Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Preparation of BMDCs
2.3. Flow Cytometry and Antibodies
2.4. Proliferation Assays and Cytokine Quantification
2.5. Protein Kinase Inhibition
2.6. Nuclear Extract Preparation
2.7. EMSA and Supershift Assays
2.8. Western Blots
2.9. Measurements of Cytokine Production
2.10. Real-Time PCR Analysis
2.11. Statistics
3. Results
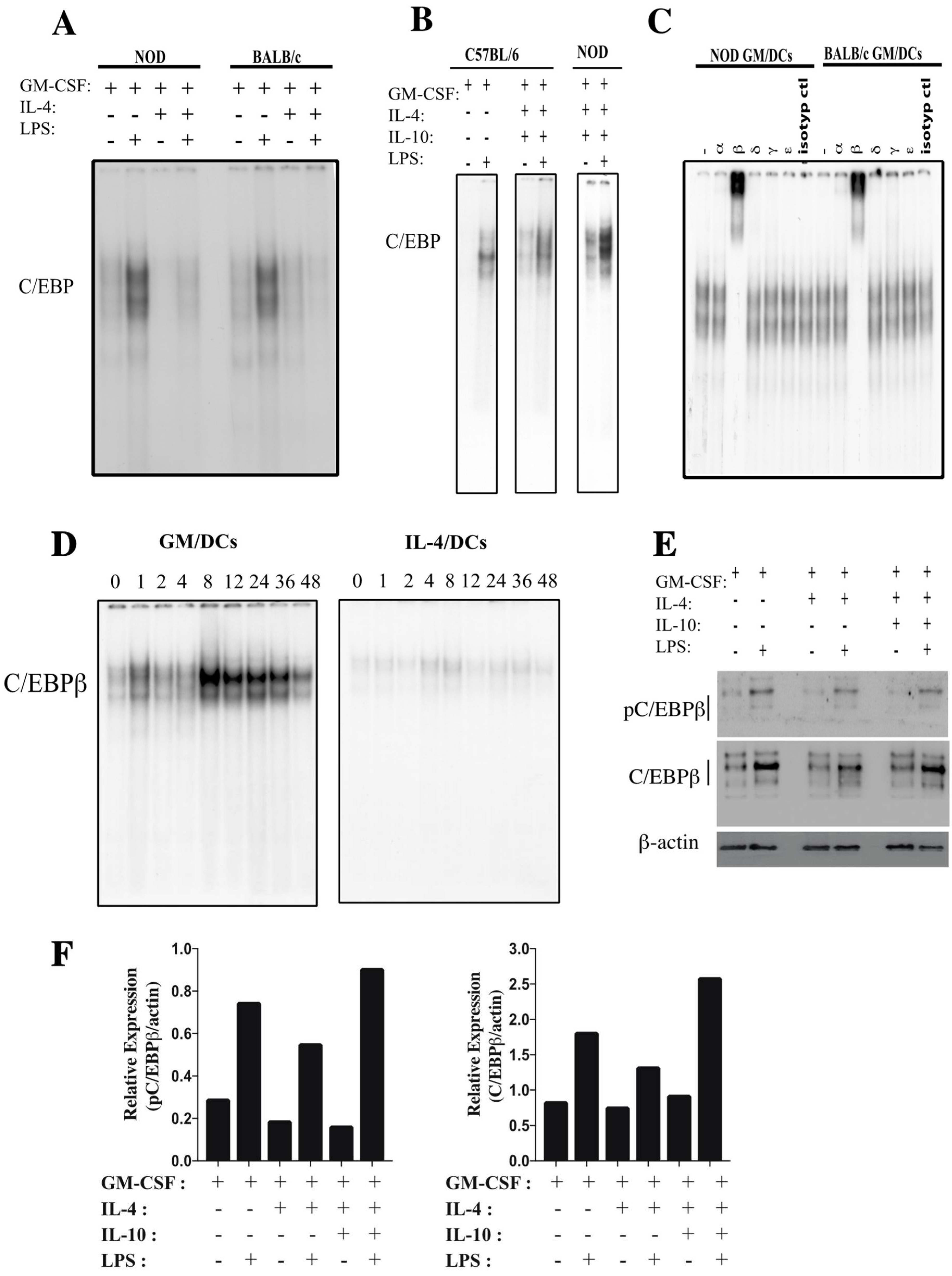
3.1. C/EBPβ DNA Binding Activity in GM/DCs and in IL-4/DCs
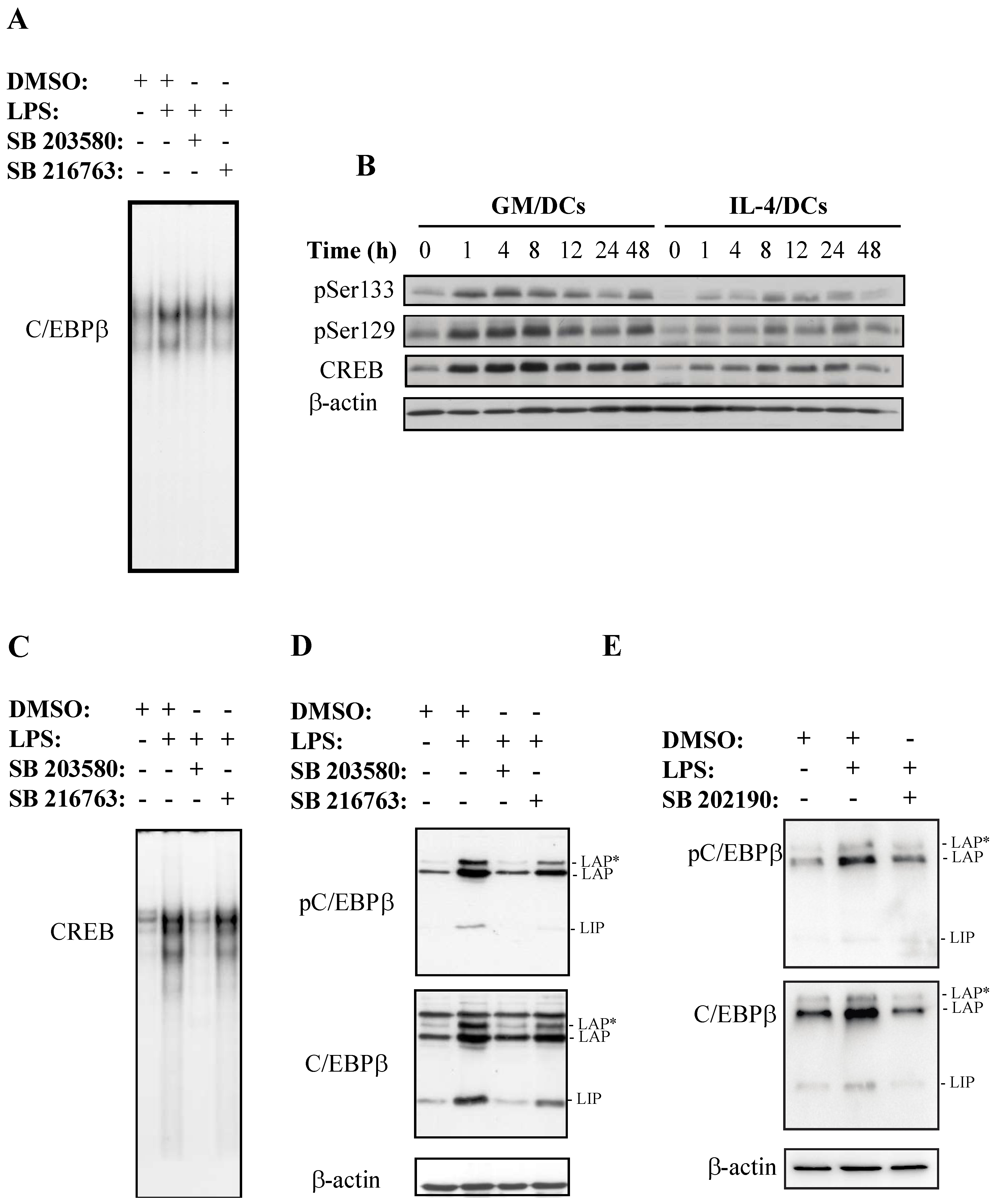
