Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Analysis
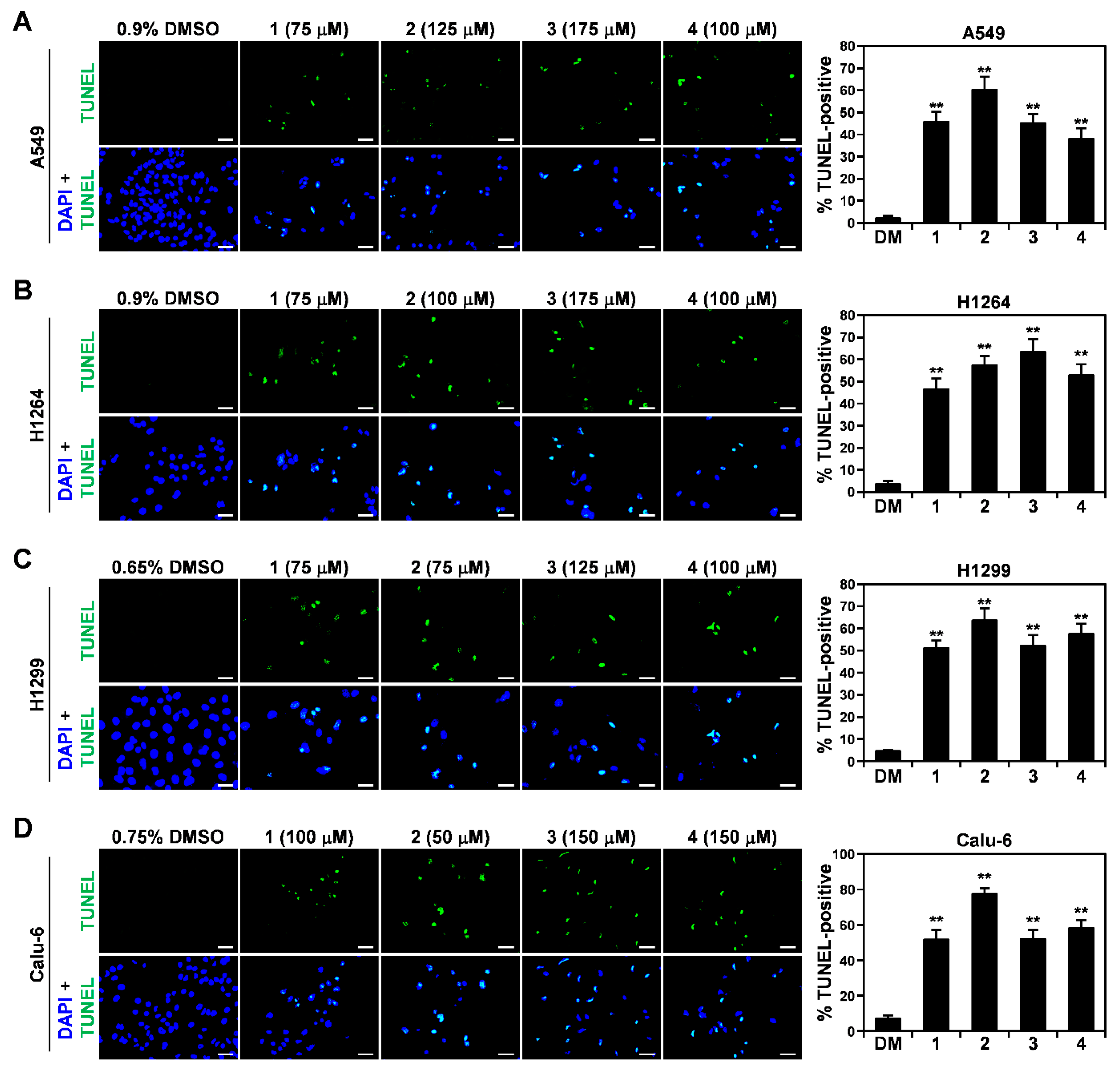
2.3. TUNEL Assay
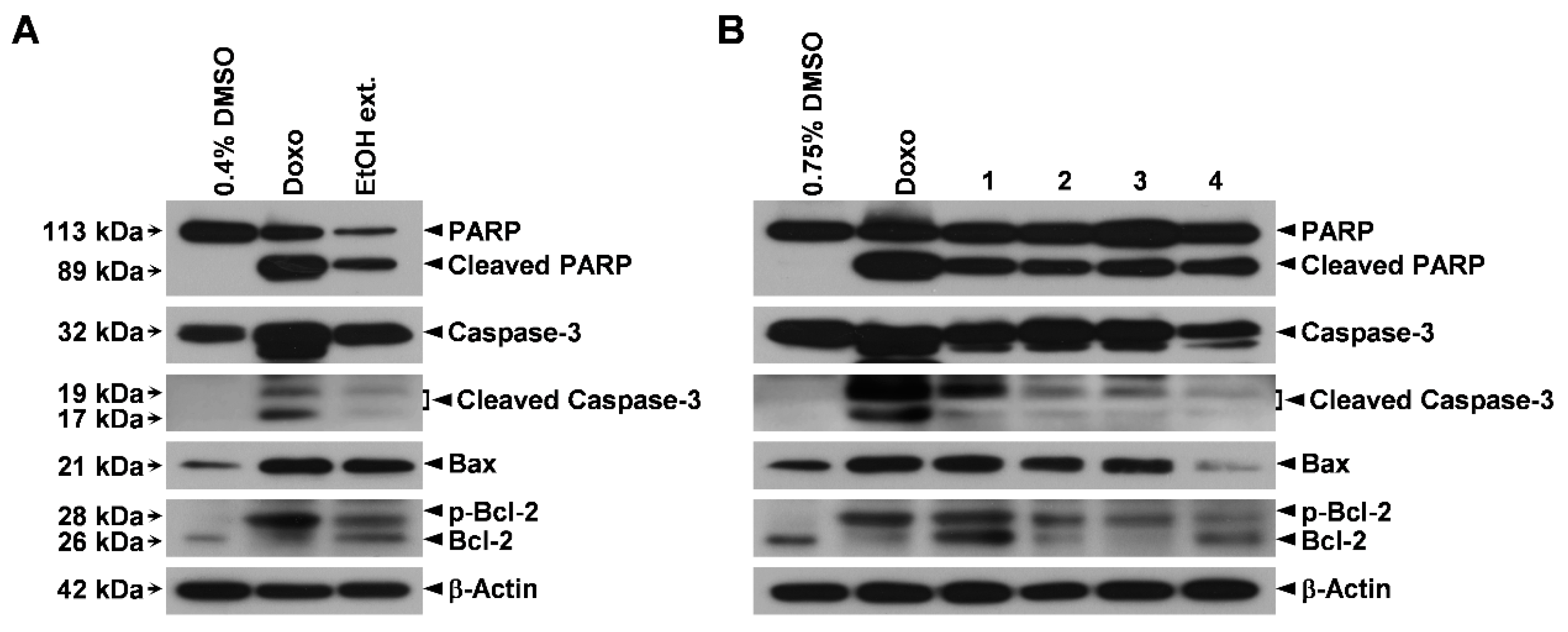
2.4. Immunoblotting
2.5. Statistical Analysis
3. Results
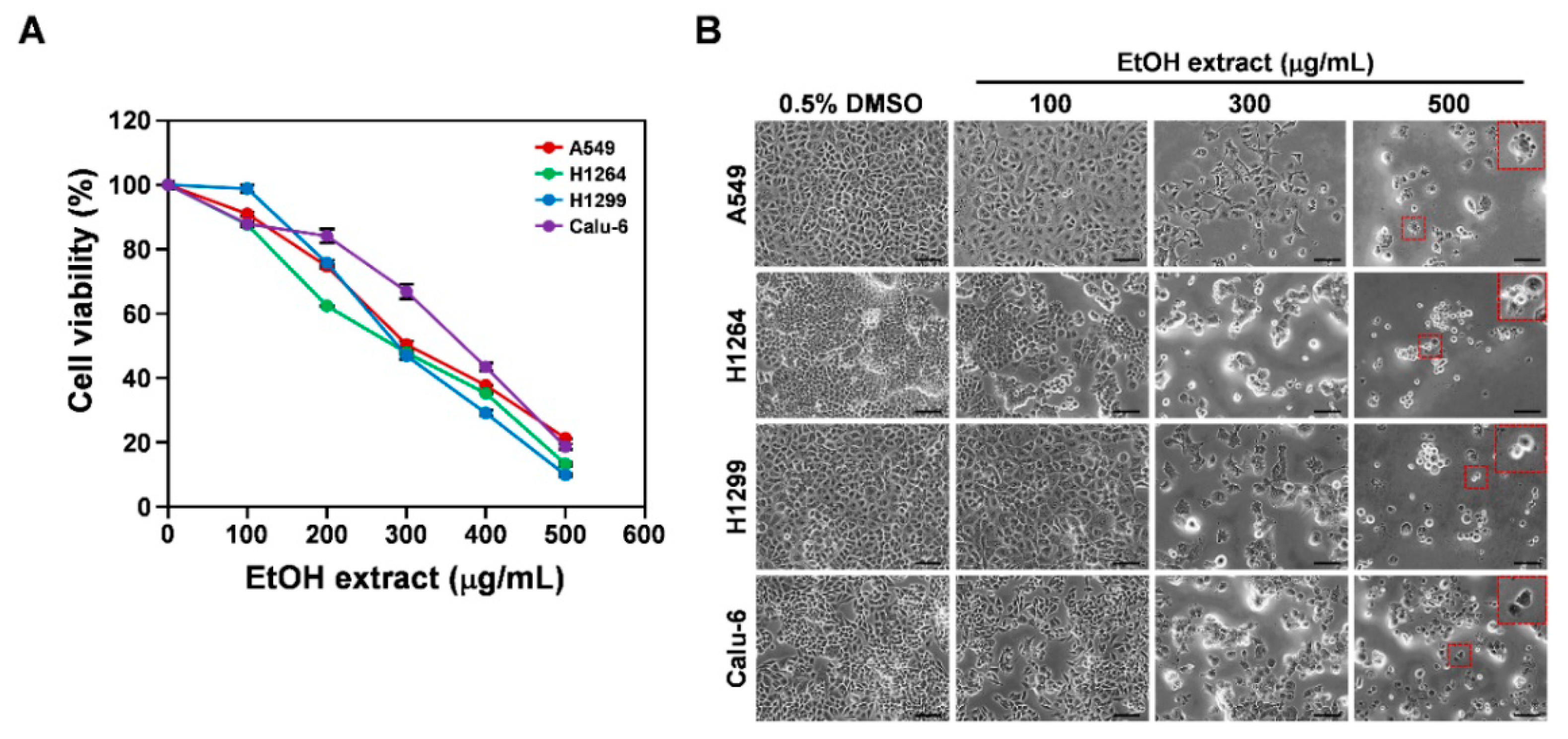
3.1. Cytotoxicity of the EtOH Extract of the Sclerotia of P. cocos toward Human Lung Adenocarcinoma Cells with Different p53 Statuses
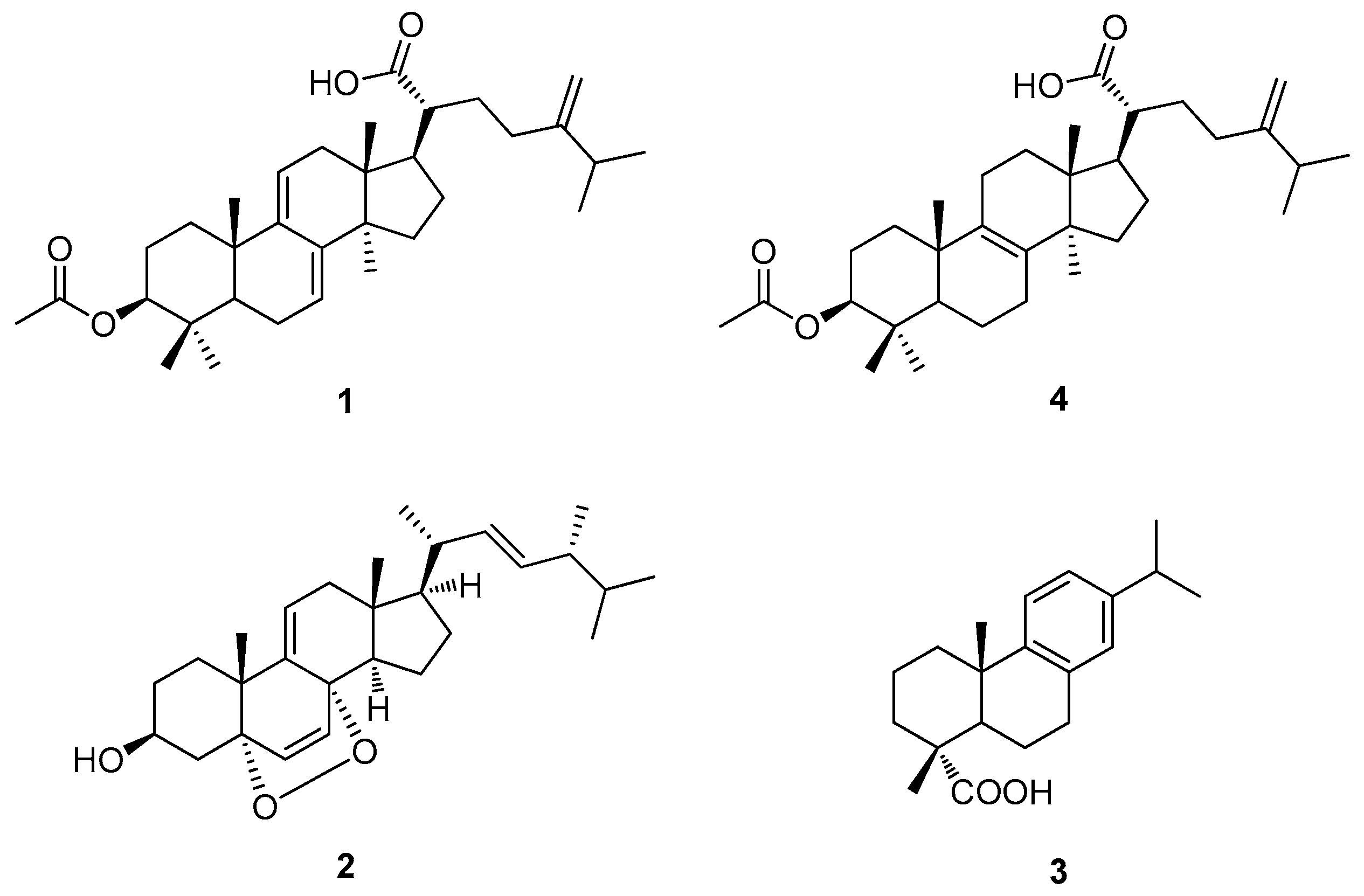
3.2. Chemical Analysis of Bioactive Compounds from the EtOH Extract of the Sclerotia of P. cocos
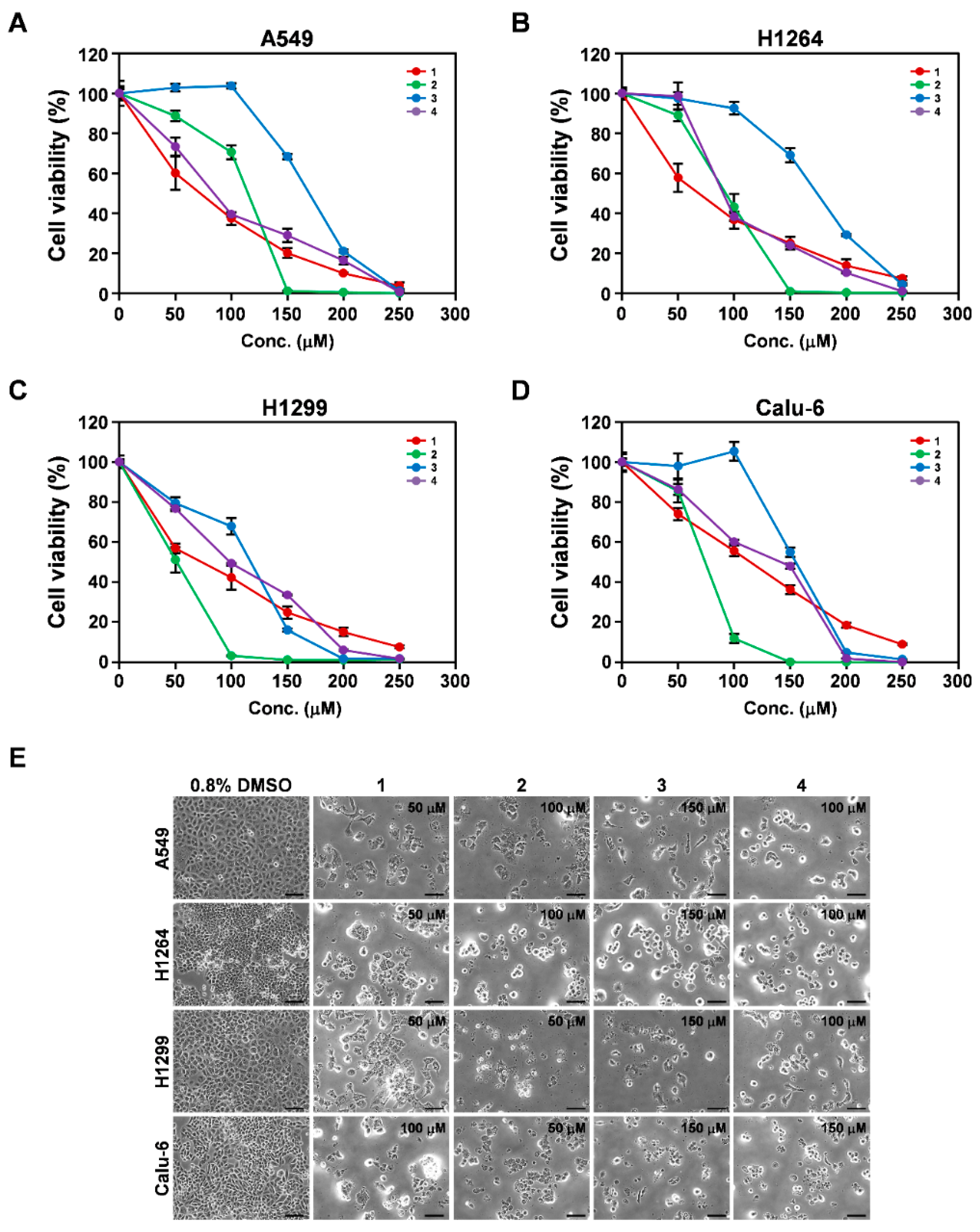
3.3. Cytotoxicity of Isolated Compounds 1–4 toward Human Lung Adenocarcinoma Cells
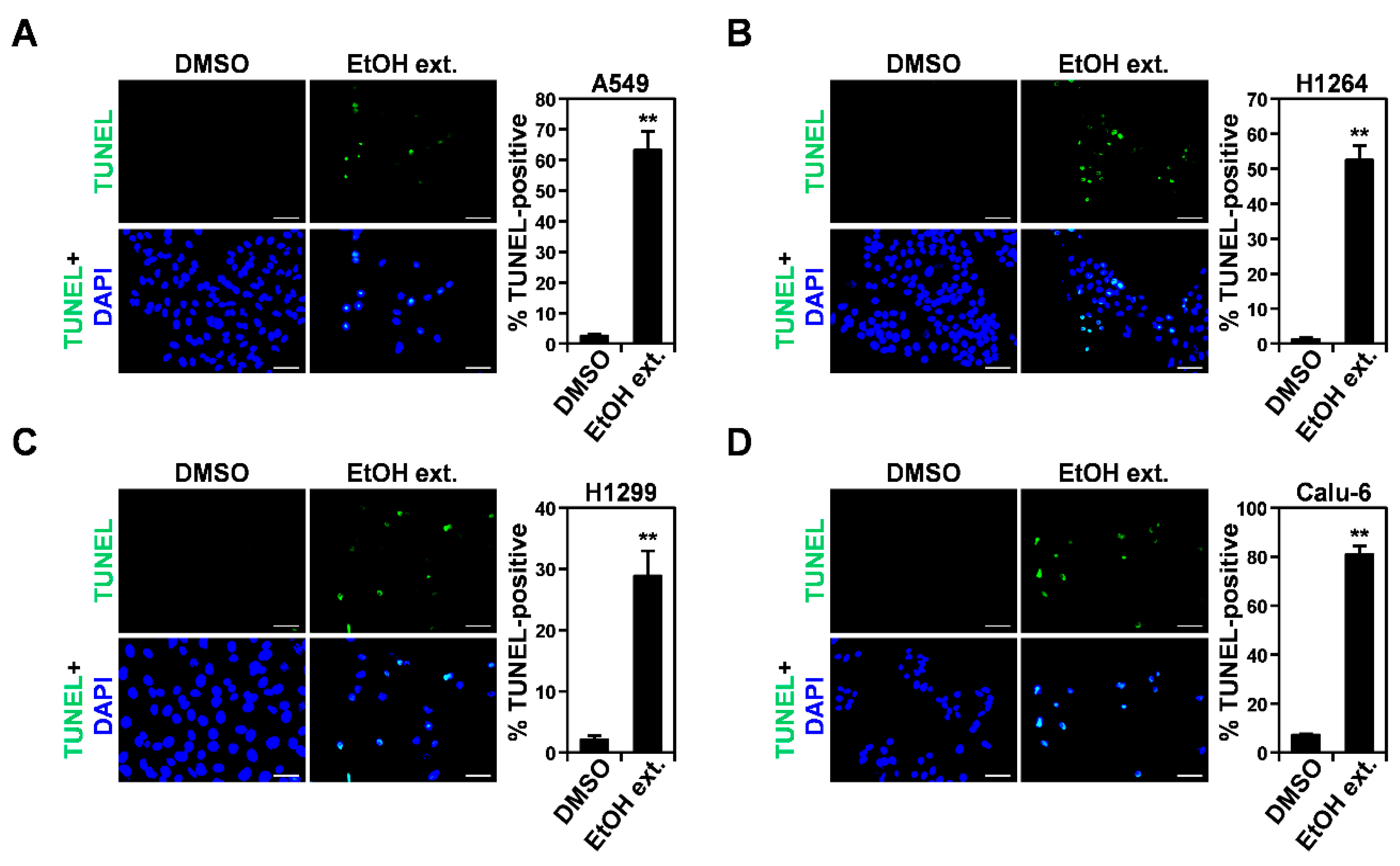
3.4. Induction of the Mitochondrial Apoptotic Pathway by the EtOH Extract of the Sclerotia of P. cocos and Its Cytotoxic Constituents in Human Lung Adenocarcinoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R.; Lima, N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN®): Review of development and future perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A.; Vurro, M.; Mathieu, V.; Cimmino, A.; Evidente, M.; van Otterlo, W.A.L.; Dasari, R.; Lefranc, F.; Kiss, R. Toward a cancer drug of fungal origin. Med. Res. Rev. 2015, 35, 937–967. