Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy
Abstract
1. Introduction
2. Material and Methods
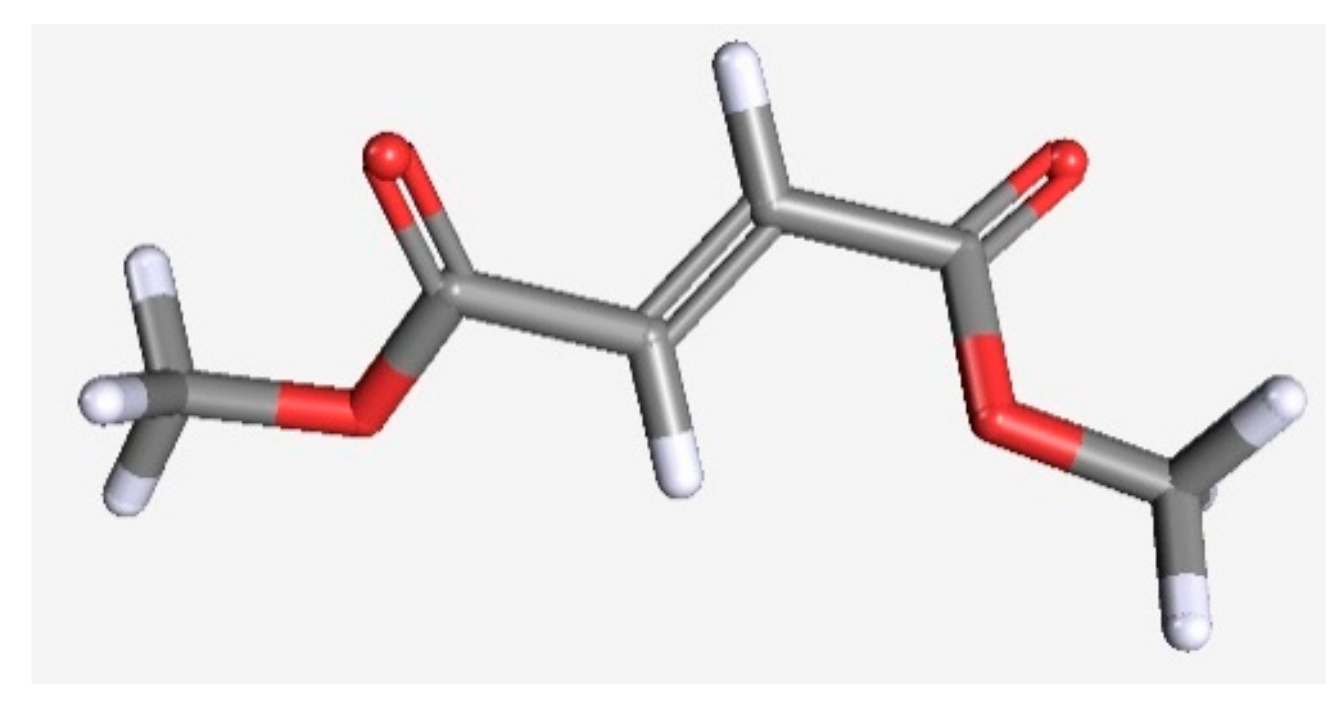
2.1. Reagents
2.2. Cell Culture
2.3. Cell Proliferation and Cytotoxicity Assay
2.4. Apoptosis Assays
2.5. Fluorescence-Activated Cell Sorting Analysis (FACS)
2.6. Detection of Autophagy
2.7. Combination of Oxaliplatin and Radiation
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
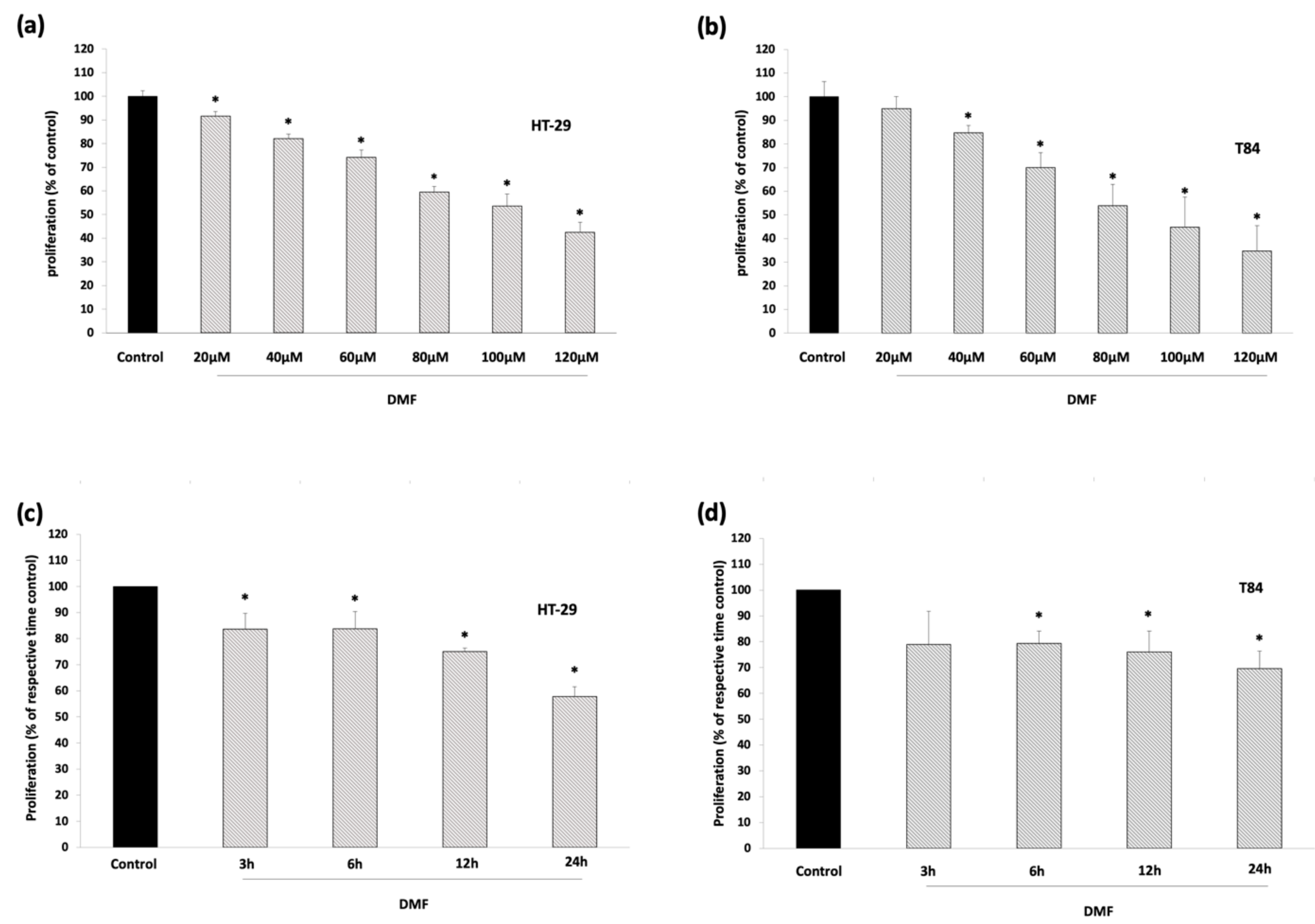
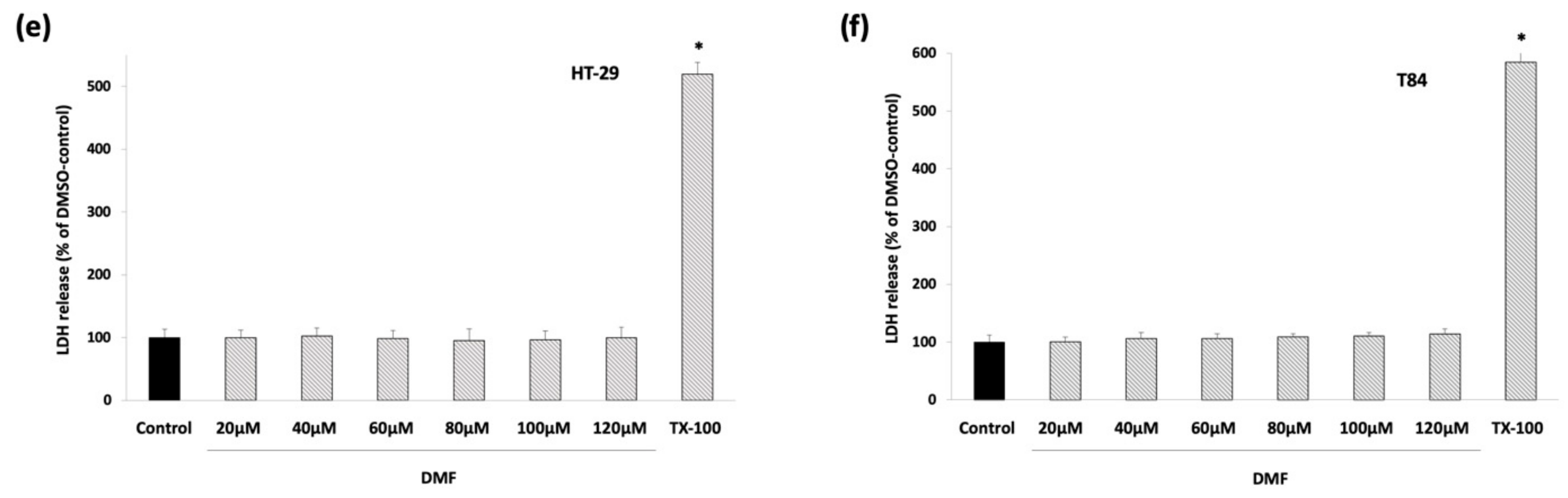
3.1. DMF Has No Cytotoxic Effects and Inhibits Colon Carcinoma Cell Proliferation
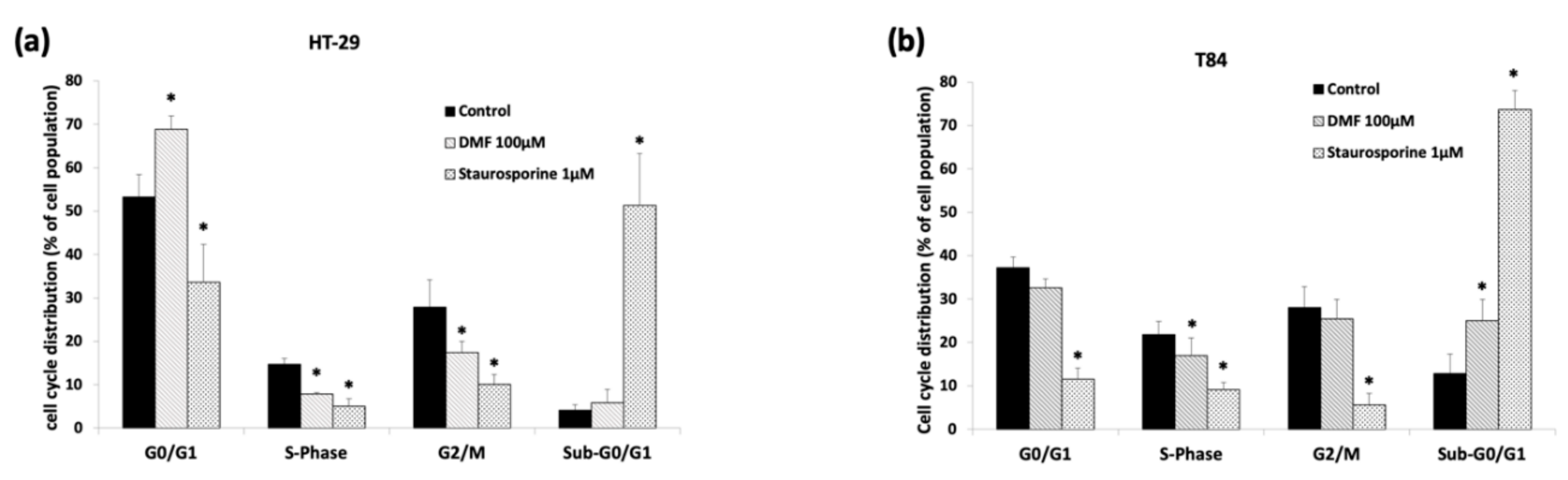
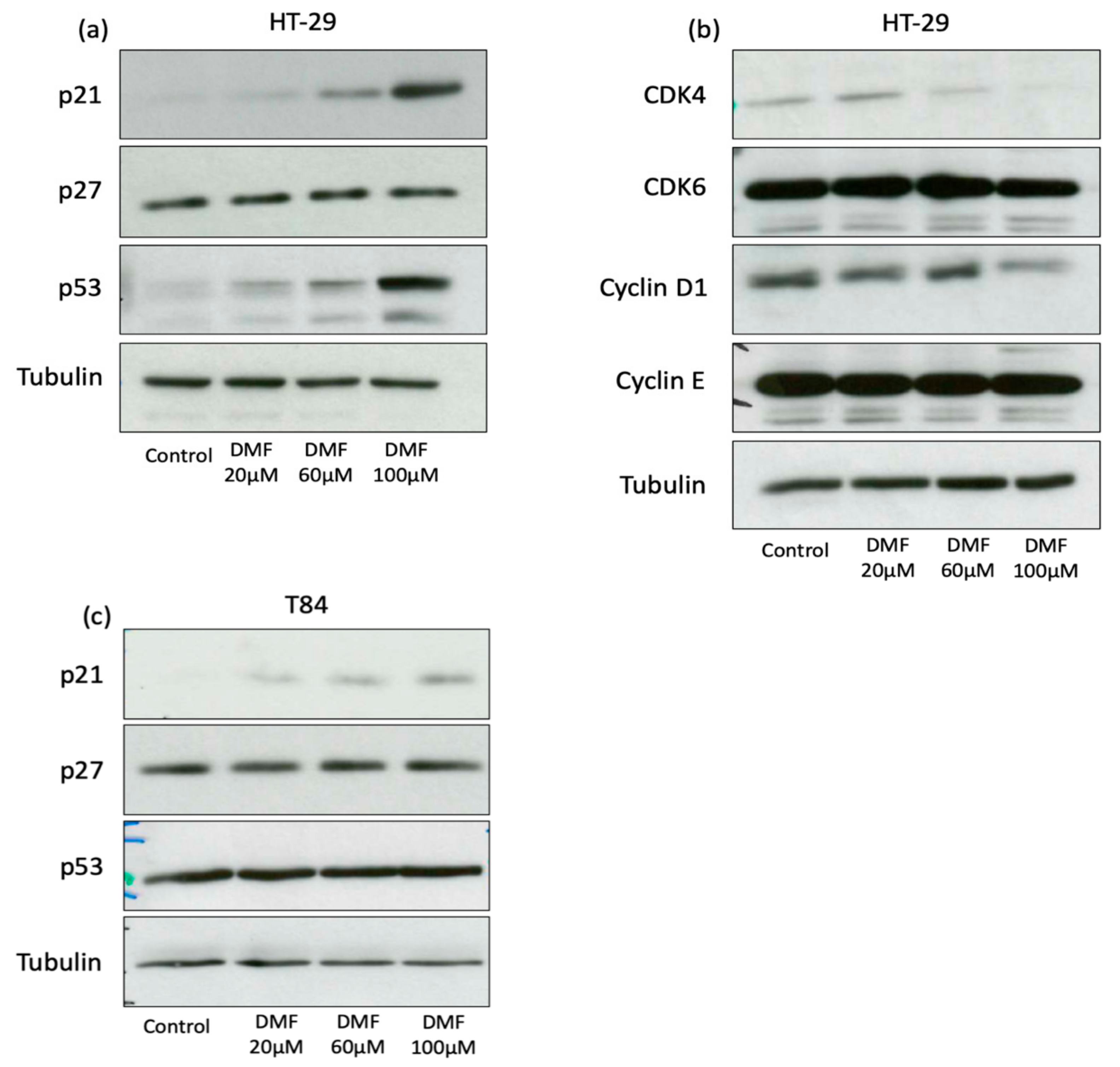
3.2. DMF Induces G0/G1 Cell Cycle Arrest in HT-29 and Augments Sub-G0/G1 Phase in T84
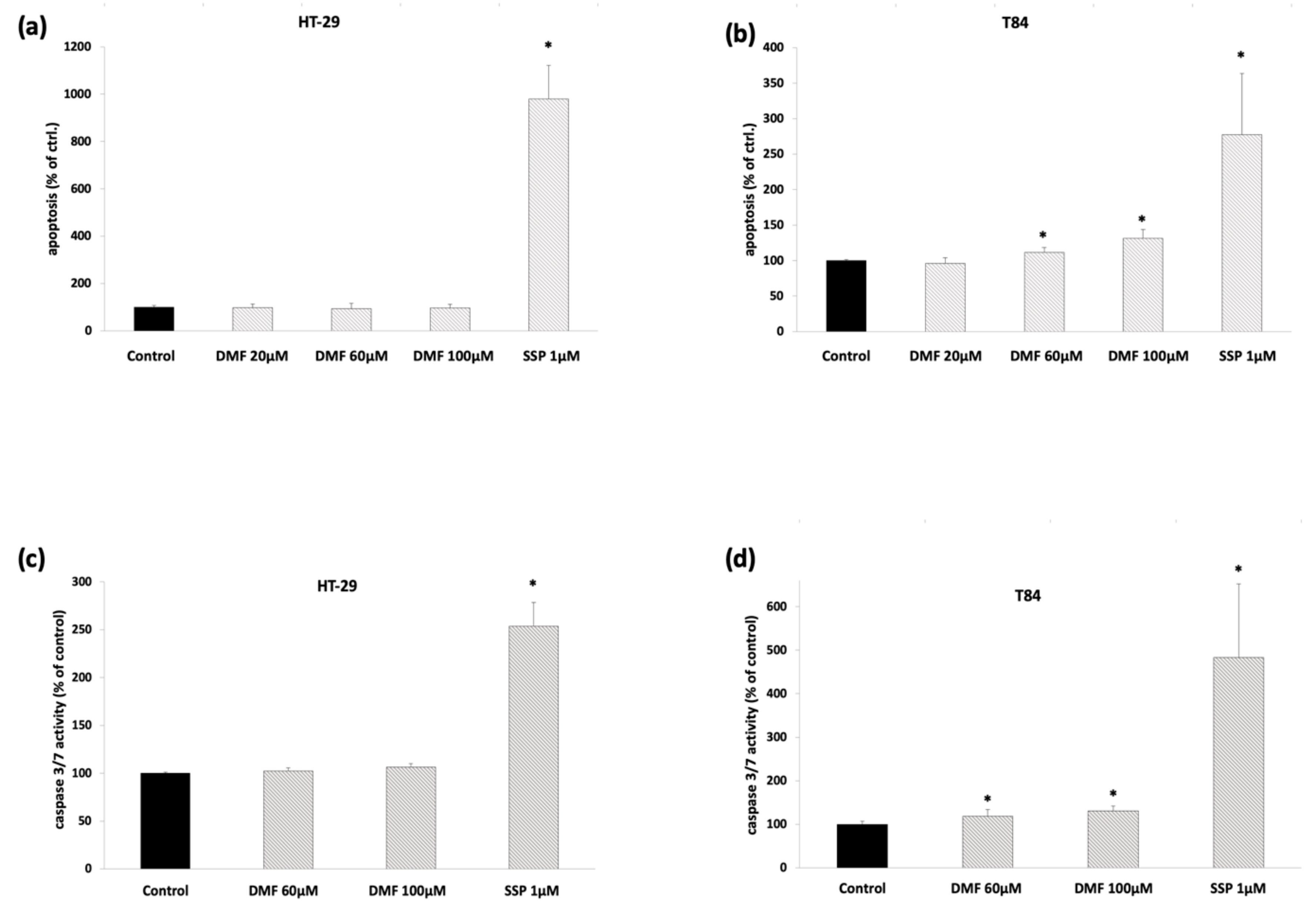
3.3. DMF Induces Cell Death-Related Mechanisms in T84
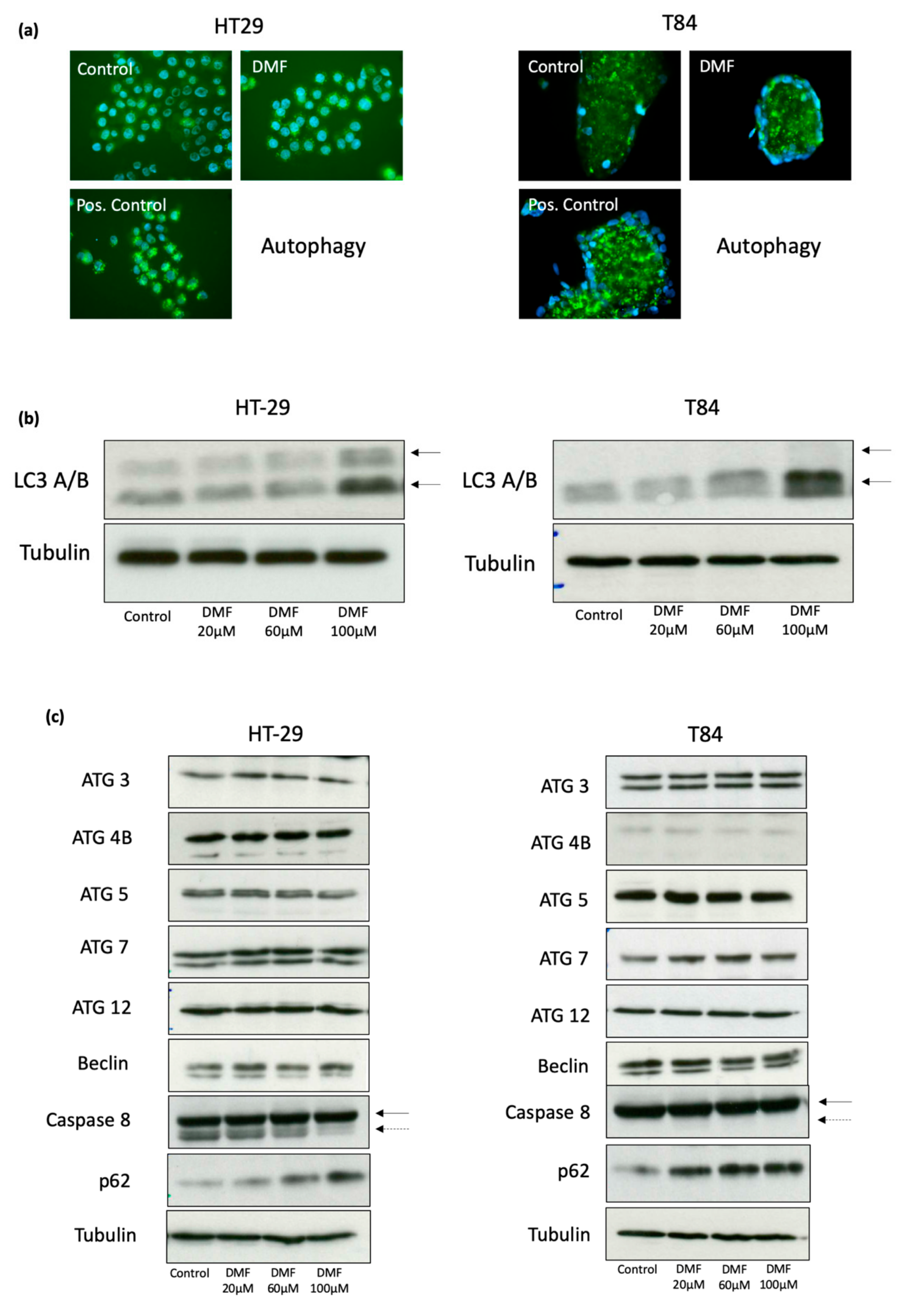
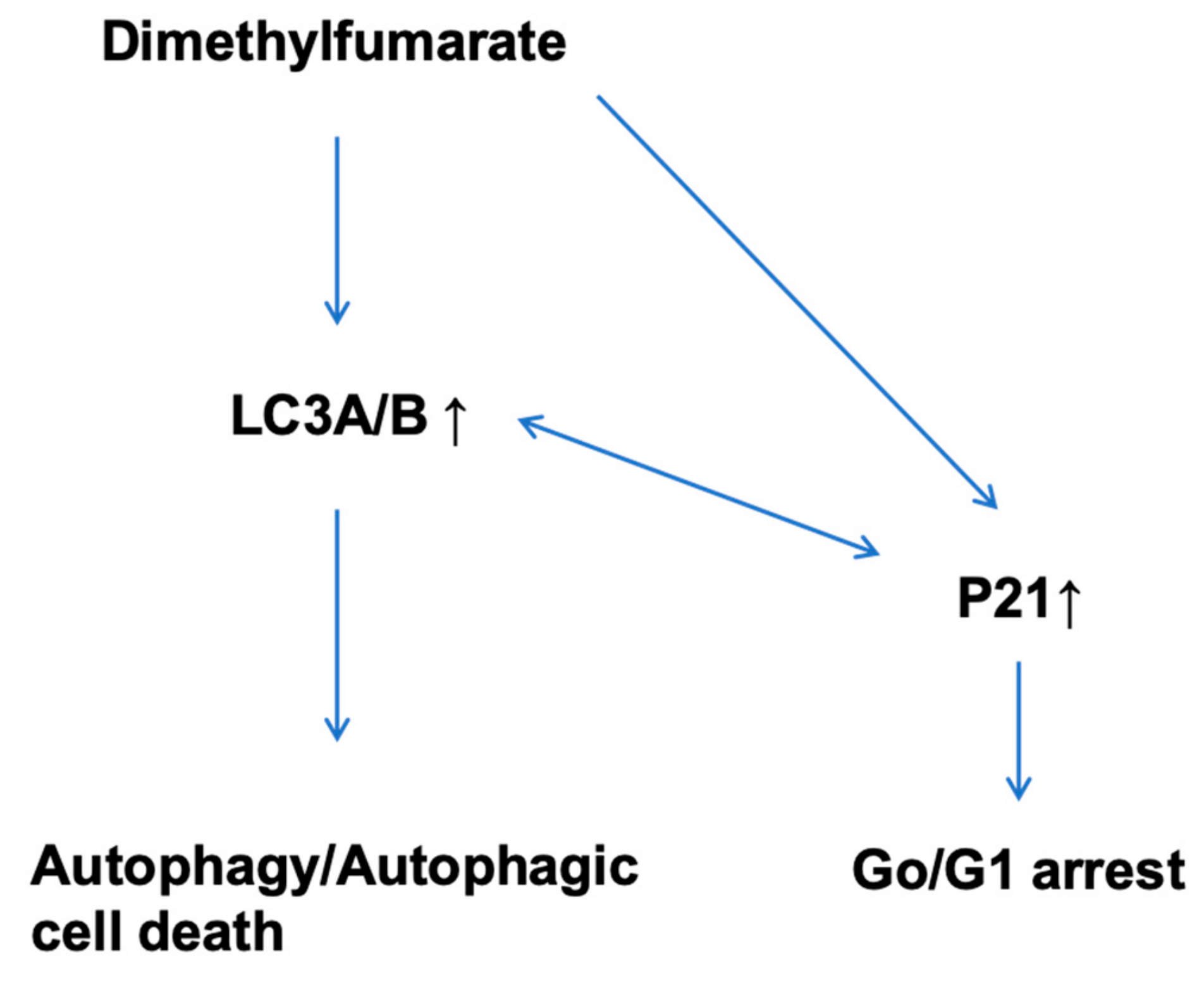
3.4. DMF Induces Autophagy and Decreases Activity of Cellular Senescence
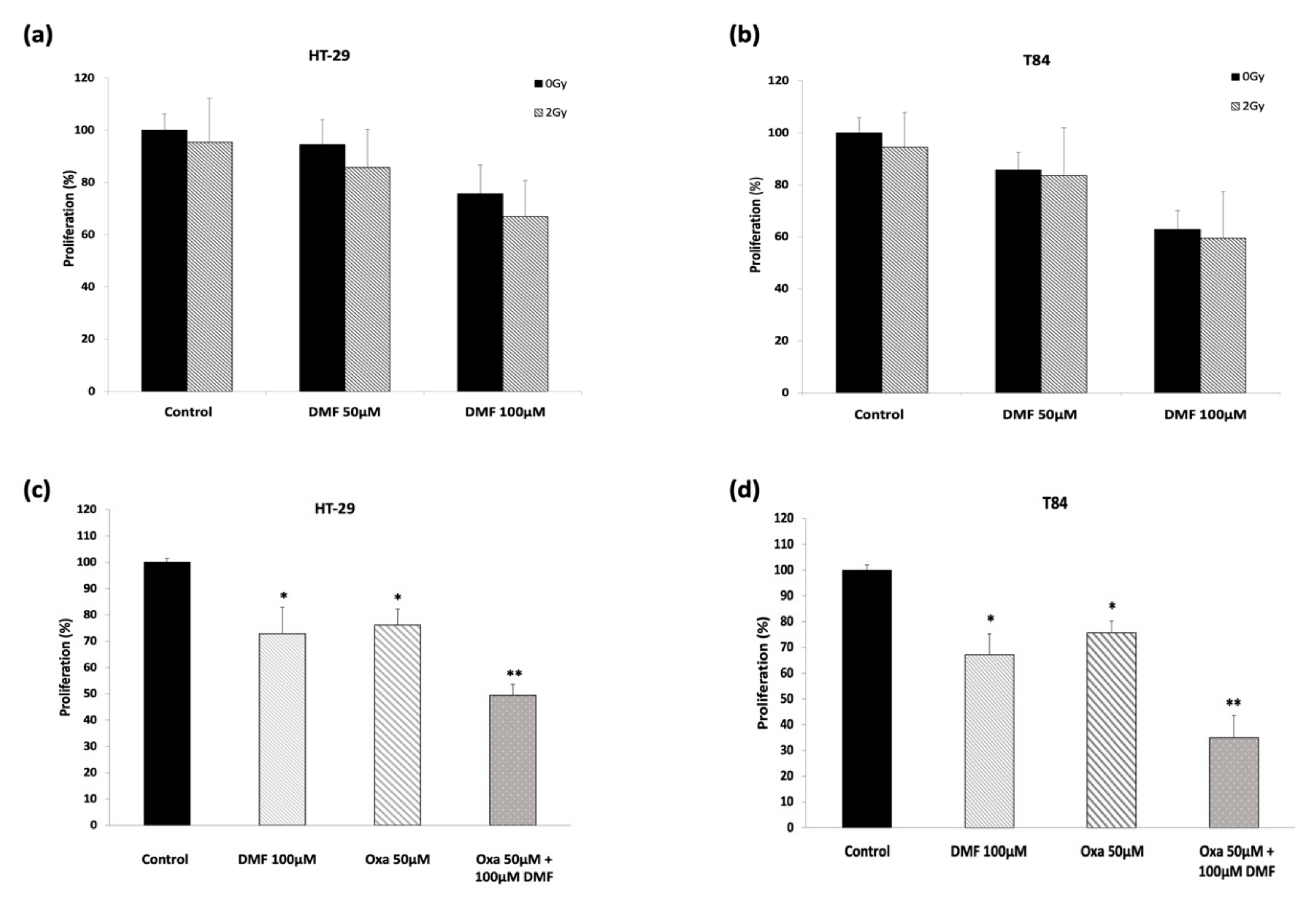
3.5. DMF Shows Synergetic Effect in Combination with Oxaliplatin but Does Not Potentiate Radiation Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMF | Dimethylfumarate |
| BrdU | Bromodeoxyuridine |
| LDH | lactatedehydrogenase |
| DMSO | Dimethylsulfoxide |
| FCS | fetal calf serum |
| NAC | N-acetylcystein |
