New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Tissue Processing
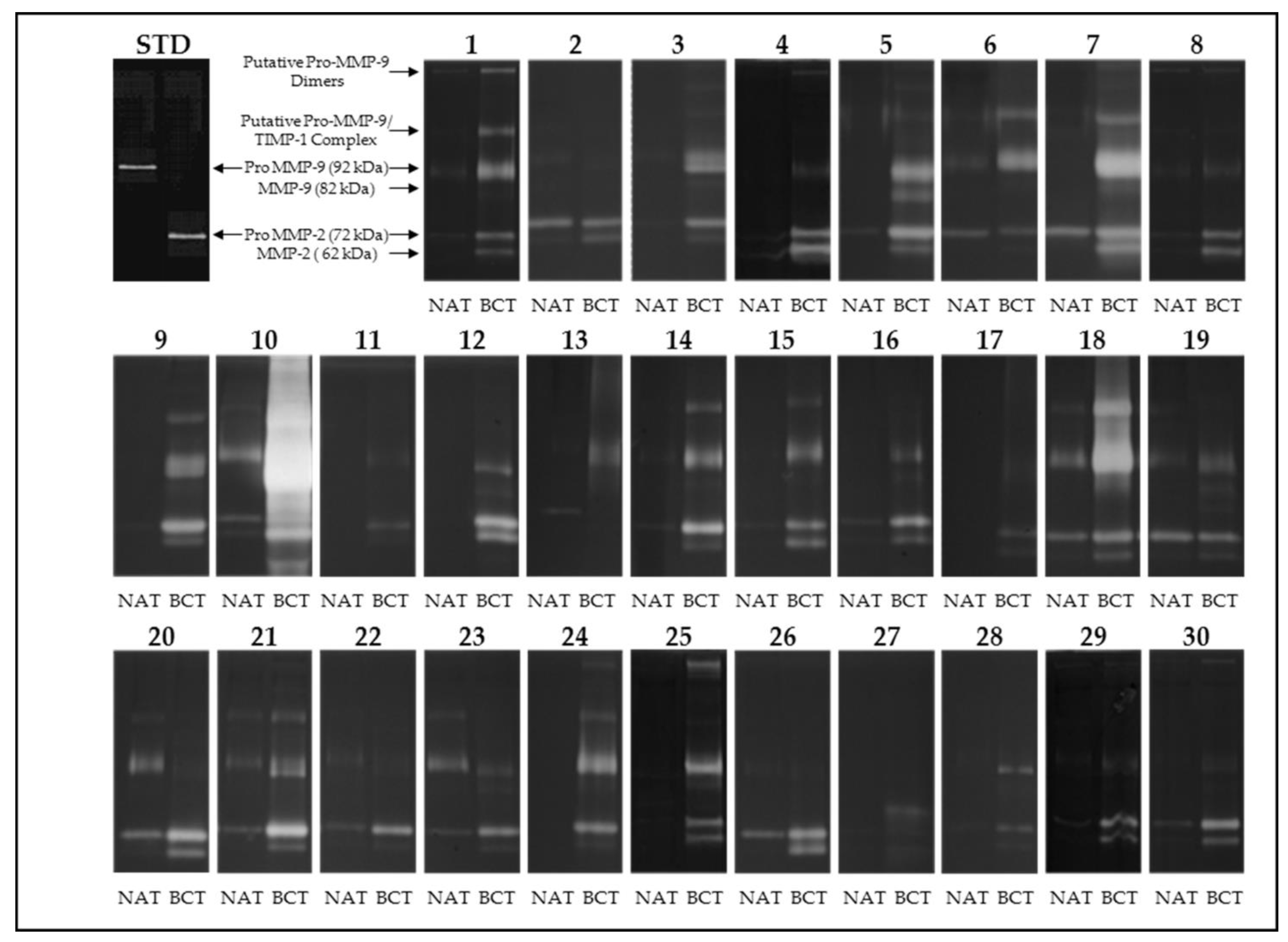
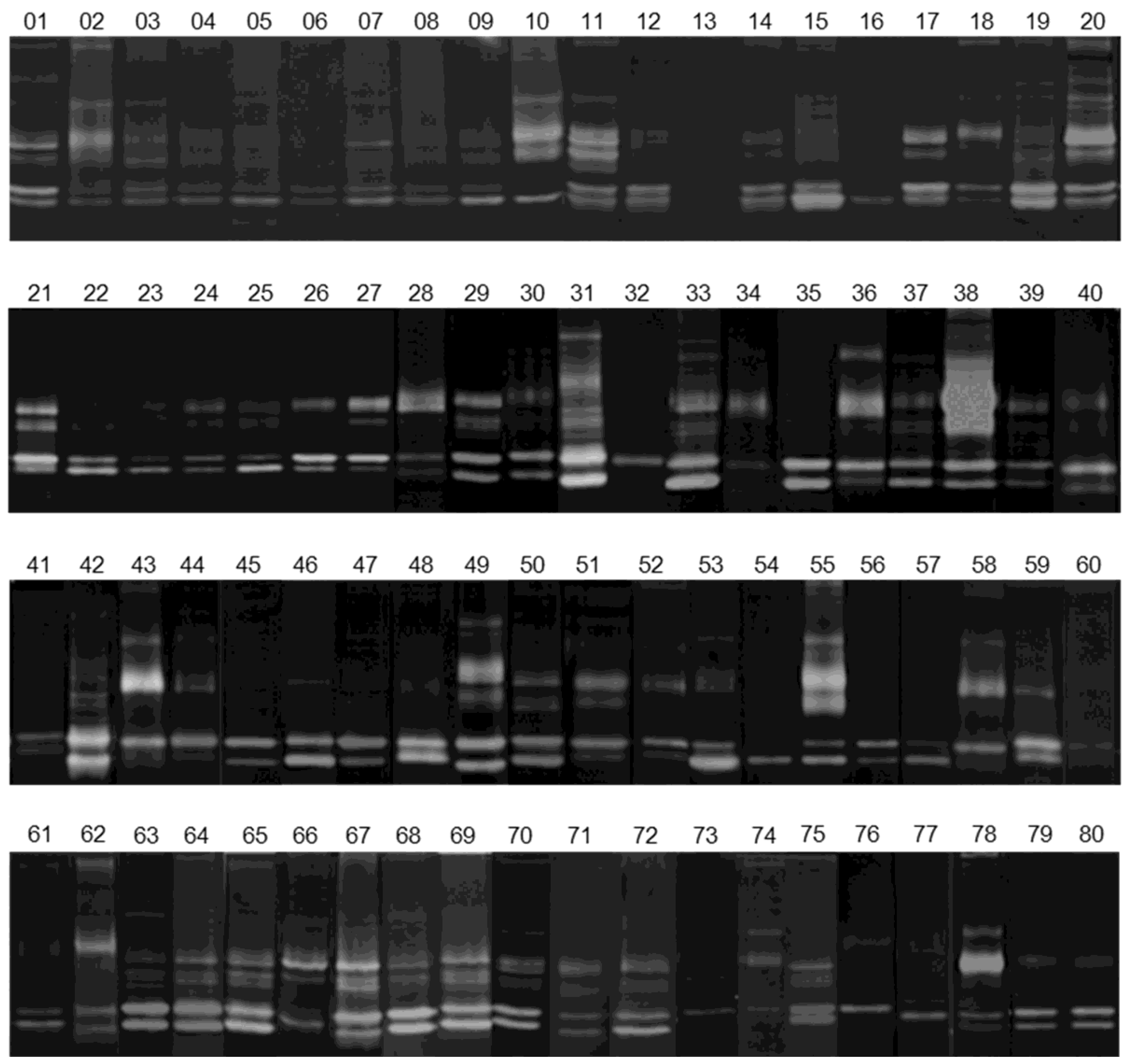
2.3. Gelatin Zymography
2.4. Quantification of Enzymatic Activity
2.5. Two-Dimensional Gel Electrophoresis
2.6. Protein Identification and Functional Association
3. Results
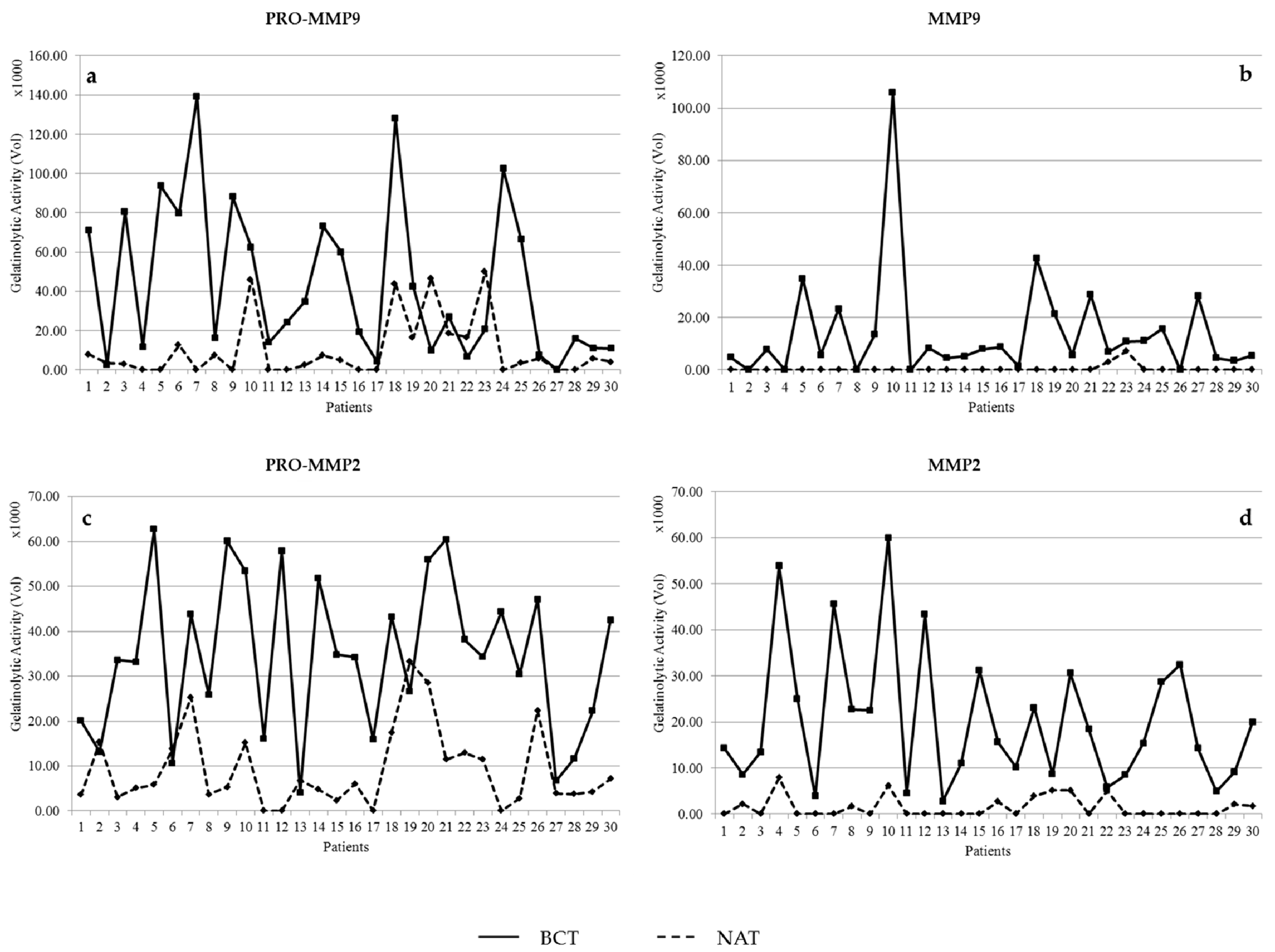
3.1. Activity Levels of MMP-2 and MMP-9 in Breast Cancer Tissues and Their Adjacent Non-Tumoral Tissues
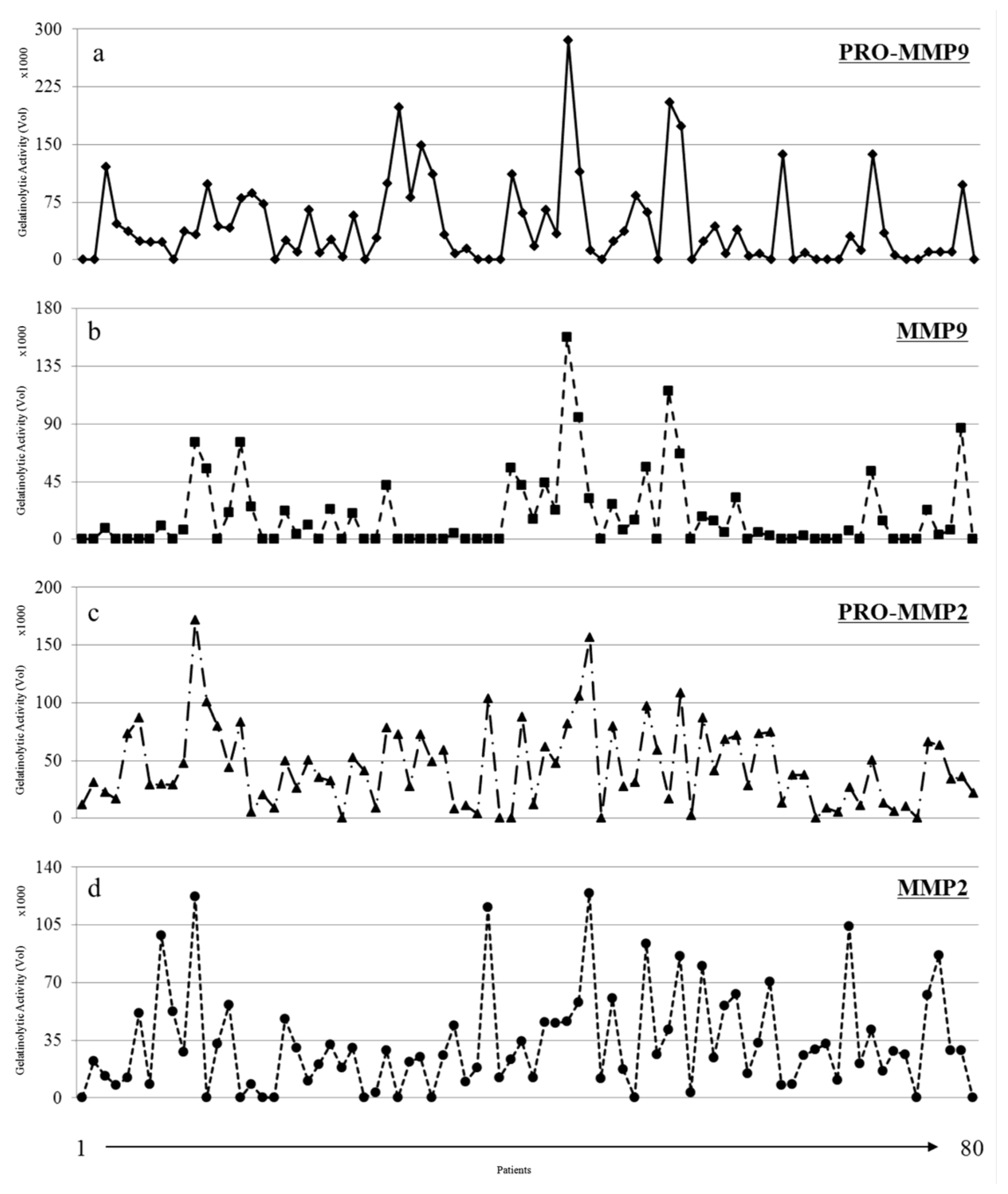
3.2. Gelatinolytic Activities of MMP-2 and MMP-9 in Breast Cancer Patients
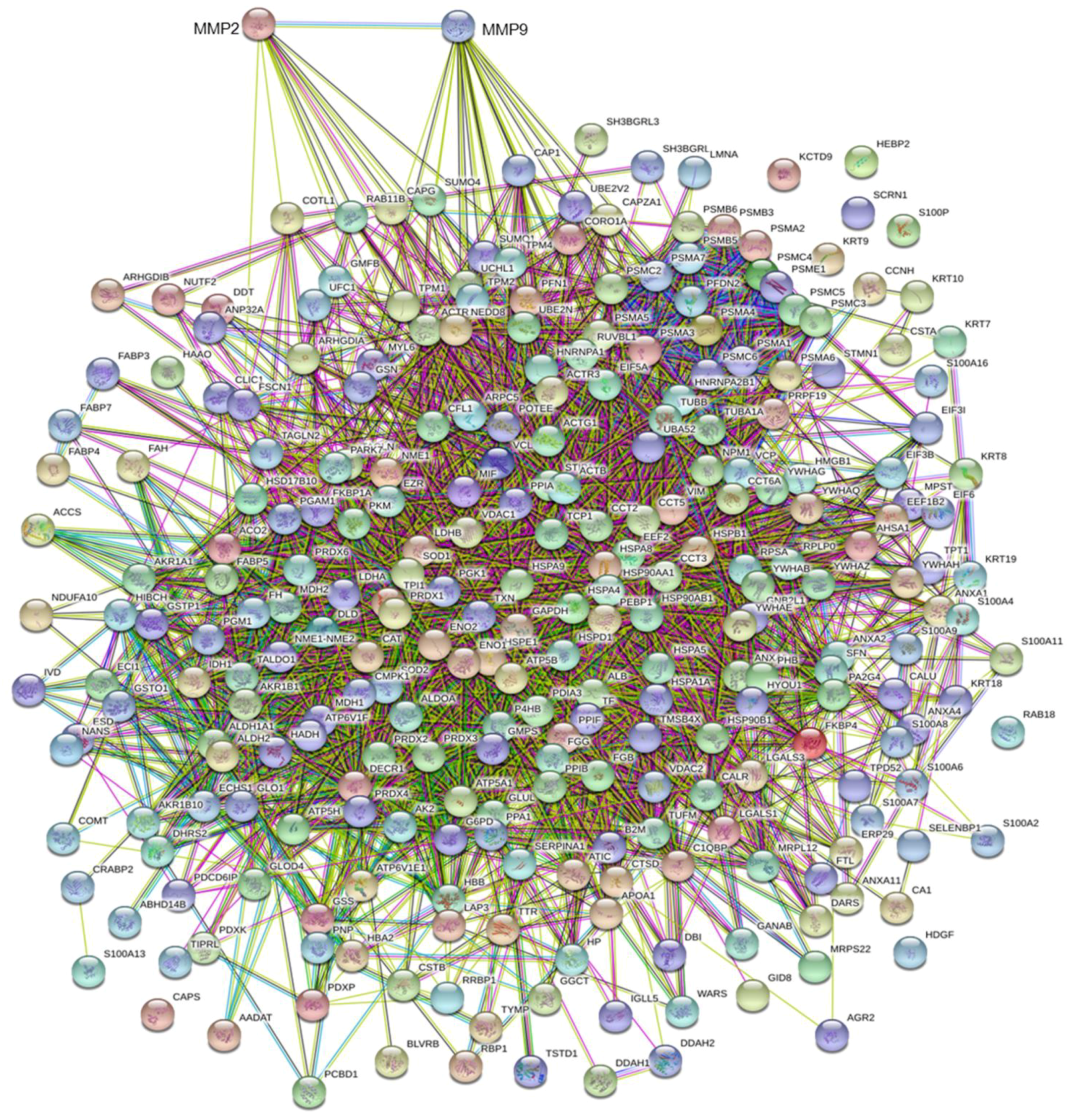
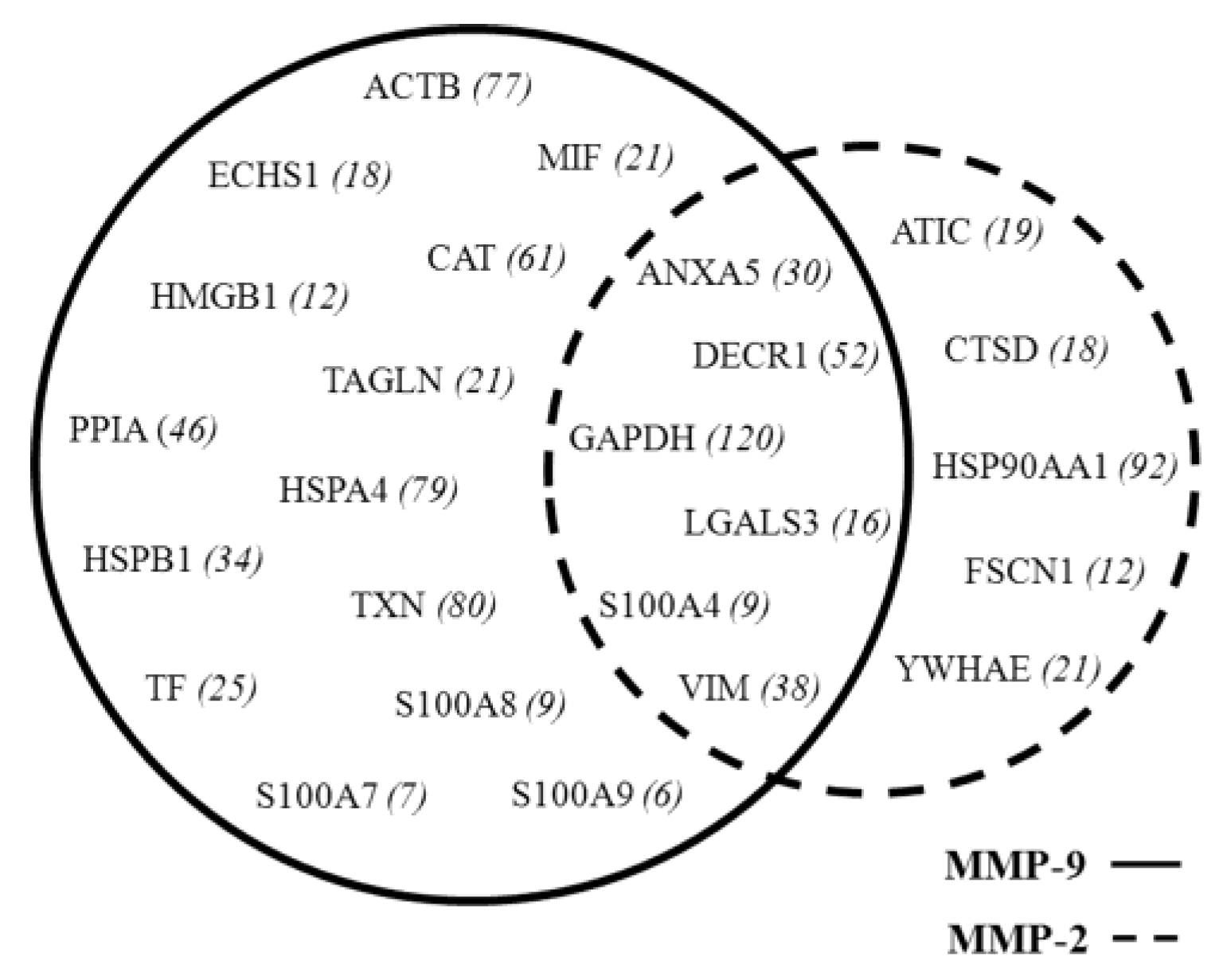
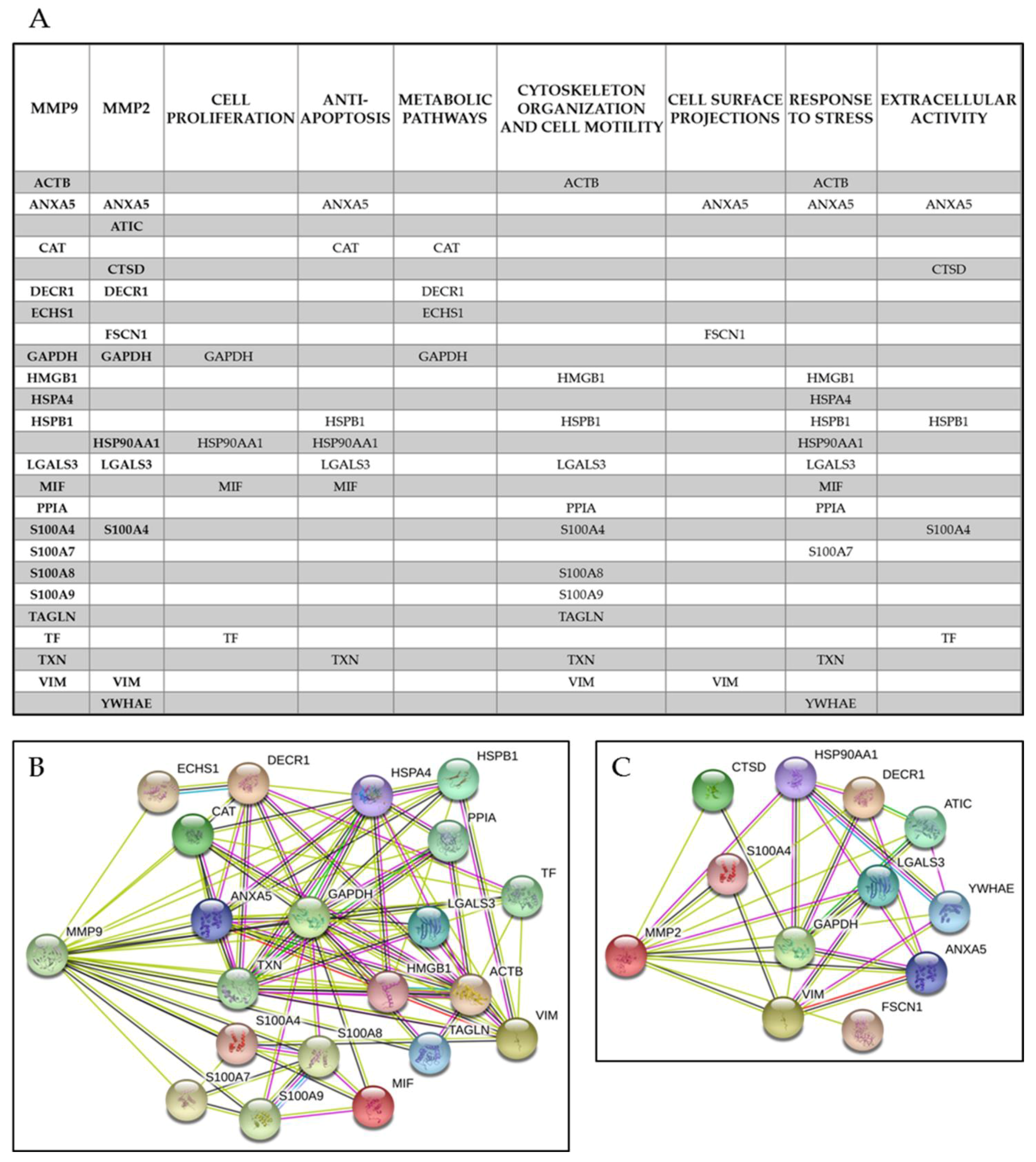
3.3. MMP Levels and Proteomic Correlations
4. Discussion
4.1. Common Interactors for MMP-2 And MMP-9
4.2. MMP-9 Direct Interactors
4.3. MMP-2 Direct Interactors
