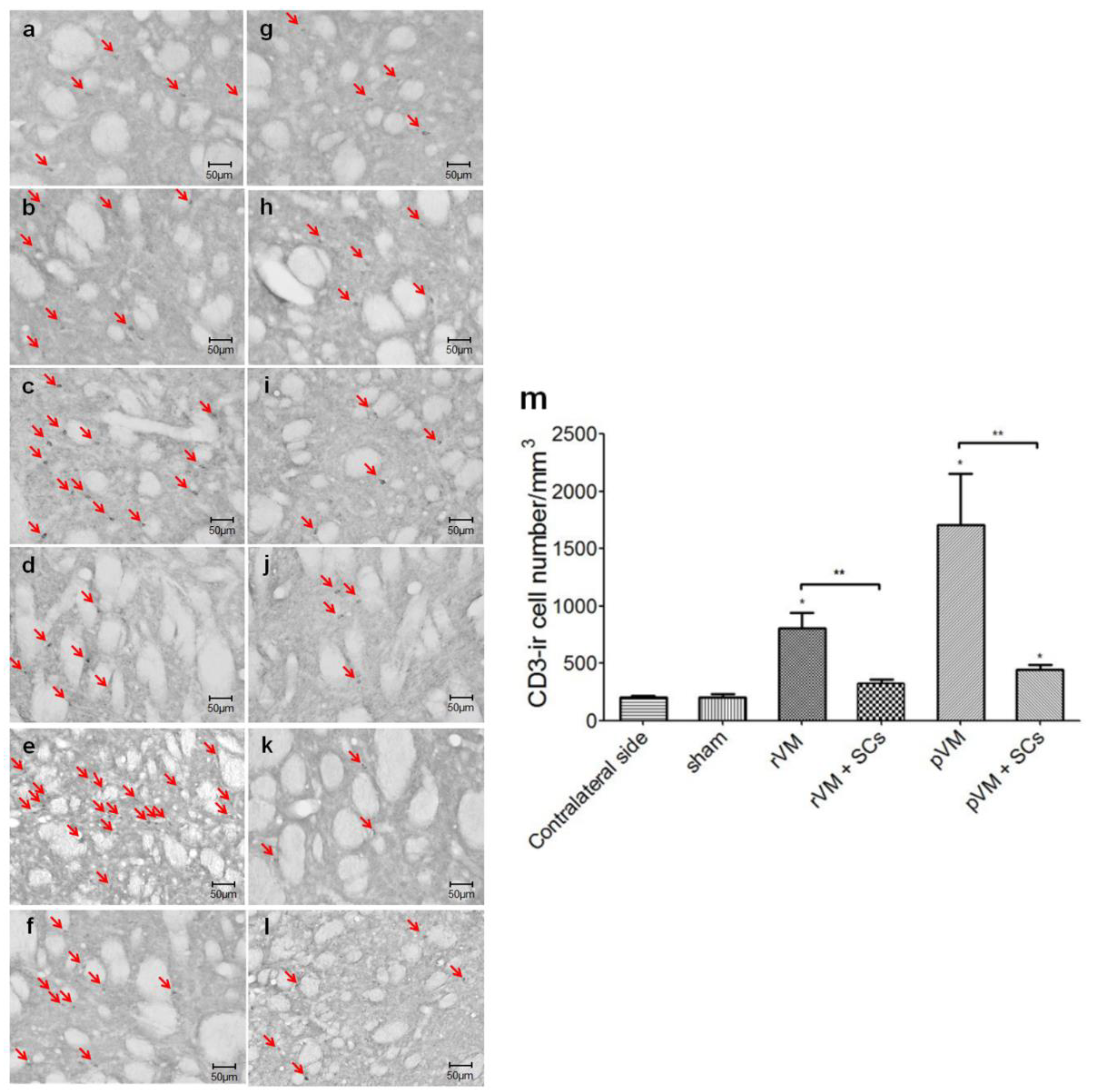
3.7. SCs Attenuate the T-Cell Infiltration Immune Response of after Allo- and Xeno-Transplantation
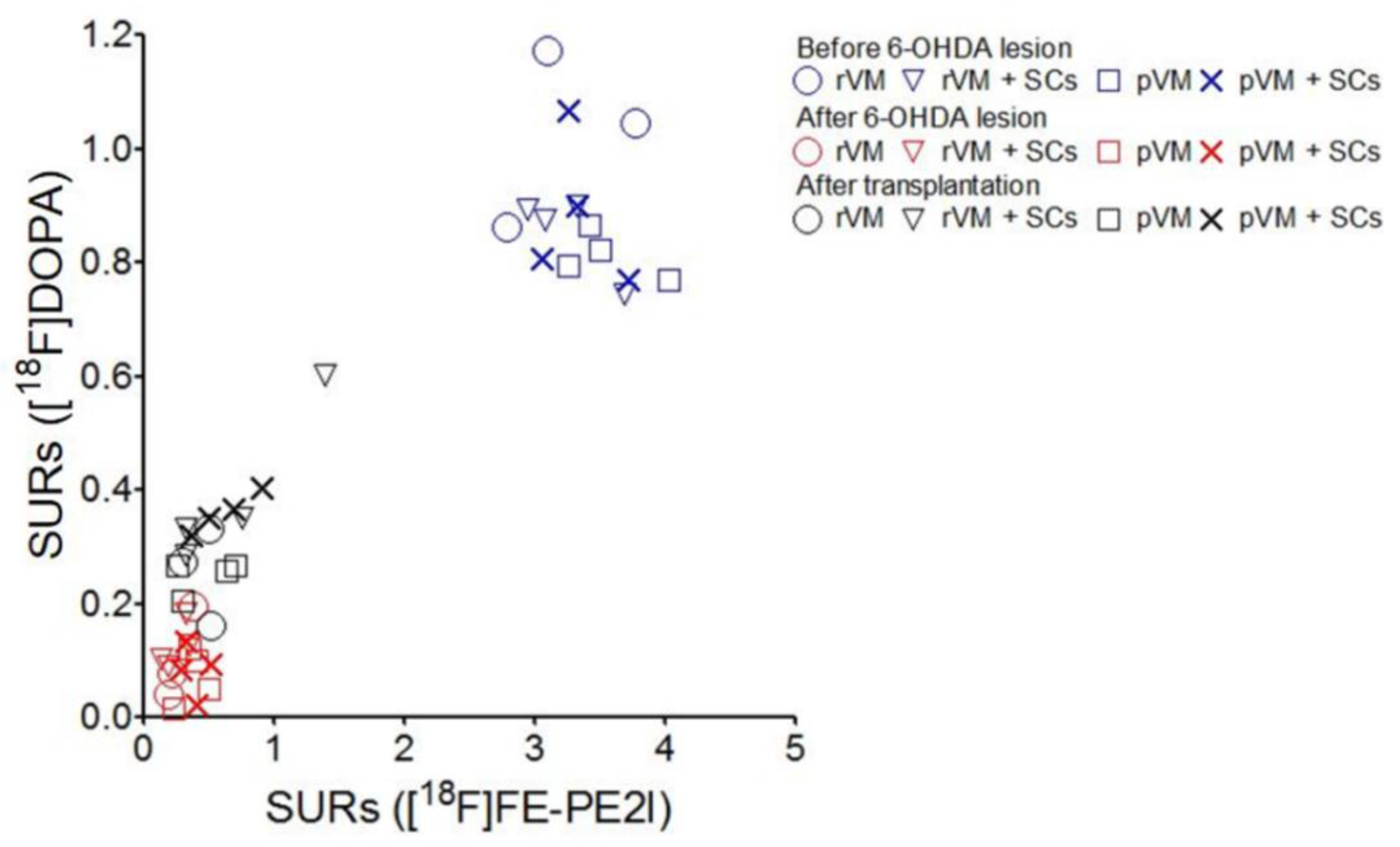
3.8. The SURs of [18F]DOPA and [18F]FE-PE2I Were Highly Correlated
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Jenkinson, N.; Owen, S.L.; Aziz, T.Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 2007, 8, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 2008, 9, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Bejjani, B.; Houeto, J.; Hariz, M.; Yelnik, J.; Mesnage, V.; Bonnet, A.; Pidoux, B.; Dormont, D.; Cornu, P.; Agid, Y. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology 2002, 59, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Drouin-Ouellet, J.; Parmar, M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat. Rev. Neurol. 2015, 11, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Barker, R.A.; Parmar, M. Neural grafting in Parkinson’s disease: Problems and possibilities. Prog. Brain Res. 2010, 184, 265–294. [Google Scholar] [PubMed]
- Kefalopoulou, Z.; Politis, M.; Piccini, P.; Mencacci, N.; Bhatia, K.; Jahanshahi, M.; Widner, H.; Rehncrona, S.; Brundin, P.; Björklund, A.; et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: Two case reports. JAMA Neurol. 2014, 71, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ambasudhan, R.; Dolatabadi, N.; Nutter, A.; Masliah, E.; McKercher, S.R.; Lipton, S.A. Potential for cell therapy in Parkinson’s disease using genetically programmed human embryonic stem cell-derived neural progenitor cells. J. Comp. Neurol. 2014, 522, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Brederlau, A.; Correia, A.S.; Anisimov, S.V.; Elmi, M.; Paul, G.; Roybon, L.; Morizane, A.; Bergquist, F.; Riebe, I.; Nannmark, U.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cells to a Rat Model of Parkinson’s Disease: Effect of In Vitro Differentiation on Graft Survival and Teratoma Formation. Stem Cells 2006, 24, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Minn, Y.K.; Lee, J.Y.; Choi, D.H.; Chang, M.Y.; Shim, J.W.; Ko, J.Y.; Koh, H.C.; Kang, M.J.; Kang, J.S.; et al. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J. Neurochem. 2005, 92, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- de Melo-Martín, I.; Hellmers, N.; Henchcliffe, C. First-in-human cell transplant trials in Parkinson’s disease: The need for an improved informed consent process. Parkinsonism Relat. Disord. 