Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Animals
2.3. Precision-cut Lung Slices
2.4. mRNA Isolation and Real-Time PCR
2.5. Western Blot
2.6. Cell Culture
2.7. Organoid Assay
2.8. Immunofluorescence
2.9. Wnt/β-catenin Activity Assay
2.10. Statistical Analysis
3. Results
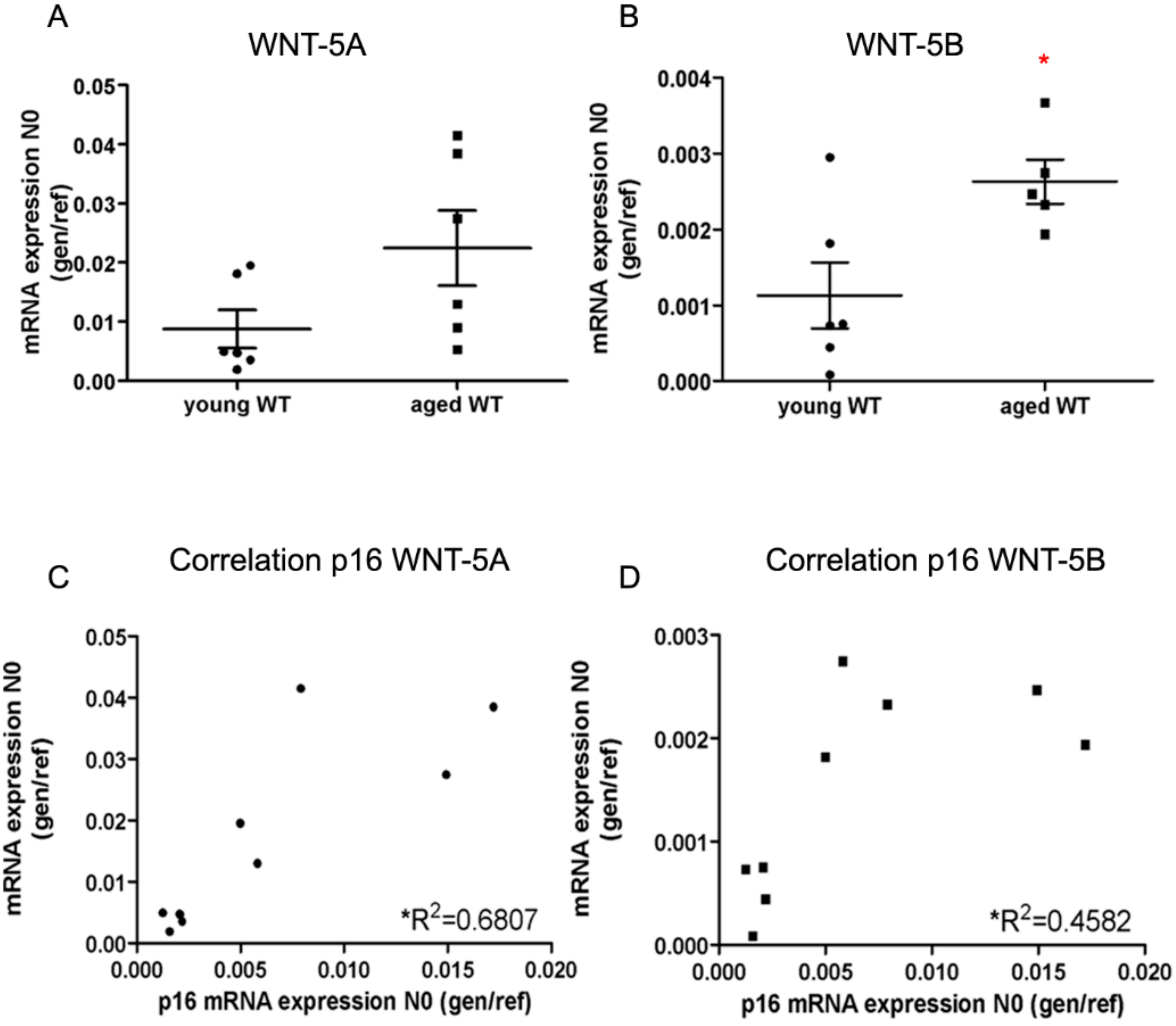
3.1. WNT-5A and WNT-5B Are Enhanced in Ageing Mice
