Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Western Blot Analysis
2.4. Immunofluorescence and Light Microscopy
2.5. Antibodies
2.6. Electron Microscopy
2.7. RT-PCR
2.8. ERG Measurements
2.9. ERG Data Analysis
3. Results
3.1. Syne-2/Nesprin-2 KO Mice Show Normal Retinal Morphology
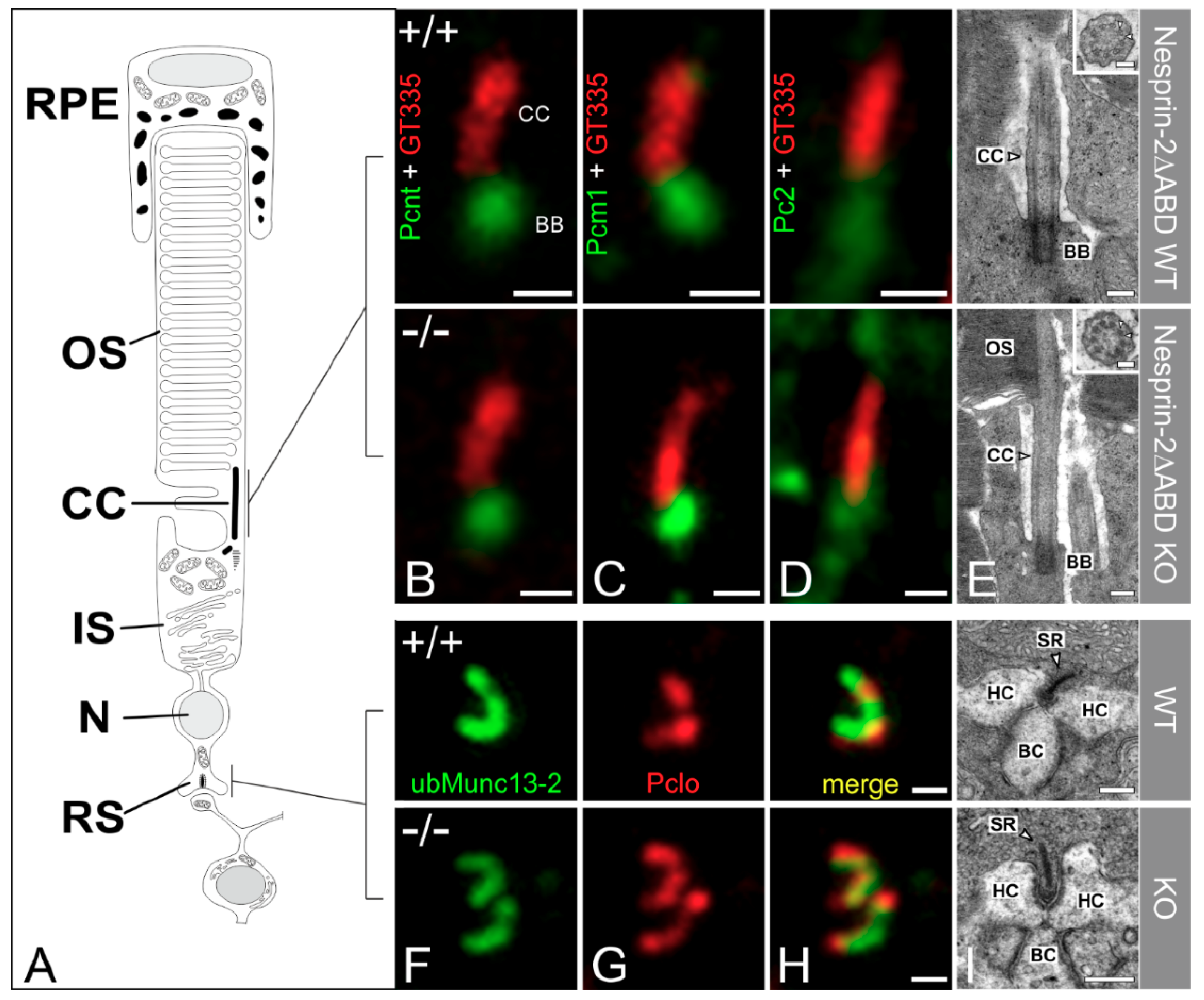
3.2. Syne-2/Nesprin-2 KO Mice Show Intact Photoreceptor Connecting Cilia
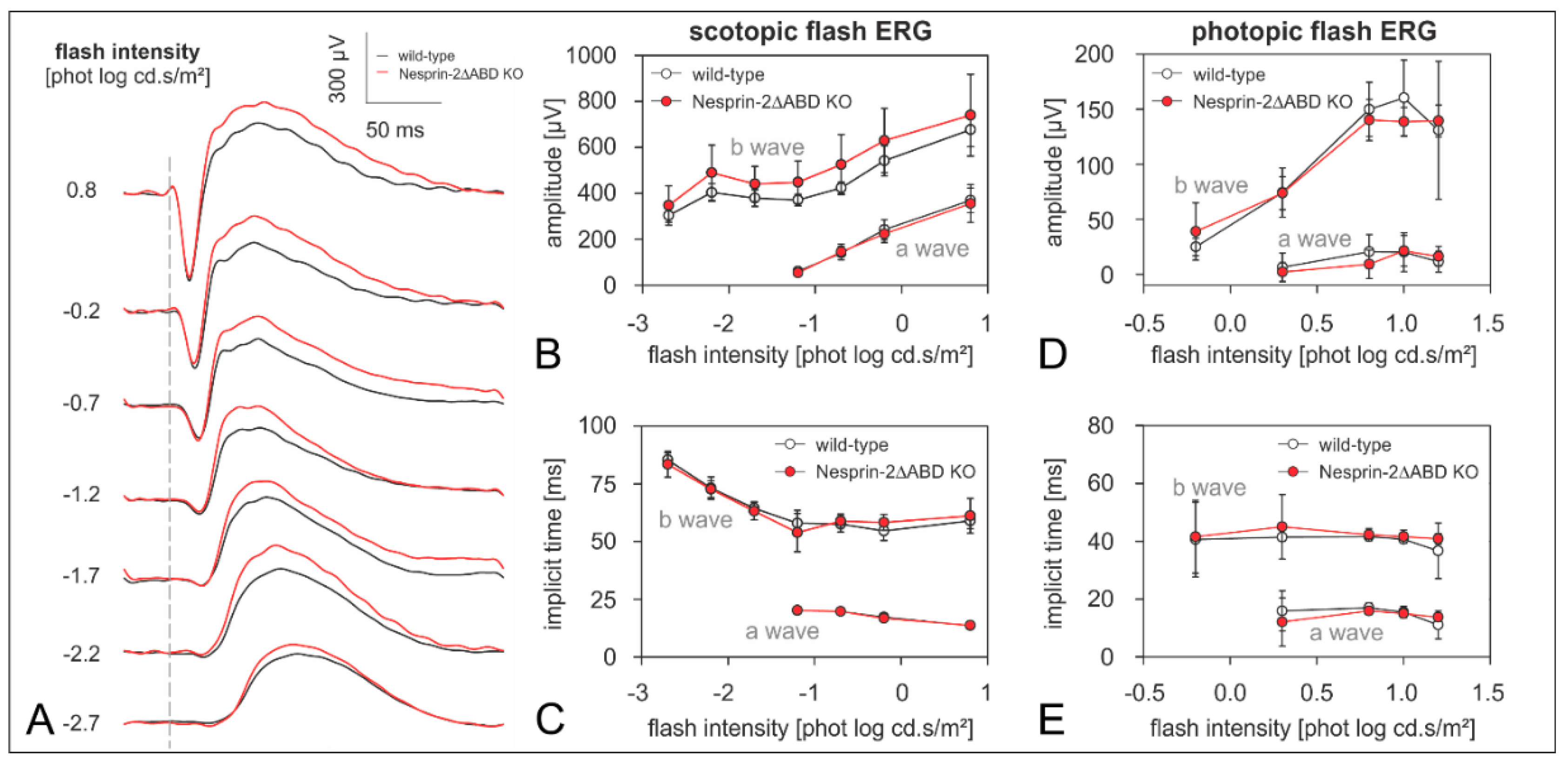
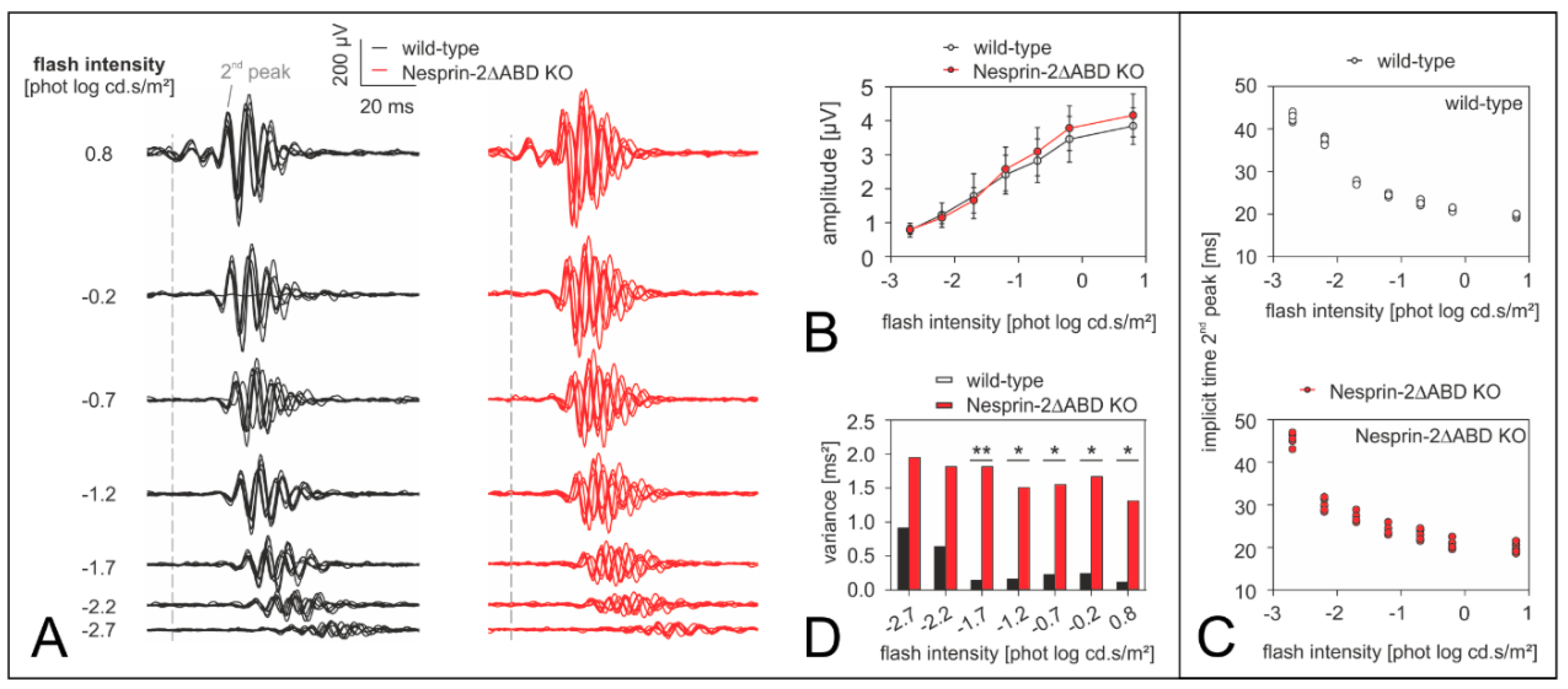
3.3. Syne-2/Nesprin-2 KO Mice Show an Intact ERG but Larger Variability in the Timing of the Oscillatory Potentials
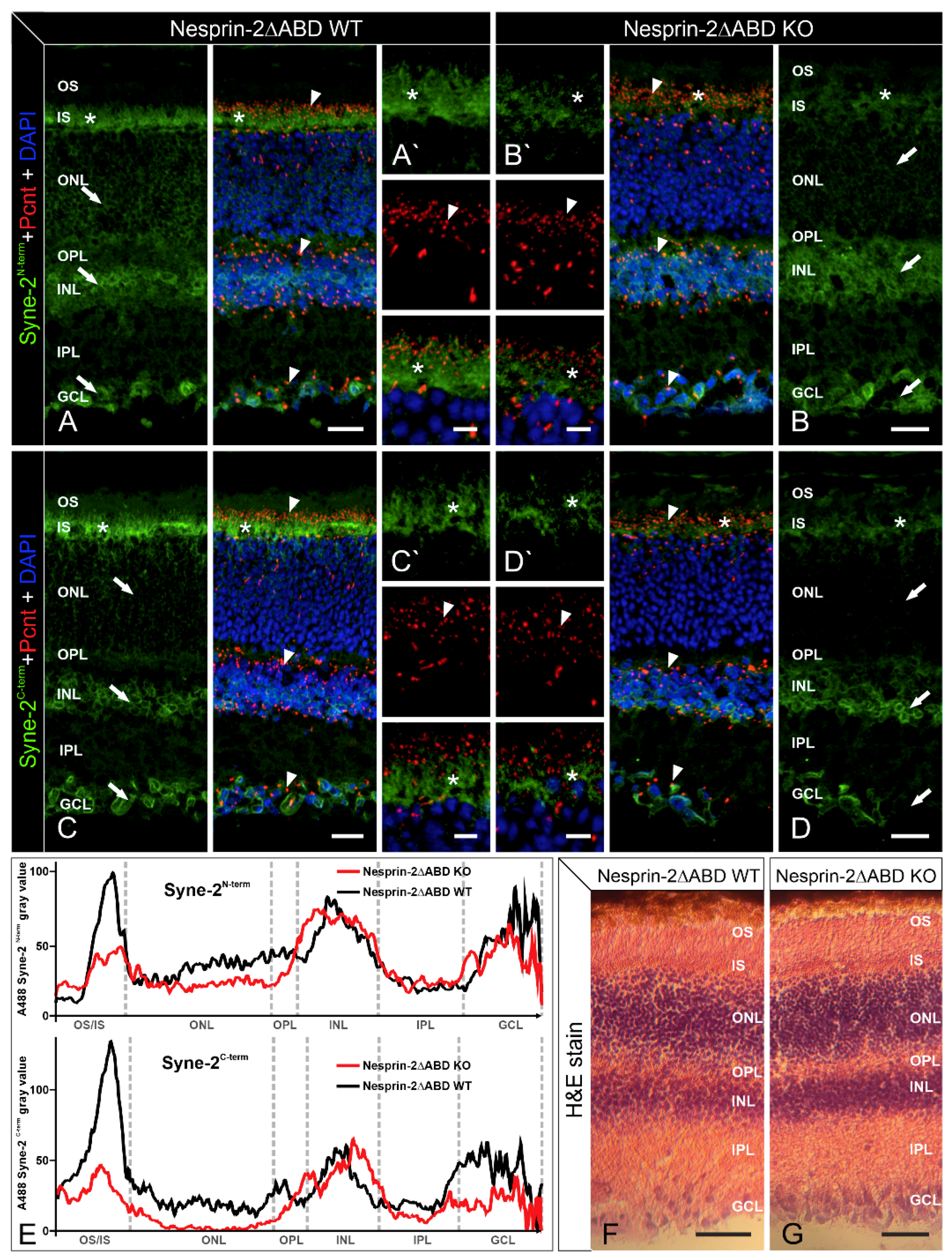
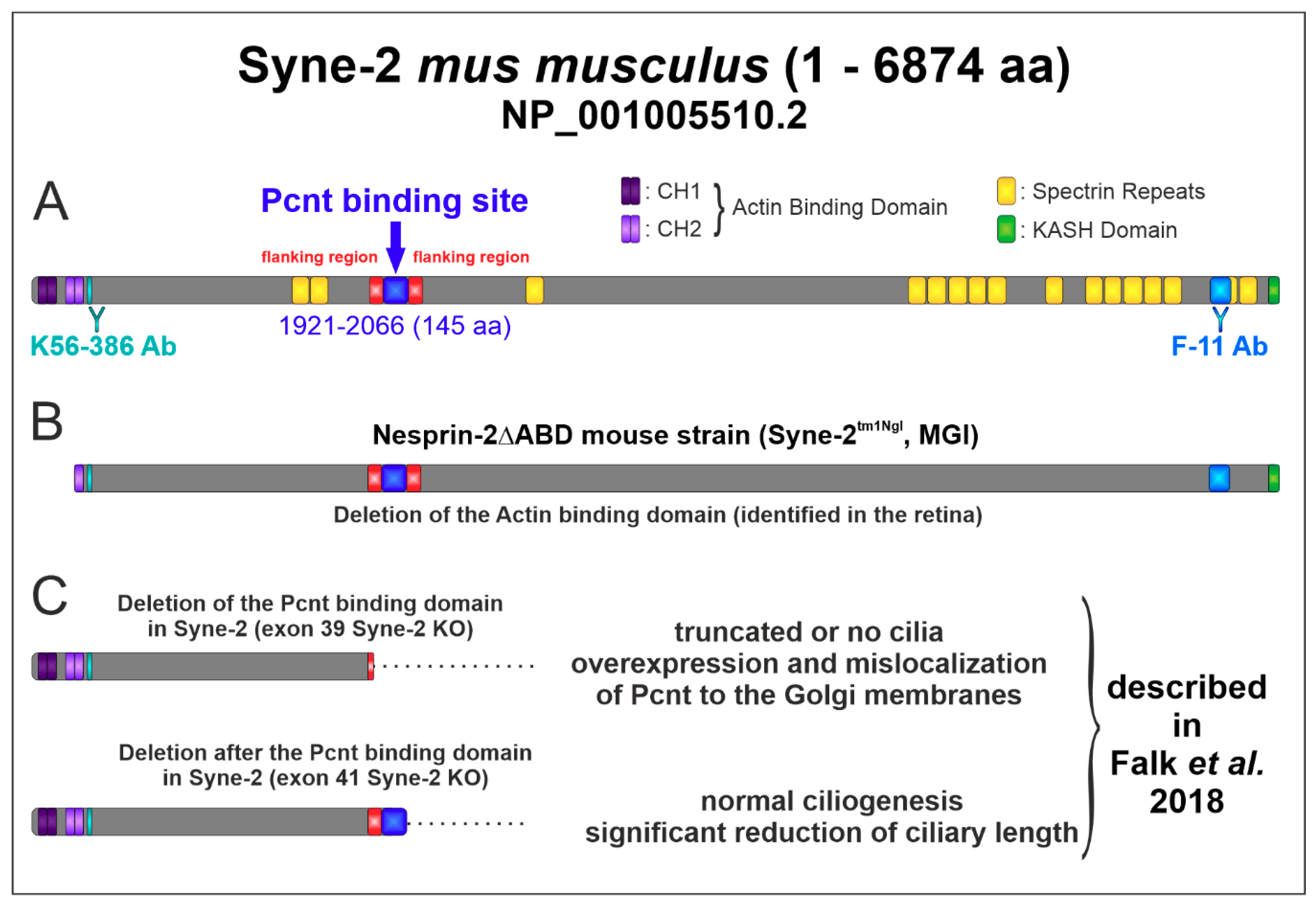
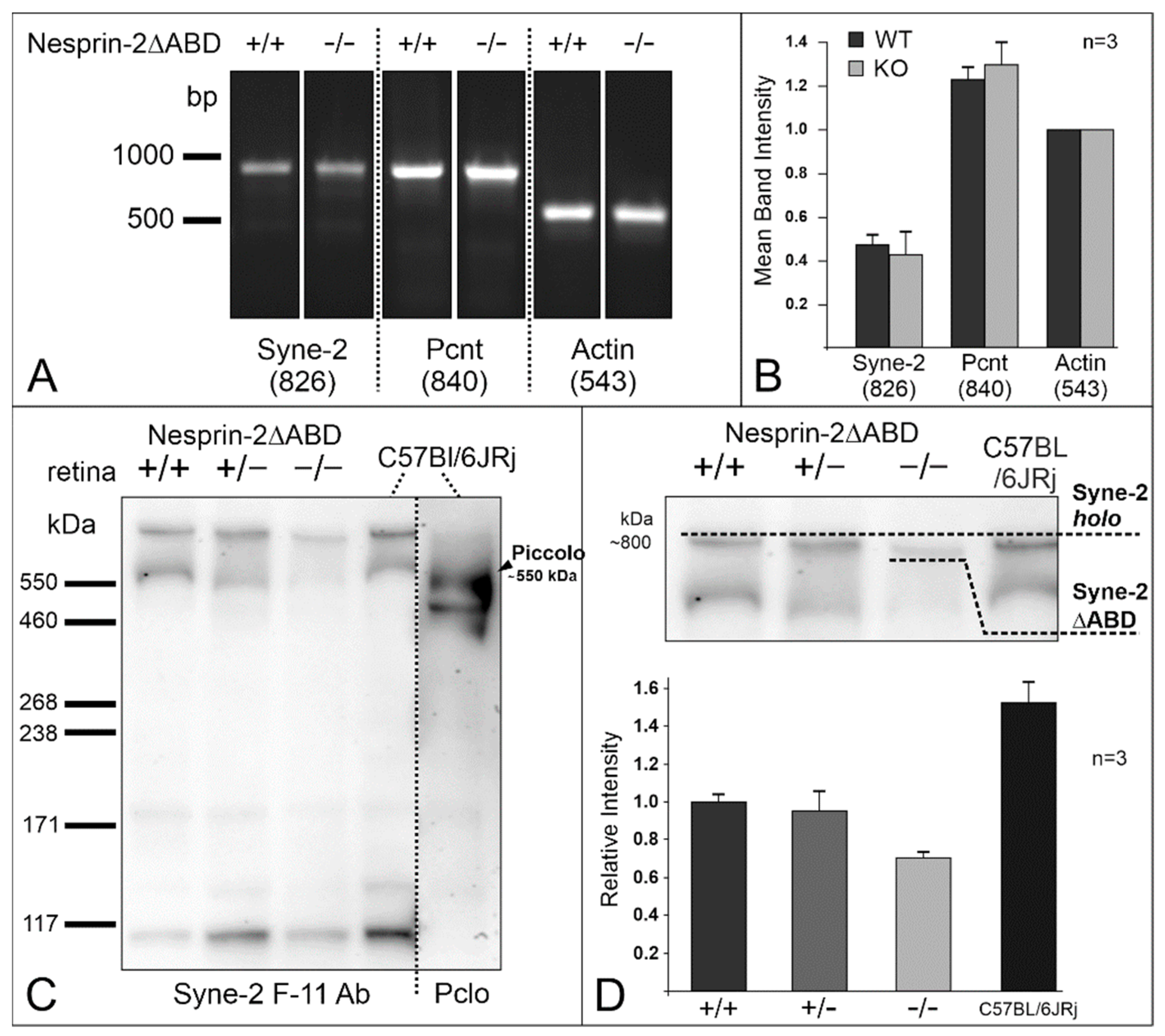
3.4. Syne-2/Nesprin-2 KO Mice Show Reduced Syne-2 Levels
4. Discussion
4.1. A Hypomorphic Mutation of Murine Syne-2 does not Affect Retina Structure