2015, 21, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Strecker, R.E.; Lindvall, O.; Isacson, O.; Nilsson, O.G.; Barbin, G.; Prochiantz, A.; Forni, C.; Nieoullon, A.; Widner, H.; et al. Intracerebral Grafting of Dopamine Neurons. Ann. N. Y. Acad. Sci. 1987, 495, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.T.H.; Clough, C.G.; Hughes, R.C.; Hitchcock, E.R.; Kenny, B.G. Implantation of Human Fetal Ventral Mesencephalon to the Right Caudate Nucleus in Advanced Parkinson’s Disease. Arch. Neurol. 1991, 48, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P.; et al. Neuropathological Evidence of Graft Survival and Striatal Reinnervation after the Transplantation of Fetal Mesencephalic Tissue in a Patient with Parkinson’s Disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Pogarell, O.; Koch, W.; Gildehaus, F.J.; Kupsch, A.; Lindvall, O.; Oertel, W.H.; Tatsch, K. Long-term assessment of striatal dopamine transporters in parkinsonian patients with intrastriatal embryonic mesencephalic grafts. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Lindvall, O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.S.; Schumacher, J.M.; Ellias, S.L.; Palmer, E.P.; Saint-Hilaire, M.; Shannon, K.; Penn, R.; Starr, P.; Vanhorne, C.; Kott, H.S.; et al. Porcine xenografts in Parkinson’s disease and Huntington’s disease patients: Preliminary results. Cell Transplant. 2000, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C. A brief history of cross-species organ transplantation. Bayl. Univ. Med Cent. Proc. 2012, 25, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.; Torper, O.; Drouin-Ouellet, J. Cell-based therapy for Parkinson’s disease: A journey through decades toward the light side of the Force. Eur. J. Neurosci. 2018, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Wakeman, D.R.; Kordower, J.H. Cell Replacement Strategies for Parkinson’s Disease. In Cell Therapy; Emerich, D.F., Orive, G., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 73–83. [Google Scholar]
- Busauschina, A.; Schnuelle, P.; van der Woude, F.J. Cyclosporine nephrotoxicity. Transplant. Proc. 2004, 36, S229–S233. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.M.; Ellias, S.; Palmer, E.P.; Kott, H.S.; Dinsmore, J.; Dempsey, P.K.; Fischman, A.J.; Thomas, C.; Feldman, R.G.; Kassissieh, S.; et al. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology 2000, 54, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Danovitch, G.M. Immunosuppressant-induced metabolic toxicities. Transplant. Rev. 2000, 14, 65–81. [Google Scholar] [CrossRef]