| CRC | colorectal carcinoma |
| ROS | reactive oxygen species |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Werdenberg, D.; Joshi, R.; Wolffram, S.; Merkle, H.P.; Langguth, P. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm. Drug Dispos. 2003, 24, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Kaluzki, I.; Hrgovic, I.; Hailemariam-Jahn, T.; Doll, M.; Kleemann, J.; Valesky, E.M.; Kippenberger, S.; Kaufmann, R.; Zoeller, N.; Meissner, M. Dimethylfumarate inhibits melanoma cell proliferation via p21 and p53 induction and bcl-2 and cyclin B1 downregulation. Tumor Boil. 2016, 37, 13627–13635. [Google Scholar] [CrossRef]
- Kirlin, W.G.; Cai, J.; Delong, M.J.; Patten, E.J.; Jones, D.P. Dietary Compounds That Induce Cancer Preventive Phase 2 Enzymes Activate Apoptosis at Comparable Doses in HT29 Colon Carcinoma Cells. J. Nutr. 1999, 129, 1827–1835. [Google Scholar] [CrossRef][Green Version]
- Xie, X.; Zhao, Y.; Ma, C.-Y.; Xu, X.-M.; Zhang, Y.-Q.; Wang, C.-G.; Jin, J.; Shen, X.; Gao, J.-L.; Li, N.; et al. Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway. Br. J. Pharmacol. 2015, 172, 3929–3943. [Google Scholar] [CrossRef]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nature 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Begleiter, A.; Sivananthan, K.; Curphey, T.J.; Bird, R.P. Induction of NAD(P)H quinone: oxidoreductase1 inhibits carcinogen-induced aberrant crypt foci in colons of Sprague-Dawley rats. Cancer Epidemiol. Biomark. Prev. 2003, 12, 566–572. [Google Scholar]