4.2. Nesprin-2△ABD KO Mice Show Altered Inner Retinal Signal Processing
4.3. Expression Analysis Revealed an Alternative Translational Start Site of Syne-2
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patterson, K.; Molofsky, A.B.; Robinson, C.; Acosta, S.; Cater, C.; Fischer, J.A. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol. Biol. Cell 2004, 15, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, M.; Omori, Y.; Biyanwila, J.; Malicki, J. Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proc. Natl. Acad. Sci. USA 2007, 104, 14819–14824. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lei, K.; Zhou, M.; Craft, C.M.; Xu, G.; Xu, T.; Zhuang, Y.; Xu, R.; Han, M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum. Mol. Genet. 2011, 20, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Dawe, H.R.; Adams, M.; Wheway, G.; Szymanska, K.; Logan, C.V.; Noegel, A.A.; Gull, K.; Johnson, C.A. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J. Cell Sci. 2009, 122, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Falk, N.; Kessler, K.; Schramm, S.F.; Boldt, K.; Becirovic, E.; Michalakis, S.; Regus-Leidig, H.; Noegel, A.A.; Ueffing, M.; Thiel, C.T.; et al. Functional analyses of Pericentrin and Syne-2 interaction in ciliogenesis. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Doxsey, S.J.; Stein, P.; Evans, L.; Calarco, P.D.; Kirschner, M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 1994, 76, 639–650. [Google Scholar] [CrossRef]
- Jurczyk, A.; Gromley, A.; Redick, S.; Agustin, J.S.; Witman, G.; Pazour, G.J.; Peters, D.J.M.; Doxsey, S. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J. Cell Biol. 2004, 166, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Onishi, K.; Asanuma, M.P.; Miyazaki, I.; Diaz-Corrales, F.J.; Ogawa, N. Embryonic