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The global decrease in cancer mortality: Trends and disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Solak, M.; Petekkaya, I.; Keskin, O.; Kertmen, N.; Sarici, F.; Arik, Z.; Babacan, T.; Ozisik, Y.; Altundag, K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013, 22, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L. Delay in breast cancer: Implications for stage at diagnosis and survival. Front. Public Health 2014, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Pondé, N.; Brandão, M.; El-Hachem, G.; Werbrouck, E.; Piccart, M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2018, 67, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Keegan, N.M.; Gleeson, J.P.; Hennessy, B.T.; Morris, P.G. PI3K inhibition to overcome endocrine resistance in breast cancer. Expert Opin. Investig. Drugs 2018, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Holanda, A.O.; Oliveira, A.R.; Cruz, K.J.; Severo, J.S.; Morais, J.B.; Silva, B.B.; Marreiro, D.D. Zinc and metalloproteinases 2 and 9: What is their relation with breast cancer? Rev. Assoc. Med. Bras. 2017, 63, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; Abbate, I.; Oakley, C.; Casamassima, P.; Savino, E.; Casamassima, A.; Sciortino, G.; Fazio, V.; Gadaleta-Caldarola, G.; Catino, A.; et al. Clinical and prognostic role of matrix metalloproteinase-2, -9 and their inhibitors in breast cancer and liver diseases: A review. Int. J. Biochem. Cell Biol. 2016, 77, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Skerenova, M.; Mikulova, V.; Capoun, O.; Zima, T.; Tesarova, P. Circulating tumor cells and serum levels of MMP-2, MMP-9 and VEGF as markers of the metastatic process in patients with high risk of metastatic progression. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, J.; Vihinen, P.; Vuoristo, M.S.; Kellokumpu-Lehtinen, P.; Kähäri, V.M.; Pyrhönen, S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin. Cancer Res. 2005, 11, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Leppä, S.; Saarto, T.; Vehmanen, L.; Blomqvist, C.; Elomaa, I. A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin. Cancer Res. 2004, 10, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pucci-Minafra, I.; Minafra, S.; La Rocca, G.; Barranca, M.; Fontana, S.; Alaimo, G.; Okada, Y. Zymographic analysis of circulating and tissue forms of colon carcinoma gelatinase A (MMP-2) and B (MMP-9) separated by mono- and two-dimensional electrophoresis. Matrix Biol. 2001, 20, 419–427. [Google Scholar] [CrossRef]
- Pucci-Minafra, I.; Cancemi, P.; Marabeti, M.R.; Albanese, N.N.; Di Cara, G.; Taormina, P.; Marrazzo, A. Proteomic profiling of 13 paired ductal infiltrating breast carcinomas and non-tumoral adjacent counterparts. Proteom. Clin. Appl. 2007, 1, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Pucci Minafra, I.; Di Cara, G.; Musso, R.; Peri, G.; Valentino, B.; D’Arienzo, M.; Martini, D.; Raspanti, M.; Minafra, S. Proteomic profiling of In Vitro bone-conditioned skbr3 breast cancer cells. J. Proteom. Bioinf. 2016, 9, 75–83. [Google Scholar]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks; made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 2, 32–40. [Google Scholar]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef] [PubMed]
- Pucci-Minafra, I.; Di Cara, G.; Musso, R.; Cancemi, P.; Albanese, N.N.; Roz, E.; Minafra, S. Retrospective Proteomic Screening of 100 Breast Cancer Tissues. Proteomes 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sirover, M.A. Subcellular dynamics of multifunctional protein regulation: Mechanisms of GAPDH intracellular translocation. J. Cell. Biochem. 2012, 113, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Mussunoor, S.; Murray, G.I. The role of annexins in tumour development and progression. J. Pathol. 2008, 216, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wehder, L.; Arndt, S.; Murzik, U.; Bosserhoff, A.K.; Kob, R.; von Eggeling, F.; Melle, C. Annexin A5 is involved in migration and invasion of oral carcinoma. Cell Cycle 2009, 8, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Guo, C.; Guan, H.; Liu, S.; Sun, M.Z. Annexin A5 as a potential marker in tumors. Clin. Chim. Acta 2014, 427, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bird, P.; Salem, H.H. Effects of annexin V on the activity of the anticoagulant proteins C and S. Thromb. Res. 1993, 69, 279–287. [Google Scholar] [CrossRef]
- Shetty, P.; Bargale, A.; Patil, B.R.; Mohan, R.; Dinesh, U.S.; Vishwanatha, J.K.; Gai, P.B.; Patil, V.S.; Amsavardani, T.S. Cell surface interaction of annexin A2 and galectin-3 modulates epidermal growth factor receptor signaling in Her-2 negative breast cancer cells. Mol. Cell. Biochem. 2016, 411, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.R.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar] [PubMed]
- HUGO Gene Nomenclature Committee (HGNC). Available online: http://www.genenames.org/cgi-bin/genefamilies/set/459 (accessed on 17 March 2017).