3.2. WNT-5 Impedes the Expression of Alveolar Epithelial Cell Markers in Whole Lung Tissue
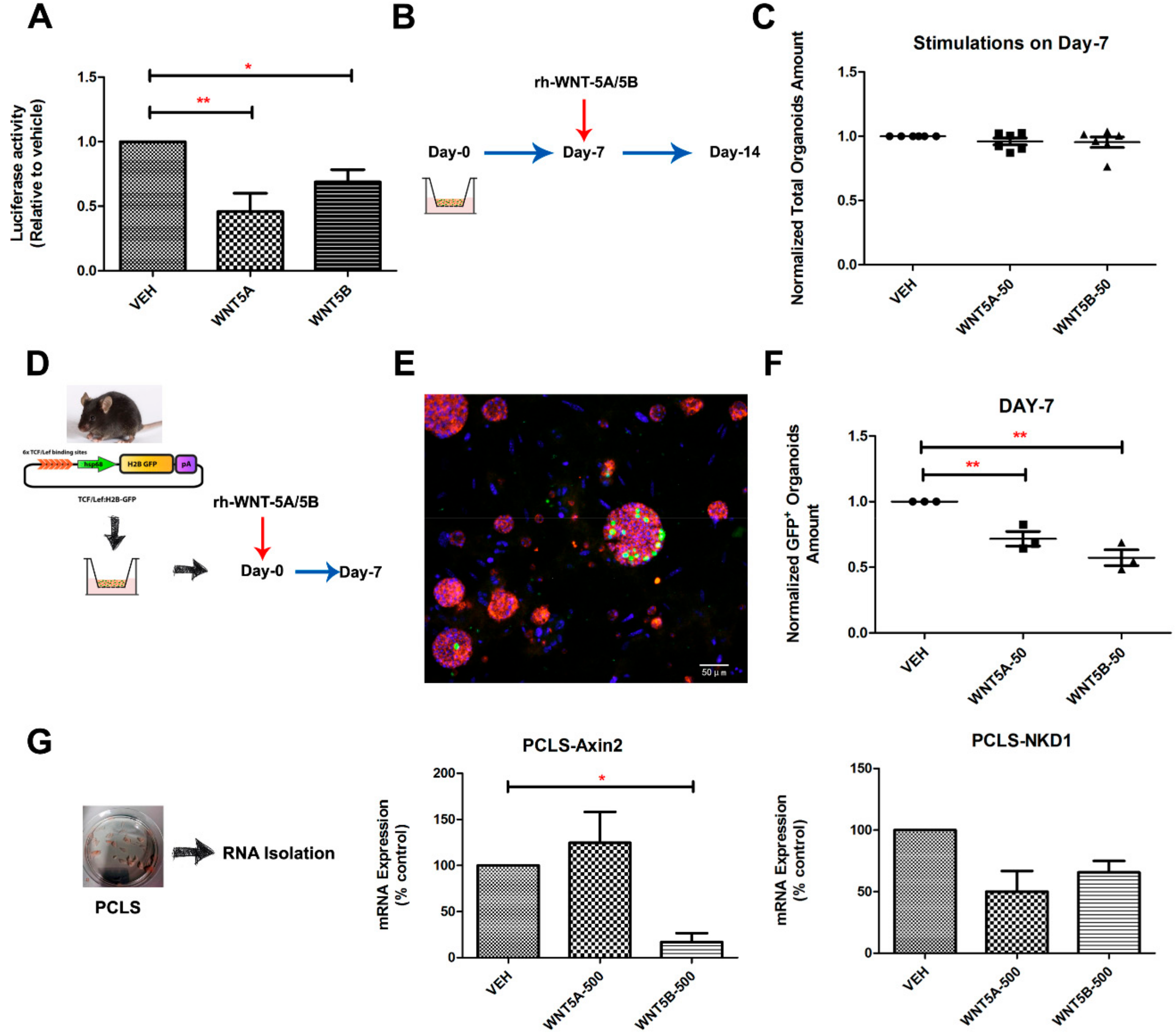
3.3. WNT-5A and WNT-5B Repress Lung Organoid Formation
3.4. WNT-5A/-5B Signaling Repress Canonical WNT/β-Catenin Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO Global Initiative for Chronic Obstructive. Available online: http://www.goldcopd.org. (accessed on 25 September 2019).