expression of pericentrin suggests universal roles in ciliogenesis. Dev. Genes Evol. 2006, 216, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Delaval, B.; Doxsey, S.J. Pericentrin in cellular function and disease. J. Cell Biol. 2010, 188, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mühlhans, J.; Brandstätter, J.H.; Gießl, A. The Centrosomal Protein Pericentrin Identified at the Basal Body Complex of the Connecting Cilium in Mouse Photoreceptors. PLoS ONE 2011, 6, e26496. [Google Scholar] [CrossRef] [PubMed]
- Falk, N.; Lösl, M.; Schröder, N.; Gießl, A. Specialized Cilia in Mammalian Sensory Systems. Cells 2015, 4, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Lüke, Y.; Zaim, H.; Karakesisoglou, I.; Jaeger, V.M.; Sellin, L.; Lu, W.; Schneider, M.; Neumann, S.; Beijer, A.; Munck, M.; et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J. Cell Sci. 2008, 121, 1887–1898. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Hemmerlein, M.; Ammermuller, J.; Imig, C.; Reim, K.; Lipstein, N.; Kalla, S.; Kawabe, H.; Brose, N.; Brandstatter, J.H.; et al. Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J. Neurosci. 2012, 32, 8040–8052. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Atorf, J.; Feigenspan, A.; Kremers, J.; Maw, M.A.; Brandstätter, J.H. Photoreceptor degeneration in two mouse models for congenital stationary night blindness type 2. PLoS ONE 2014, 9, e86769. [Google Scholar] [CrossRef] [PubMed]
- Frishman, L.J. Origins of the electroretinogram. In Principles and Practice of Clinical Electrophysiology of Vision; Heckenlively, J.R., Arden, G.B., Eds.; The MIT Press: Cambridge, UK, 2006. [Google Scholar]