- Vego, H.; Sand, K.L.; Høglund, R.A.; Fallang, L.E.; Gundersen, G.; Holmøy, T.; Maghazachi, A.A. Monomethyl fumarate augments NK cell lysis of tumor cells through degranulation and the upregulation of NKp46 and CD107a. Cell Mol. Immunol. 2016, 13, 57–64. [Google Scholar] [CrossRef]
- Valesky, E.M.; Hrgovic, I.; Doll, M.; Wang, X.; Pintér, A.; Kleemann, J.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Dimethylfumarate effectively inhibits lymphangiogenesis via p21 induction and G1 cell cycle arrest. Exp. Dermatol. 2016, 25, 200–205. [Google Scholar] [CrossRef]
- Meissner, M.; Doll, M.; Hrgovic, I.; Reichenbach, G.; König, V.; Hailemariam-Jahn, T.; Gille, J.; Kaufmann, R. Suppression of VEGFR2 expression in human endothelial cells by dimethylfumarate treatment: Evidence for anti-angiogenic action. J. Invest. Dermatol. 2011, 131, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Loewe, R.; Holnthoner, W.; Gröger, M.; Pillinger, M.; Gruber, F.; Mechtcheriakova, D.; Hofer, E.; Wolff, K.; Petzelbauer, P. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J. Immunol. 2002, 168, 4781–4787. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, S.; König, V.; Doll, M.; Hailemariam-Jahn, T.; Hrgovic, I.; Zöller, N.; Kaufmann, R.; Kippenberger, S.; Meissner, M. Dimethylfumarate protects against TNF-α-induced secretion of inflammatory cytokines in human endothelial cells. J. Inflamm. (Lond.) 2015, 6, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, Y.; Tsubaki, M.; Matsuoka, H.; Satou, T.; Itoh, T.; Kusunoki, T.; Kidera, Y.; Tanimori, Y.; Shoji, K.; Nakamura, H.; et al. Dimethylfumarate inhibits tumor cell invasion and metastasis by suppressing the expression and activities of matrix metalloproteinases in melanoma cells. Cell Biol. Int. 2009, 33, 1087–1094. [Google Scholar] [CrossRef]
- Oh, C.J.; Park, S.; Kim, J.-Y.; Kim, H.-J.; Jeoung, N.H.; Choi, Y.-K.; Go, Y.; Park, K.-G.; Lee, I.-K. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Boil. 2014, 2, 855–864. [Google Scholar] [CrossRef]
- Odom, R.Y.; Dansby, M.Y.; Rollins-Hairston, A.M.; Jackson, K.M.; Kirlin, W.G. Phytochemical induction of cell cycle arrest by glutathione oxidation and reversal by N-acetylcysteine in human colon carcinomacarcinoma cells. Nutr. Cancer 2009, 61, 332–339. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Ma, R.; Xiao, X.-L.; Zhang, Y.-H.; Zhang, X.-Z.; Hu, N.; Gao, J.-L.; Zheng, Y.-F.; Dong, D.-L.; Sun, Z.-J. Azo polymeric micelles designed for colon-targeted dimethyl fumarate delivery for colon cancer therapy. Acta Biomater. 2016, 44, 323–331. [Google Scholar] [CrossRef]