- Salama, I.; Malone, P.S.; Mihaimeed, F.; Jones, J.L. A review of the S100 proteins in cancer. Eur. J. Surg. Oncol. 2008, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Fritz, G.; Vetter, S.W.; Heizmann, C.W. Binding of S100 proteins to RAGE: An update. Biochim. Biophys. Acta 2009, 1793, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, M.; Zhou, Y.; Wang, F.; Li, X.; Wang, L.; Fan, Q. S100A4 participates in epithelial-mesenchymal transition in breast cancer via targeting MMP2. Tumour Biol. 2016, 37, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.M.; Bennett, D.; Platt-Higgins, A.M.; Al-Medhity, M.; Barraclough, R.; Rudland, P.S. S100A4 Elevation Empowers Expression of Metastasis Effector Molecules in Human Breast Cancer. Cancer Res. 2017, 77, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Buetti-Dinh, A.; Pivkin, I.V.; Friedman, R. S100A4 and its role in metastasis—computational integration of data on biological networks. Mol. Biosyst. 2015, 11, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Buetti-Dinh, A.; Pivkin, I.V.; Friedman, R. S100A4 and its role in metastasis–simulations of knockout and amplification of epithelial growth factor receptor and matrix metalloproteinases. Mol. Biosyst. 2015, 11, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Solway, J.; Boyd, D.D. Expression cloning identifies transgelin (SM22) as a novel repressor of 92-kDa type IV collagenase (MMP-9) expression. J. Biol. Chem. 2006, 281, 26424–26436. [Google Scholar] [CrossRef] [PubMed]
- Conroy, H.; Mawhinney, L.; Donnelly, S.C. Inflammation and cancer: Macrophage migration inhibitory factor (MIF)—The potential missing link. Q. J. Med. 2010, 103, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; de Araújo, J.M.; Fernandes, T.A.; Cobucci, R.N.; Lanza, D.C.; Andrade, V.S.; Fernandes, J.V. Macrophage migration inhibitory factor (MIF): Biological activities and relation with cancer. Pathol. Oncol. Res. 2016, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Kindt, N.; Saussez, S. Macrophage migration inhibitory factor involvement in breast cancer (Review). Int. J. Oncol. 2015, 47, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Srivastava, P. Heat-Shock Proteins. Curr. Protoc. Immunol. 2004. [Google Scholar] [CrossRef]
- Abisambra, J.F.; Jinwal, U.K.; Jones, J.R.; Blair, L.J.; Koren, J.; Dickey, C.A. Exploiting the diversity of the heat-shock protein family for primary and secondary tauopathy therapeutics. Curr. Neuropharmacol. 2011, 9, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins Role in cellular functions and pathology. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2015, 1854, 291–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat shock proteins and cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.R. Peptidyl-prolyl isomerase (PPIase): An emerging area in tumor biology. Cancer Res. Front. 2017, 3, 126–143. [Google Scholar] [CrossRef]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 9, 30293–30296. [Google Scholar] [CrossRef]
- Takeuchi, A.; Miyamoto, T.; Yamaji, K.; Masuho, Y.; Hayashi, M.; Hayashi, H.; Onozaki, K. A human erythrocyte-derived growth-promoting factor with a wide target cell spectrum: Identification as catalase. Cancer Res. 1995, 55, 1586–1589. [Google Scholar] [PubMed]
- Zhang, J.; Ibrahim, M.M.; Sun, M.; Tang, J. Enoyl-coenzyme A hydratase in cancer. Clin. Chim. Acta 2015, 448, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.S.; Gao, P.; Dai, Y.C.; Xie, J.P.; Zeng, W.; Lian, Q.N. Attenuation of enoyl coenzyme A hydratase short chain 1 expression in gastric cancer cells inhibits cell proliferation and migration in vitro. Cell. Mol. Biol. Lett. 2014, 19, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Raninga, P.V.; Trapani, G.D.; Tonissen, K.F. Cross Talk between Two Antioxidant Systems, Thioredoxin and DJ-1: Consequences for Cancer. Oncoscience 2014, 1, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, H.; Lu, Y.; Yin, L. Diagnostic and prognostic value of serum thioredoxin and DJ-1 in non-small cell lung carcinoma patients. Tumour Biol. 2016, 37, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers (Basel) 2010, 2, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Laskey, J.; Webb, I.; Schulman, H.M.; Ponka, P. Evidence that transferrin supports cell proliferation by supplying iron for DNA synthesis. Exp. Cell Res. 1988, 176, 87–95. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, A.; Song, I.H.; Park, I.A.; Yu, J.H.; Ahn, J.H.; Gong, G. Cytoplasmic expression of high mobility group B1 (HMGB1) is associated with tumor-infiltrating lymphocytes (TILs) in breast cancer. Pathol. Int. 2016, 66, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cancemi, P.; Di Cara, G.; Albanese, N.N.; Costantini, F.; Marabeti, M.R.; Musso, R.; Riili, I.; Lupo, C.; Roz, E.; Pucci-Minafra, I. Differential occurrence of S100A7 in breast cancer tissues: A proteomic-based investigation. Proteom. Clin. Appl. 2012, 6, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Curtis, L.; Pind, M.; Murphy, L.C.; Watson, P.H. S100A7 (psoriasin) influences immune response genes in human breast cancer. Exp. Cell Res. 2007, 313, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Watson, P.H. S100A7 (psoriasin) oncostatin-M and interleukin-6 in human breast cancer. Oncogene 2010, 29, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Ravi, J.; Powell, C.A.; Trikha, P.; Schwendener, R.A.; Bai, X.F.; Shilo, K.; Zou, X.; et al. S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res. 2012, 72, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, C.; Jin, Q.; Liu, Z. S100 protein family in human cancer. Am. J. Cancer Res. 2014, 4, 89–115. [Google Scholar] [PubMed]
- Bildyug, N. Matrix metalloproteinases: An emerging role in regulation of actin microfilament system. Biomol. Concepts 2016, 7, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sanka, K.; Maddala, R.; Epstein, D.L.; Rao, P.V. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Boutchueng-Djidjou, M.; Collard-Simard, G.; Fortier, S.; Hébert, S.S.; Kelly, I.; Landry, C.R.; Faure, R.L. The last enzyme of the de novo purine synthesis pathway 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase (ATIC) plays a central role in insulin signaling and the Golgi/endosomes protein network. Mol. Cell. Proteom. 2015, 14, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Platet, N.; Liaudet, E.; Laurent, V.; Derocq, D.; Brouillet, J.P.; Rochefort, H. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 1996, 14, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Pranjol, M.Z.I.; Gutowski, N.J.; Hannemann, M.; Whatmore, J.L. Cathepsin D non-proteolytically induces proliferation and migration in human omental microvascular endothelial cells via activation of the ERK1/2 and PI3K/AKT pathways. Biochim. Biophys. Acta 2018, 1865, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; An, H.J.; Kim, T.H.; Kim, G.; Kang, H.; Heo, J.H.; Kwon, A.Y.; Kim, S. Fascin expression is inversely correlated with breast cancer metastasis suppressor 1 and predicts a worse survival outcome in node-negative breast cancer patients. J. Cancer 2017, 8, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, D. Hsp90: A global regulator of the genotype-to-phenotype map in cancers. Adv. Cancer Res. 2016, 129, 225–247. [Google Scholar] [PubMed]
- Calderwood, S.K.; Neckers, L. Hsp90 in Cancer: Transcriptional roles in the nucleus. Adv. Cancer Res. 2016, 129, 89–106. [Google Scholar] [PubMed]
- Aghazadeh, Y.; Papadopoulos, V. The role of the 14-3-3 protein family in health, disease, and drug development. Drug Discov. Today 2016, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Zuo, D.; Xue, B.; Muthuswamy, S.; Muller, W.J. A novel role for 14-3-3 sigma in regulating epithelial cell polarity. Genes Dev. 2010, 24, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.K.; Morrison, D.K. 14-3-3 Proteins: Diverse functions in cell proliferation and cancer progression. Semin. Cell Dev. Biol. 2011, 22, 681–687. [Google Scholar] [CrossRef] [PubMed]
| PRO-MMP-9/PRO-MMP-2 | PRO-MMP-9/MMP-9 | PRO-MMP-2/MMP-2 | MMP-9/MMP-2 | |
|---|---|---|---|---|
| Number of XY Pairs | 80 | 80 | 80 | 80 |
| Pearson r | 0.249 | 0.6805 | 0.6244 | 0.292 |
| 95% confidence interval | 0.03091 to 0.4444 | 0.5418 to 0.7831 | 0.4690 to 0.7423 | 0.07722 to 0.4809 |
| p value (two-tailed) | 0.0259 | p < 0.0001 | p < 0.0001 | 0.0086 |
| p value summary | * | *** | *** | ** |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | Yes | Yes |
| R squared | 0.06199 | 0.4631 | 0.3899 | 0.08529 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cara, G.; Marabeti, M.R.; Musso, R.; Riili, I.; Cancemi, P.; Pucci Minafra, I. New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations. Cells 2018, 7, 89. https://doi.org/10.3390/cells7080089
Di Cara G, Marabeti MR, Musso R, Riili I, Cancemi P, Pucci Minafra I. New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations. Cells. 2018; 7(8):89. https://doi.org/10.3390/cells7080089
Chicago/Turabian StyleDi Cara, Gianluca, Maria Rita Marabeti, Rosa Musso, Ignazio Riili, Patrizia Cancemi, and Ida Pucci Minafra. 2018. "New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations" Cells 7, no. 8: 89. https://doi.org/10.3390/cells7080089
APA StyleDi Cara, G., Marabeti, M. R., Musso, R., Riili, I., Cancemi, P., & Pucci Minafra, I. (2018). New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations. Cells, 7(8), 89. https://doi.org/10.3390/cells7080089