- Harazny, J.; Scholz, M.; Buder, T.; Lausen, B.; Kremers, J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc. Ophthalmol. 2009, 119, 181–197. [Google Scholar] [CrossRef] [PubMed]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstätter, J.H. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Regus-Leidig, H.; Ott, C.; Löhner, M.; Atorf, J.; Fuchs, M.; Sedmak, T.; Kremers, J.; Fejtova, A.; Gundelfinger, E.D.; Brandstätter, J.H. Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS ONE 2013, 8, e70373. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, F.; Wehman, A.M.; Link, B.A.; Baier, H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell 2008, 134, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Heynen, H.; Wachtmeister, L.; van Norren, D. Origin of the oscillatory potentials in the primate retina. Vision Res. 1985, 25, 1365–1373. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falk, N.; Joachimsthaler, A.; Kessler, K.; Lux, U.T.; Noegel, A.A.; Kremers, J.; Brandstätter, J.H.; Gießl, A. Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model. Cells 2019, 8, 1238. https://doi.org/10.3390/cells8101238
Falk N, Joachimsthaler A, Kessler K, Lux UT, Noegel AA, Kremers J, Brandstätter JH, Gießl A. Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model. Cells. 2019; 8(10):1238. https://doi.org/10.3390/cells8101238
Chicago/Turabian StyleFalk, Nathalie, Anneka Joachimsthaler, Kristin Kessler, Uwe Thorsten Lux, Angelika Anna Noegel, Jan Kremers, Johann Helmut Brandstätter, and Andreas Gießl. 2019. "Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model" Cells 8, no. 10: 1238. https://doi.org/10.3390/cells8101238
APA StyleFalk, N., Joachimsthaler, A., Kessler, K., Lux, U. T., Noegel, A. A., Kremers, J., Brandstätter, J. H., & Gießl, A. (2019). Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model. Cells, 8(10), 1238. https://doi.org/10.3390/cells8101238