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, H.; Togami, M.; Adachi, N.; Fukai, Y.; Okumoto, T. Studies on the antitumor active polysaccharides from the mycelia of Poria cocos Wolf. III. Antitumor activity against mouse tumors. Yakugaku Zasshi 1986, 106, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kaminaga, T.; Yasukawa, K.; Kanno, H.; Tai, T.; Nunoura, Y.; Takido, M. Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 1996, 53, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Andújar, I.; Recio, M.C.; Giner, R.M. Lanostanoids from fungi: A group of potential anticancer compounds. J. Nat. Prod. 2012, 75, 2016–2044. [Google Scholar] [CrossRef] [PubMed]
- Gapter, L.; Wang, Z.; Glinski, J.; Ng, K.Y. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem. Biophys. Res. Commun. 2005, 332, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Uchiyama, E.; Ukiya, M.; Tabata, K.; Kimura, Y.; Suzuki, T.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J. Nat. Prod. 2011, 74, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chiu, L.C.; Cheung, P.C.; Ooi, V.E.C. Growth-inhibitory effects of a β-glucan from the mycelium of Poria cocos on human breast carcinoma MCF-7 cells: Cell-cycle arrest and apoptosis induction. Oncol. Rep. 2006, 15, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Y.; Gapter, L.A.; Ling, H.; Agarwal, R.; Ng, K. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem. Pharm. Bull. 2008, 56, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Zhou, L.; Jia, X.; Gapter, L.A.; Agarwal, R.; Ng, K. Polyporenic acid C induces caspase-8-mediated apoptosis in human lung cancer A549 cells. Mol. Carcinog. 2009, 48, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, J.; Lu, C.; Cai, D. Pachymic acid induces apoptosis via activating ROS-dependent JNK and ER stress pathways in lung cancer cells. Cancer Cell Int. 2015, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.F.; Lin, H.C.; Huang, X.W.; Huang, H.Y.; Wu, C.P.; Kao, M.C. An ethanol extract of Poria cocos inhibits the proliferation of non-small cell lung cancer A549 cells via the mitochondria-mediated caspase activation pathway. J. Funct. Foods 2016, 23, 614–627. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y. Targeting p53 for novel anticancer therapy. Transl. Oncol. 2010, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, H.M.; Roh, H.S.; Kim, J.; Yu, J.S.; Lee, S.; Seok, S.; Pang, C.; Baek, K.H.; Kim, K.H. Vulpinic acid contributes to the cytotoxicity of Pulveroboletus ravenelii to human cancer cells by inducing apoptosis. RSC Adv. 2017, 7, 35297–35304. [Google Scholar] [CrossRef]