- Weissmann, N. Chronic obstructive pulmonary disease and pulmonary vascular disease a comorbidity? Ann. Am. Thorac. Soc. 2018, 15, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.C.; Makena, P.S.; Kennedy, J.; Teng, B.; Luellen, C.; Sinclair, S.E.; Waters, C.M. A heteromeric molecular complex regulates the migration of lung alveolar epithelial cells during wound healing. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Zheng, D.; Soh, B.S.; Yin, L.; Hu, G.; Chen, Q.; Choi, H.; Han, J.; Chow, V.T.K.; Chen, J. Differentiation of Club Cells to Alveolar Epithelial Cells in Vitro. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Gotoh, S.; Tateishi, K.; Yamamoto, Y.; Korogi, Y.; Nagasaki, T.; Matsumoto, H.; Muro, S.; Hirai, T.; Ito, I.; et al. Directed Induction of Functional Multi-ciliated Cells in Proximal Airway Epithelial Spheroids from Human Pluripotent Stem Cells. Stem. Cell Rep. 2016, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Tamò, L.; Hibaoui, Y.; Kallol, S.; Alves, M.P.; Albrecht, C.; Hostettler, K.E.; Feki, A.; Rougier, J.-S.; Abriel, H.; Knudsen, L.; et al. Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L921–L932. [Google Scholar] [CrossRef]
- Jain, R.; Barkauskas, C.E.; Takeda, N.; Bowie, E.J.; Aghajanian, H.; Wang, Q.; Padmanabhan, A.; Manderfield, L.J.; Gupta, M.; Li, D.; et al. Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015, 6, 6727. [Google Scholar] [CrossRef]
- Yuan, T.; Volckaert, T.; Redente, E.F.; Hopkins, S.; Klinkhammer, K.; Wasnick, R.; Chao, C.M.; Yuan, J.; Zhang, J.S.; Yao, C.; et al. FGF10-FGFR2B Signaling Generates Basal Cells and Drives Alveolar Epithelial Regeneration by Bronchial Epithelial Stem Cells after Lung Injury. Stem. Cell Rep. 2019, 12, 1041–1055. [Google Scholar] [CrossRef]
- Salwig, I.; Spitznagel, B.; Vazquez-Armendariz, A.I.; Khalooghi, K.; Guenther, S.; Herold, S.; Szibor, M.; Braun, T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019, 12, e102099. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immun. 2016, 38, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Ng-Blichfeldt, J.-P.; Gosens, R.; Dean, C.; Griffiths, M.; Hind, M. Regenerative pharmacology for COPD: Breathing new life into old lungs. Thorax 2019, 74, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The Pathology of Chronic Obstructive Pulmonary Disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem. Cell 2017, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Shichino, S.; Ueha, S.; Nakajima, T.; Hashimoto, S.; Yamazaki, S.; Matsushima, K. Mesenchymal-Epithelial Interactome Analysis Reveals Essential Factors Required for Fibroblast-Free Alveolosphere Formation. Science 2018, 11, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chu, X.; Chen, C.; Bellusci, S. Role of Fibroblast Growth Factor 10 in Mesenchymal Cell Differentiation During Lung Development and Disease. Front. Genet. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- McQualter, J.L.; McCarty, R.C.; Van der Velden, J.; O’Donoghue, R.J.J.; Asselin-Labat, M.L.; Bozinovski, S.; Bertoncello, I. TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem. Cell Res. 2013, 11, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hong, G.; Kim, D.H.; Kim, Y.M.; Kim, Y.K.; Oh, Y.M.; Jee, Y.K. The role of FGF-2 in smoke-induced emphysema and the therapeutic potential of recombinant FGF-2 in patients with COPD. Exp. Mol. Med. 2018, 50, 150. [Google Scholar] [CrossRef]
- Skronska-Wasek, W.; Gosens, R.; Königshoff, M.; Baarsma, H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018, 187, 150–166. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Königshoff, M. “WNT-er is coming”: WNT signalling in chronic lung diseases. Thorax 2017, 72, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Stabler, C.T.; Morrisey, E.E. Developmental pathways in lung regeneration. Cell Tissue Res. 2017, 367, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sanchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.S.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef]
- Zuriaga, M.A.; Fuster, J.J.; Farb, M.G.; MacLauchlan, S.; Bretón-Romero, R.; Karki, S.; Hess, D.T.; Apovian, C.M.; Hamburg, N.M.; Gokce, N.; et al. Activation of non-canonical WNT signaling in human visceral adipose tissue contributes to local and systemic inflammation. Sci. Rep. 2017, 7, 17326. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Kim, Y.C.; Yu, B.; Moroishi, T.; Mo, J.S.; Plouffe, S.W.; Meng, Z.; Lin, K.C.; Yu, F.X.; Alexander, C.M.; et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell 2015, 162, 780–794. [Google Scholar] [CrossRef]