- Kang, H.-J.; Seo, H.-A.; Go, Y.; Oh, C.J.; Jeoung, N.H.; Park, K.-G.; Lee, I.-K. Dimethylfumarate Suppresses Adipogenic Differentiation in 3T3-L1 Preadipocytes through Inhibition of STAT3 Activity. PLoS ONE 2013, 8, e61411. [Google Scholar] [CrossRef]
- Singh, P.; Blatt, A.; Feld, S.; Zohar, Y.; Saadi, E.; Barki-Harrington, L.; Hammond, E.; Ilan, N.; Vlodavsky, I.; Chowers, Y.; et al. The Heparanase Inhibitor PG545 Attenuates Colon Cancer Initiation and Growth, Associating with Increased p21 Expression. Neoplasia 2017, 19, 175–184. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Rodríguez, P.R.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: the suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, P.F.; Fouladdel, S.; Ghaffari, S.M.; Amin, G.; Azizi, E. Induction of G1 cell cycle arrest and cyclin D1 downregulation in response to pericarp extract of Baneh in human breast cancer T47D cells. DARU J. Pharm. Sci. 2012, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Loewe, R.; Valero, T.; Kremling, S.; Pratscher, B.; Kunstfeld, R.; Pehamberger, H.; Petzelbauer, P. Dimethylfumarate Impairs Melanoma Growth and Metastasis. Cancer Res. 2006, 66, 11888–11896. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Ogawa, N.; Takeda, T.; Sakamoto, K.; Shimaoka, H.; Fujita, A.; Itoh, T.; Imano, M.; Satou, T.; Nishida, S. Dimethyl fumarate induces apoptosis of hematopoietic tumor cells via inhibition of NF-κB nuclear translocation and downregulation of Bcl-xL and XIAP. Biomed. Pharmacother. 