- Lee, S.R.; Roh, H.S.; Lee, S.; Park, H.B.; Jang, T.S.; Ko, Y.J.; Baek, K.H.; Kim, K.H. Bioactivity-guided isolation and chemical characterization of antiproliferative constituents from morel mushroom (Morchella esculenta) in human lung adenocarcinoma cells. J. Funct. Foods 2018, 40, 249–260. [Google Scholar] [CrossRef]
- Baek, J.; Roh, H.S.; Baek, K.H.; Lee, S.; Lee, S.; Song, S.S.; Kim, K.H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 2018, 224, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Roh, H.S.; Yu, J.S.; Baek, J.; Lee, S.; Ra, M.; Kim, S.Y.; Baek, K.H.; Kim, K.H. Pinecone of Pinus koraiensis inducing apoptosis in human lung cancer cells by activating caspase-3 and its chemical constituents. Chem. Biodivers. 2017, 14, e1600412. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Steinberg, S.M.; Nau, M.M.; Carbone, D.; D’Amico, D.; Bodner, S.; Oie, H.K.; Linnoila, R.I.; Mulshine, J.L.; Minna, J.D. p53 Gene mutations in non-small-cell-lung-cancer cell-lines and their correlation with the presence of ras mutations and clinical features. Oncogene 1992, 7, 171–180. [Google Scholar] [PubMed]