- Harada, T.; Yamamoto, H.; Kishida, S.; Kishida, M.; Awada, C.; Takao, T.; Kikuchi, A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017, 108, 42–52. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Skronska-Wasek, W.; Mutze, K.; Ciolek, F.; Wagner, D.E.; John-Schuster, G.; Heinzelmann, K.; Günther, A.; Bracke, K.R.; Dagouassat, M.; et al. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017, 214, 143–163. [Google Scholar] [CrossRef]
- Heijink, I.H.; De Bruin, H.G.; Dennebos, R.; Jonker, M.R.; Noordhoek, J.A.; Brandsma, C.A.; Van Den Berge, M.; Postma, D.S. Cigarette smoke-induced epithelial expression of WNT-5B: Implications for COPD. Eur. Respir. J. 2016, 48, 504–515. [Google Scholar] [CrossRef]
- Van Dijk, E.M.; Menzen, M.H.; Spanjer, A.I.R.; Middag, L.D.C.; Brandsma, C.-A.A.; Gosens, R. Noncanonical WNT-5B signaling induces inflammatory responses in human lung fibroblasts. Am. J. Physiol. Cell. Mol. Physiol. 2016, 310, 1166–1176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nabhan, A.; Brownfield, D.G.; Krasnow, M.A.; Desai, T.J. A single cell Wnt signaling niche maintains stemness of alveolar type 2 cells HHS Public Access. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Xu, C.; Lu, M.; Wu, X.; Tang, L.; Wu, X. Wnt/β-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. ACTA Mol. Basis Dis. 2017, 1863, 3226–3242. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.; Gosens, R. WNT-5A: Signaling and functions in health and disease. Cell. Mol. Life Sci. 2016, 73, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; van DijkI, E.M.; Bos, I.S.T.; Kistemaker, L.E.M.; Gosens, R. Mouse Lung Tissue Slice Culture. In Mouse Cell Culture; Humana Press: New York, NY, USA, 2019; pp. 297–311. ISBN 978-1-4939-9086-3. [Google Scholar]
- Ng-Blichfeldt, J.P.; Schrik, A.; Kortekaas, R.K.; Noordhoek, J.A.; Heijink, I.H.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 2018, 36, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.B.; Peng, T.; Zepp, J.A.; Snitow, M.; Vincent, T.L.; Penkala, I.J.; Cui, Z.; Herriges, M.J.; Morley, M.P.; Zhou, S.; et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016, 17, 2312–2325. [Google Scholar] [CrossRef]
- Ferrer-Vaquer, A.; Piliszek, A.; Tian, G.; Aho, R.J.; Dufort, D.; Hadjantonakis, A.K. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev. Biol. 2010, 10, 121. [Google Scholar] [CrossRef]
- Ng-Blichfeldt, J.P.; de Jong, T.; Kortekaas, R.K.; Wu, X.; Lindner, M.; Guryev, V.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. TGF-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, 14–28. [Google Scholar] [CrossRef]
- McCauley, K.B.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem. Cell 2017, 20, 844–857. [Google Scholar] [CrossRef]
- Wansleeben, C.; Barkauskas, C.E.; Rock, J.R.; Hogan, B.L.M. Stem cells of the adult lung: Their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 131–148. [Google Scholar] [CrossRef]
- Chen, F.; Fine, A. Stem Cells in Lung Injury and Repair. Am. J. Pathol. 2016, 186, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S. Understanding the developmental pathways pulmonary fibroblasts may follow during alveolar regeneration. Cell Tissue Res. 2017, 367, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Morrisey, E.E.; Cavanaugh, C.A.; Zepp, J.A.; Frank, D.B.; Zacharias, W.J.; Morley, M.P. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar]
- Lemjabbar-Alaoui, H.; Dasari, V.; Sidhu, S.S.; Mengistab, A.; Finkbeiner, W.; Gullup, M.; Basbaum, C. Wnt and hedgehog are critical mediators of cigarette smoke-induced lung cancer. PLoS ONE 2006, 1, 93. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; van Dijk, E.M.; Ng-Blichfeldt, J.-P.; Bos, I.S.T.; Ciminieri, C.; Königshoff, M.; Kistemaker, L.E.M.; Gosens, R. Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors. Cells 2019, 8, 1147. https://doi.org/10.3390/cells8101147
Wu X, van Dijk EM, Ng-Blichfeldt J-P, Bos IST, Ciminieri C, Königshoff M, Kistemaker LEM, Gosens R. Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors. Cells. 2019; 8(10):1147. https://doi.org/10.3390/cells8101147
Chicago/Turabian StyleWu, Xinhui, Eline M. van Dijk, John-Poul Ng-Blichfeldt, I. Sophie T. Bos, Chiara Ciminieri, Melanie Königshoff, Loes E.M. Kistemaker, and Reinoud Gosens. 2019. "Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors" Cells 8, no. 10: 1147. https://doi.org/10.3390/cells8101147
APA StyleWu, X., van Dijk, E. M., Ng-Blichfeldt, J.-P., Bos, I. S. T., Ciminieri, C., Königshoff, M., Kistemaker, L. E. M., & Gosens, R. (2019). Mesenchymal WNT-5A/5B Signaling Represses Lung Alveolar Epithelial Progenitors. Cells, 8(10), 1147. https://doi.org/10.3390/cells8101147