2014, 68, 999–1005. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, C.; Huang, M.-Y.; Guo, Y.-X.; Hu, G.-Q. Crosstalk between Beclin-1-dependent autophagy and caspase-dependent apoptosis induced by tanshinone IIA in human osteosarcoma MG-63 cells. Oncol. Rep. 2016, 36, 1807–1818. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BUON 2019, 24, 488–493. [Google Scholar]
- Son, Y.; An, Y.; Jung, J.; Shin, S.; Park, I.; Gwak, J.; Ju, B.G.; Chung, Y.; Na, M.; Oh, S. Protopine isolated from Nandina domestica induces apoptosis and autophagy in colon cancer cells by stabilizing p53. Phytother. Res. 2019, 33, 1689–1696. [Google Scholar] [CrossRef]
- Gao, G.-Y.; Ma, J.; Lu, P.; Jiang, X.; Chang, C. Ophiopogonin B induces the autophagy and apoptosis of colon cancer cells by activating JNK/c-Jun signaling pathway. Biomed. Pharmacother. 2018, 108, 1208–1215. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wang, J.; Ye, Z.; Li, J.C. Oridonin upregulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int. J. Biol Sci. 2012, 8, 901–912. [Google Scholar] [CrossRef]
- Wang, Y.; Kuramitsu, Y.; Baron, B.; Kitagawa, T.; Tokuda, K.; Akada, J.; Nakamura, K. CGK733-induced LC3 II formation is positively associated with the expression of cyclin-dependent kinase inhibitor p21Waf1/Cip1 through modulation of the AMPK and PERK/CHOP signaling pathways. Oncotarget 2015, 6, 39692–39701. [Google Scholar]
- Fan, J.D.; Lei, P.J.; Zheng, J.Y.; Wang, X.; Li, S.; Liu, H.; He, Y.L.; Wang, Z.N.; Wei, G.; Zhang, X.; et al. The selective activation of p53 target genes regulated by SMYD2 in BIX-01294 induced autophagy-related cell death. PLoS ONE 2015, 10, e0116782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Giacomo, V.; Di Valerio, V.; Rapino, M.; Bosco, D.; Cacciatore, I.; Ciulla, M.; Marrazzo, A.; Fiorito, J.; Di Stefano, A.; Cataldi, A. MRJF4, a novel histone deacetylase inhibitor, induces p21 mediated autophagy in PC3 prostate cancer cells. Cell Mol. Biol. 2015, 61, 17–23. [Google Scholar] [PubMed]
- Hanot, M.; Boivin, A.; Malésys, C.; Beuve, M.; Colliaux, A.; Foray, N.; Douki, T.; Ardail, D.; Rodriguez-Lafrasse, C. Glutathione Depletion and Carbon Ion Radiation Potentiate Clustered DNA Lesions, Cell Death and Prevent Chromosomal Changes in Cancer Cells Progeny. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Boivin, A.; Hanot, M.; Malésys, C.; Maalouf, M.; Rousson, R.; Rodriguez-Lafrasse, C.; Ardail, D. Transient Alteration of Cellular Redox Buffering before Irradiation Triggers Apoptosis in Head and Neck Carcinoma Stem and Non-Stem Cells. PLoS ONE 2011, 6, 6. [Google Scholar] [CrossRef][Green Version]