- Abouljoud, M.S.; Kumar, M.S.A.; Brayman, K.L.; Emre, S.; Bynon, J.S. For the OLN-452 Study Group Neoral rescue therapy in transplant patients with intolerance to tacrolimus. Clin. Transplant. 2002, 16, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.C.; Czech, K.A.; Brundin, P.; Widner, H. Intrastriatal ventral mesencephalic xenografts of porcine tissue in rats: Immune responses and functional effects. Cell Transplant. 2000, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wenker, S.D.; Leal, M.C.; Farías, M.I.; Zeng, X.; Pitossi, F.J. Cell therapy for Parkinson’s disease: Functional role of the host immune response on survival and differentiation of dopaminergic neuroblasts. Brain Res. 2016, 1638, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.C.; Frielingsdorf, H.; Mirza, B.; Sophia, J.; Anderson, P.; Czech, K.A.; Strandberg, M.; Widner, H. Porcine neural xenografts in rats and mice: Donor tissue development and characteristics of rejection. Exp. Neurol. 2001, 172, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Mital, P.; Kaur, G.; Dufour, J.M. Immunoprotective Sertoli cells: Making allogeneic and xenogeneic transplantation feasible. Reproduction 2010, 139, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Loftis, J.M. Sertoli cell therapy: A novel possible treatment strategy for treatment-resistant major depressive disorder. Med Hypotheses. 2011, 77, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shamekh, R.; El-Badri, N.S.; Saporta, S.; Pascual, C.; Sanberg, P.R.; Cameron, D.F. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006, 15, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinzon, W.; Korbutt, G.S.; Power, R.; Hooton, J.; Rajotte, R.V.; Rabinovitch, A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000, 49, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.M.; Diakanov, I.; Selden, C.; Hodgson, H. Co-transplantation of encapsulated HepG2 and rat Sertoli cells improves outcome in a thioacetamide induced rat model of acute hepatic failure. Transpl. Int. 2005, 18, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-G.; Lee, H.-M.; Oh, B.C.; Lee, J.R. Cell-mediated Immunomodulation of Chemokine Receptor 7–expressing Porcine Sertoli Cells in Murine Heterotopic Heart Transplantation. J. Hear. Lung Transplant. 2009, 28, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Shamekh, R.; Mallery, J.; Newcomb, J.; Hushen, J.; Saporta, S.; Cameron, D.F.; Sanberg, C.D.; Sanberg, P.R.; Willing, A.E. Enhancing tyrosine hydroxylase expression and survival of fetal ventral mesencephalon neurons with rat or porcine Sertoli cells in vitro. Brain Res. 2006, 1096, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shamekh, R.; Newcomb, J.; Mallery, J.; Cassady, C.J.; Saporta, S.; Cameron, D.F.; Sanberg, P.R.; Willing, A.E. Survival of Rat or Mouse Ventral Mesencephalon Neurons after Cotransplantation with Rat Sertoli Cells in the Mouse Striatum. Cell Transplant. 2005, 14, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Shi, B.; Zhuang, Y.; Chu, J.; Shi, X.; Zhang, S.; Guo, M. Performance and Mechanism of Neuroleukin in the Growth and Survival of Sertoli Cell-Induced Neurons in a Coculture System. Cell Transplant. 