- Rämet, M.; Castrén, K.; Järvinen, K.; Pekkala, K.; Turpeenniemi-Hujanen, T.; Soini, Y.; Pääkkö, P.; Vähäkangas, K. p53 protein expression is correlated with benzo[α]pyrene-DNA adducts in carcinoma cell lines. Carcinogenesis 1995, 16, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Shen, Y.C.; Chen, C.H. Steroids and triterpenoids of Antodia cinnamomea—A fungus parasitic on Cinnamomum micranthum. Phytochemistry 1996, 41, 1389–1392. [Google Scholar] [CrossRef]
- León, F.; Quintana, J.; Rivera, A.; Estévez, F.; Bermejo, J. Lanostanoid triterpenes from Laetiporus sulphureus and apoptosis induction on HL-60 human myeloid leukemia cells. J. Nat. Prod. 2004, 67, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Kuo, Y.H.; Chiang, B.H.; Lo, J.M.; Sheen, L.Y. Cytotoxic activities of 9,11-dehydroergosterol peroxide and ergosterol peroxide from the fermentation mycelia of Ganoderma lucidum cultivated in the medium containing leguminous plants on Hep 3B cells. J. Agric. Food Chem. 2009, 57, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A.; Claassen, F.W.; Dorado, J.; Godejohann, M.; Sierra-Alvarez, R.; Wijnberg, J.B.P.A. Fungal biotransformation products of dehydroabietic acid. J. Nat. Prod. 2007, 70, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Shim, S.H.; Lee, H.J.; Choi, Y.; Jang, T.S.; Kim, K.H.; Kang, K.S. Protective effect of lanostane triterpenoids from the sclerotia of Poria cocos Wolf against cisplatin-induced apoptosis in LLC-PK1 cells. Bioorg. Med. Chem. Lett. 2017, 27, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Kim, K.H. Anti-inflammatory activity of the sclerotia of edible fungus, Poria cocos Wolf and their active lanostane triterpenoids. J. Funct. Foods 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Dixit, V.M. Caspases: Intracellular signaling by proteolysis. Cell 1997, 91, 443–446. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly (ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar] [PubMed]
- Chipuk, J.E.; Green, D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell. Biol. 2008, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Deng, X.; May, W.S. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 2001, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Velez, J.M.; Miriyala, S.; Nithipongvanitch, R.; Noel, T.; Plabplueng, C.D.; Oberley, T.; Jungsuwadee, P.; Remmen, H.V.; Vore, M.; St. Clair, D.K. p53 regulates oxidative stress-mediated retrograde signaling: A novel mechanism for chemotherapy-induced cardiac injury. PLoS ONE 2011, 6, e18005. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, T.; Chen, T.; Duan, J.; Yu, L.; Tang, Y. Cytotoxicity activity of extracts and compounds from Commiphora myrrha resin against human gynecologic cancer cells. J. Med. Plants Res. 2011, 5, 1382–1389. [Google Scholar]
- Ukiya, M.; Kawaguchi, T.; Ishii, K.; Ogihara, E.; Tachi, Y.; Kurita, M.; Ezaki, Y.; Fukatsu, M.; Kushi, Y.; Akihisa, T. Cytotoxic activities of amino acid-conjugate derivatives of abietane-type diterpenoids against human cancer cell lines. Chem. Biodivers. 2013, 10, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Hirano, M.; Oshikubo, M.; Nobukuni, Y.; Kimura, Y.; Tai, T.; Kondo, S.; Nishino, H. Inhibition of tumor-promoting effects by poricoic acids G and H and other lanostane-type triterpenes and cytotoxic activity of poricoic acids A and G from Poria cocos. J. Nat. Prod. 2002, 65, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wong, Y.S.; Shao, M.; Huang, S.; Wang, F.; Chen, J. Apoptosis induced by 9,11-dehydroergosterol peroxide from Ganoderma lucidum mycelium in human malignant melanoma cells is Mcl-1 dependent. Mol. Med. Rep. 2018, 18, 938–944. [Google Scholar] [PubMed]