- Booth, L.; Cruickshanks, N.; Tavallai, S.; Roberts, J.L.; Peery, M.; Poklepovic, A.; Dent, P. Regulation of dimethyl-fumarate toxicity by proteasome inhibitors. Cancer Boil. Ther. 2014, 15, 1646–1657. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluzki, I.; Hailemariam-Jahn, T.; Doll, M.; Kaufmann, R.; Balermpas, P.; Zöller, N.; Kippenberger, S.; Meissner, M. Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy. Cells 2019, 8, 1329. https://doi.org/10.3390/cells8111329
Kaluzki I, Hailemariam-Jahn T, Doll M, Kaufmann R, Balermpas P, Zöller N, Kippenberger S, Meissner M. Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy. Cells. 2019; 8(11):1329. https://doi.org/10.3390/cells8111329
Chicago/Turabian StyleKaluzki, Irina, Tsige Hailemariam-Jahn, Monika Doll, Roland Kaufmann, Panagiotis Balermpas, Nadja Zöller, Stefan Kippenberger, and Markus Meissner. 2019. "Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy" Cells 8, no. 11: 1329. https://doi.org/10.3390/cells8111329
APA StyleKaluzki, I., Hailemariam-Jahn, T., Doll, M., Kaufmann, R., Balermpas, P., Zöller, N., Kippenberger, S., & Meissner, M. (2019). Dimethylfumarate Inhibits Colorectal Carcinoma Cell Proliferation: Evidence for Cell Cycle Arrest, Apoptosis and Autophagy. Cells, 8(11), 1329. https://doi.org/10.3390/cells8111329