2014, 23, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Fazio, P.; Svenningsson, P.; Cselenyi, Z.; Halldin, C.; Farde, L.; Varrone, A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov. Disord. 2018, 33, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.J.; Li, I.H.; Huang, Y.S.; Chueh, S.H.; Chou, T.K.; Huang, S.Y.; Shiue, C.Y.; Cheng, C.Y.; Ma, K.H. KA-bridged transplantation of mesencephalic tissue and olfactory ensheathing cells in a Parkinsonian rat model. J. Tissue Eng. Regen. Med. 2017, 11, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-J.; Shiue, C.-Y.; Huang, W.-S.; Cheng, C.-Y.; Huang, S.-Y.; Li, I.-H.; Tao, C.-C.; Chou, T.-K.; Liao, M.-H.; Chang, Y.-P.; et al. PET Imaging of Serotonin Transporters with 4-[18F]-ADAM in a Parkinsonian Rat Model. Cell Transplant. 2013, 22, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Allbutt, H.; Kassiou, M.; Henderson, J. Developing a preclinical model of Parkinson’s disease: A study of behaviour in rats with graded 6-OHDA lesions. Behav. Brain Res. 2006, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Folmer, J.; Wright, W.W.; Zirkin, B.R. Isolation of sertoli cells from adult rat testes: An approach to ex vivo studies of Sertoli cell function. Biol. Reprod. 2003, 68, 996–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeuchi, Y.; Sawada, T.; Blunt, S.; Jenner, P.; Marsden, C. Effects of 6-hydroxydopamine lesions of the nigrostriatal pathway on striatal serotonin innervation in adult rats. Brain Res. 1991, 562, 301–305. [Google Scholar] [CrossRef]
- Galpern, W.R.; Burns, L.H.; Deacon, T.W.; Dinsmore, J.; Isacson, O. Xenotransplantation of Porcine Fetal Ventral Mesencephalon in a Rat Model of Parkinson’s Disease: Functional Recovery and Graft Morphology. Exp. Neurol. 1996, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Björklund, A. Dissecting embryonic neural tissues for transplantation. In Neural Transplantation Methods; Dunnett, S.B., Boulton, A.A., Baker, G.B., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 3–25. [Google Scholar]
- Bang, J.-I.; Jung, I.S.; Song, Y.S.; Park, H.S.; Moon, B.S.; Lee, B.C.; Kim, S.E. PET imaging of dopamine transporters with [ 18 F]FE-PE2I: Effects of anti-Parkinsonian drugs. Nucl. Med. Biol. 2016, 43, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.-H.; Huang, W.-S.; Kuo, Y.-Y.; Peng, C.-J.; Liou, N.-H.; Liu, R.-S.; Hwang, J.-J.; Liu, J.-C.; Chen, H.-J.; Shiue, C.-Y. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. NeuroImage 2009, 45, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kyono, K.; Takashima, T.; Katayama, Y.; Kawasaki, T.; Zochi, R.; Gouda, M.; Kuwahara, Y.; Takahashi, K.; Wada, Y.; Onoe, H.; et al. Use of [18F] FDOPA-PET for in vivo evaluation of dopaminergic dysfunction in unilaterally 6-OHDA-lesioned rats. EJNMMI Res. 2011, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Forsback, S.; Niemi, R.; Marjamäki, P.; Eskola, O.; Bergman, J.; Grönroos, T.; Haaparanta, M.; Haapalinna, A.; Rinne, J.; Solin, O. Uptake of 6-[18F]fluoro-L-dopa and [18F]CFT reflect nigral neuronal loss in a rat model of Parkinson’s disease. Synapse 2004, 51, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, Hard cover Edition; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Inaji, M.; Yoshizaki, T.; Okauchi, T.; Maeda, J.; Nagai, Y.; Nariai, T.; Ohno, K.; Ando, K.; Okano, H.; Obayashi, S.; et al. In vivo PET measurements with [11C]PE2I to evaluate fetal mesencephalic transplantations to unilateral 6-OHDA-lesioned rats. Cell Transplant. 2005, 14, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Willing, A.E.; Othberg, A.I.; Saporta, S.; Anton, A.; Sinibaldi, S.; Poulos, S.G.; Cameron, D.F.; Freeman, T.B.; Sanberg, P.R. Sertoli cells enhance the survival of co-transplanted dopamine neurons. Brain Res. 1999, 822, 246–250. [Google Scholar] [CrossRef]
- Wakeman, D.R.; Redmond, D.E.; Dodiya, H.B.; Sladek, J.R.; Leranth, C.; Teng, Y.D.; Samulski, R.J.; Snyder, E.Y. Human neural stem cells survive long term in the midbrain of dopamine-depleted monkeys after GDNF overexpression and project neurites toward an appropriate target. Stem Cells Transl. Med. 2014, 3, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Tu, J.; Wan, J.; Zhang, J.; Wu, B.; Chen, S.; Zhou, J.; Mu, Y.; Wang, L. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat. Commun. 2014, 5, 5627. [Google Scholar] [CrossRef] [PubMed]
- Garnett, S.; Firnau, G.; Nahmias, C.; Chirakal, R. Striatal dopamine metabolism in living monkeys examined by positron emission tomography. Brain Res. 1983, 280, 169–171. [Google Scholar] [CrossRef]
- Varrone, A.; Steiger, C.; Schou, M.; Takano, A.; Finnema, S.J.; Guilloteau, D.; Gulyás, B.; Halldin, C. In vitro autoradiography and in vivo evaluation in cynomolgus monkey of [18F]FE-PE2I, a new dopamine transporter PET radioligand. Synapse 2009, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Schou, M.; Steiger, C.; Varrone, A.; Guilloteau, D.; Halldin, C. Synthesis, radiolabeling and preliminary in vivo evaluation of [18F]FE-PE2I, a new probe for the dopamine transporter. Bioorg. Med. Chem. Lett. 2009, 19, 4843–4845. [Google Scholar] [CrossRef] [PubMed]