| Cell Lines | EtOH Ext. (μg/mL) | Compounds (μM) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| A549 | 301.1 ± 7.2 1 | 64.4 ± 13.0 | 121.9 ± 5.2 | 163.8 ± 1.7 | 75.8 ± 3.8 |
| H1264 | 284.0 ± 3.6 | 63.6 ± 12.3 | 92.3 ± 7.9 | 171.0 ± 4.1 | 82.5 ± 4.6 |
| H1299 | 285.5 ± 4.9 | 66.6 ± 2.5 | 50.6 ± 6.7 | 122.5 ± 3.5 | 96.8 ± 0.8 |
| Calu-6 | 372.7 ± 2.0 | 108.0 ± 8.5 | 71.2 ± 3.4 | 154.5 ± 2.2 | 141.7 ± 6.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, S.; Roh, H.-S.; Song, S.-S.; Ryoo, R.; Pang, C.; Baek, K.-H.; Kim, K.H. Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis. Cells 2018, 7, 116. https://doi.org/10.3390/cells7090116
Lee S, Lee S, Roh H-S, Song S-S, Ryoo R, Pang C, Baek K-H, Kim KH. Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis. Cells. 2018; 7(9):116. https://doi.org/10.3390/cells7090116
Chicago/Turabian StyleLee, Seulah, Seul Lee, Hyun-Soo Roh, Seong-Soo Song, Rhim Ryoo, Changhyun Pang, Kwan-Hyuck Baek, and Ki Hyun Kim. 2018. "Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis" Cells 7, no. 9: 116. https://doi.org/10.3390/cells7090116
APA StyleLee, S., Lee, S., Roh, H.-S., Song, S.-S., Ryoo, R., Pang, C., Baek, K.-H., & Kim, K. H. (2018). Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis. Cells, 7(9), 116. https://doi.org/10.3390/cells7090116