- Fiszman, M.L.; Zuddas, A.; Masana, M.I.; Barker, J.L.; Porzio, U.; Di Porzio, U. Dopamine Synthesis Precedes Dopamine Uptake in Embryonic Rat Mesencephalic Neurons. J. Neurochem. 1991, 56, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, W.; Xue, S.; Han, D. Testicular defense systems: Immune privilege and innate immunity. Cell. Mol. Immunol. 2014, 11, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Garcı́a, R.; Aguiar, J.; Alberti, E.; de la Cuétara, K.; Pavón, N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem. Biophys. Res. Commun. 2004, 316, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lu, D.; Chopp, M. Intravenous Administration of Marrow Stromal Cells (MSCs) Increases the Expression of Growth Factors in Rat Brain after Traumatic Brain Injury. J. Neurotrauma 2004, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, U.; Del Dotto, P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology 2006, 67, S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, N.K.; Pal, R.; Rao, S.A.; Naik, A.L.; Jan, M.; Nair, R.; Sanjeev, C.C.; Kamble, R.B.; Murthy, D.P.; Chaitanya, K. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: A pilot clinical study. Stem Cells Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Park, H.J. Bone Marrow-Derived Mesenchymal Stem Cell Therapy as a Candidate Disease-Modifying Strategy in Parkinson’s Disease and Multiple System Atrophy. J. Clin. Neurol. 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhao, Y.-T.; Chiu, C.-H.; Chen, C.-F.F.; Chou, T.-K.; Lin, Y.-W.; Ju, Y.-T.; Wu, S.-C.; Yan, R.-F.; Shiue, C.-Y.; Chueh, S.-H.; et al. The Effect of Sertoli Cells on Xenotransplantation and Allotransplantation of Ventral Mesencephalic Tissue in a Rat Model of Parkinson’s Disease. Cells 2019, 8, 1420. https://doi.org/10.3390/cells8111420
Jhao Y-T, Chiu C-H, Chen C-FF, Chou T-K, Lin Y-W, Ju Y-T, Wu S-C, Yan R-F, Shiue C-Y, Chueh S-H, et al. The Effect of Sertoli Cells on Xenotransplantation and Allotransplantation of Ventral Mesencephalic Tissue in a Rat Model of Parkinson’s Disease. Cells. 2019; 8(11):1420. https://doi.org/10.3390/cells8111420
Chicago/Turabian StyleJhao, Yun-Ting, Chuang-Hsin Chiu, Chien-Fu F. Chen, Ta-Kai Chou, Yi-Wen Lin, Yu-Ten Ju, Shinn-Chih Wu, Ruoh-Fang Yan, Chyng-Yann Shiue, Sheau-Huei Chueh, and et al. 2019. "The Effect of Sertoli Cells on Xenotransplantation and Allotransplantation of Ventral Mesencephalic Tissue in a Rat Model of Parkinson’s Disease" Cells 8, no. 11: 1420. https://doi.org/10.3390/cells8111420
APA StyleJhao, Y.-T., Chiu, C.-H., Chen, C.-F. F., Chou, T.-K., Lin, Y.-W., Ju, Y.-T., Wu, S.-C., Yan, R.-F., Shiue, C.-Y., Chueh, S.-H., Halldin, C., Cheng, C.-Y., & Ma, K.-H. (2019). The Effect of Sertoli Cells on Xenotransplantation and Allotransplantation of Ventral Mesencephalic Tissue in a Rat Model of Parkinson’s Disease. Cells, 8(11), 1420. https://doi.org/10.3390/cells8111420