3.2. Effect of p38 MAPK and GSK3 Inhibitors on C/EBPβ and CREB Phosphorylation and DNA Binding Activity in GM/DCs
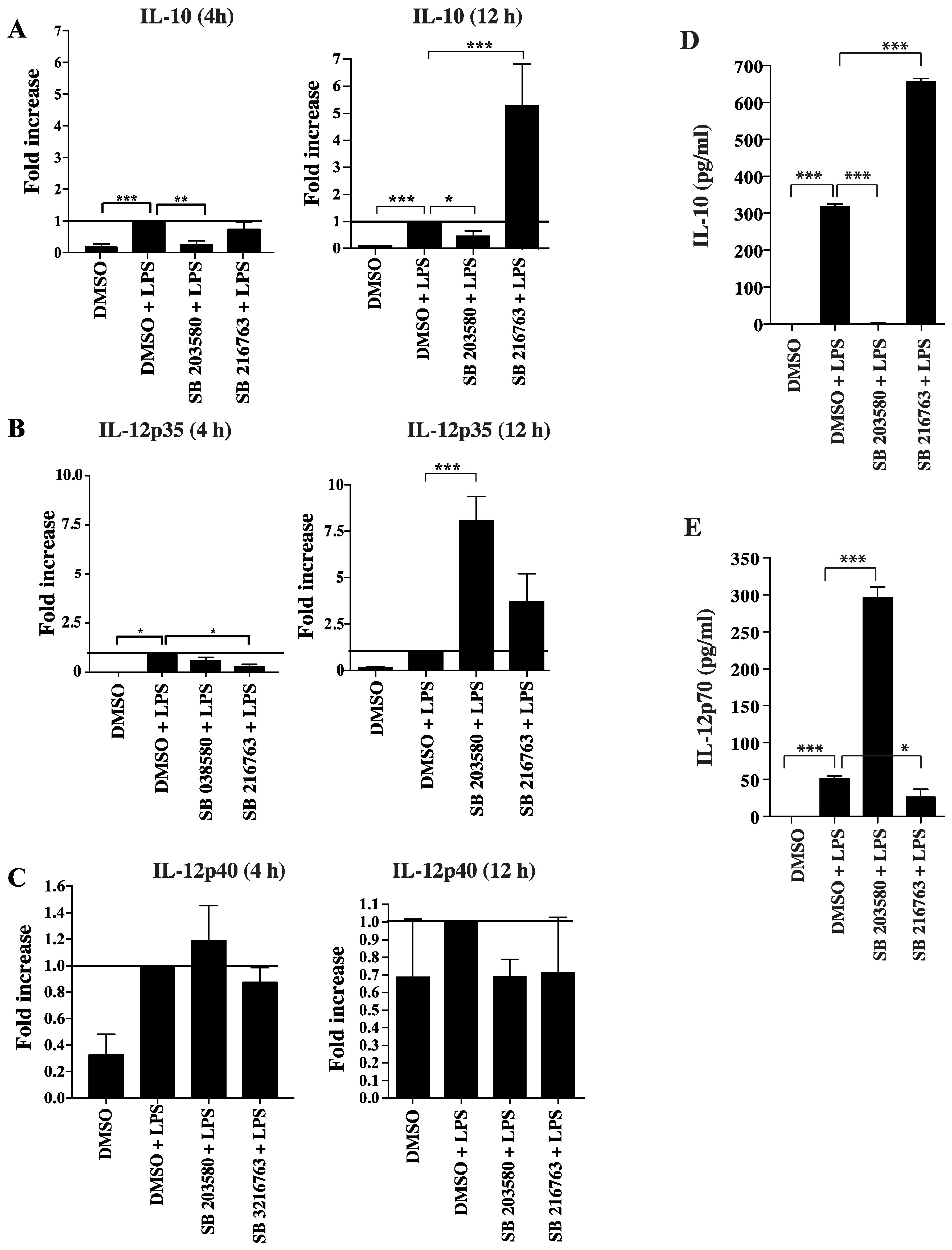
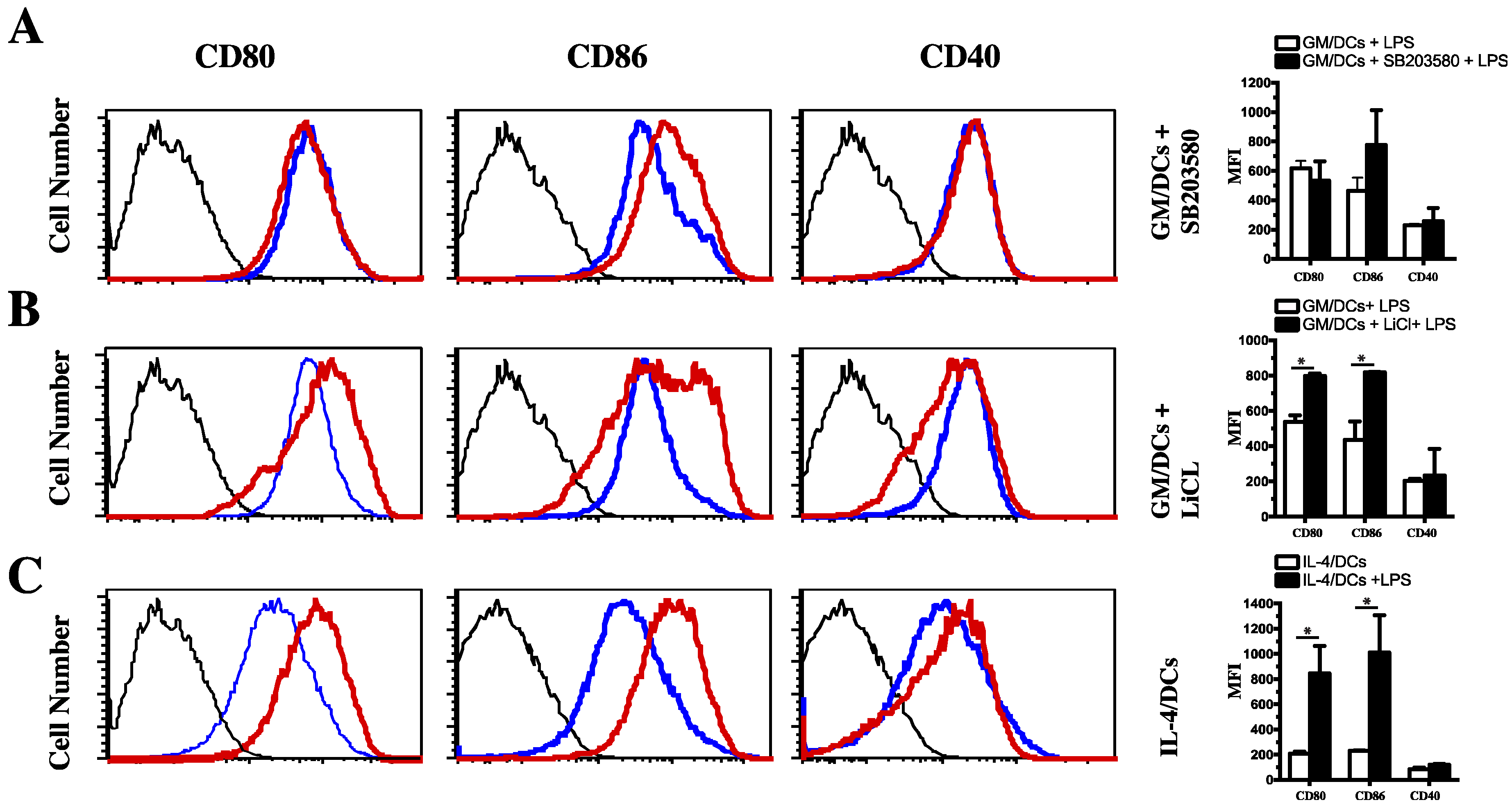
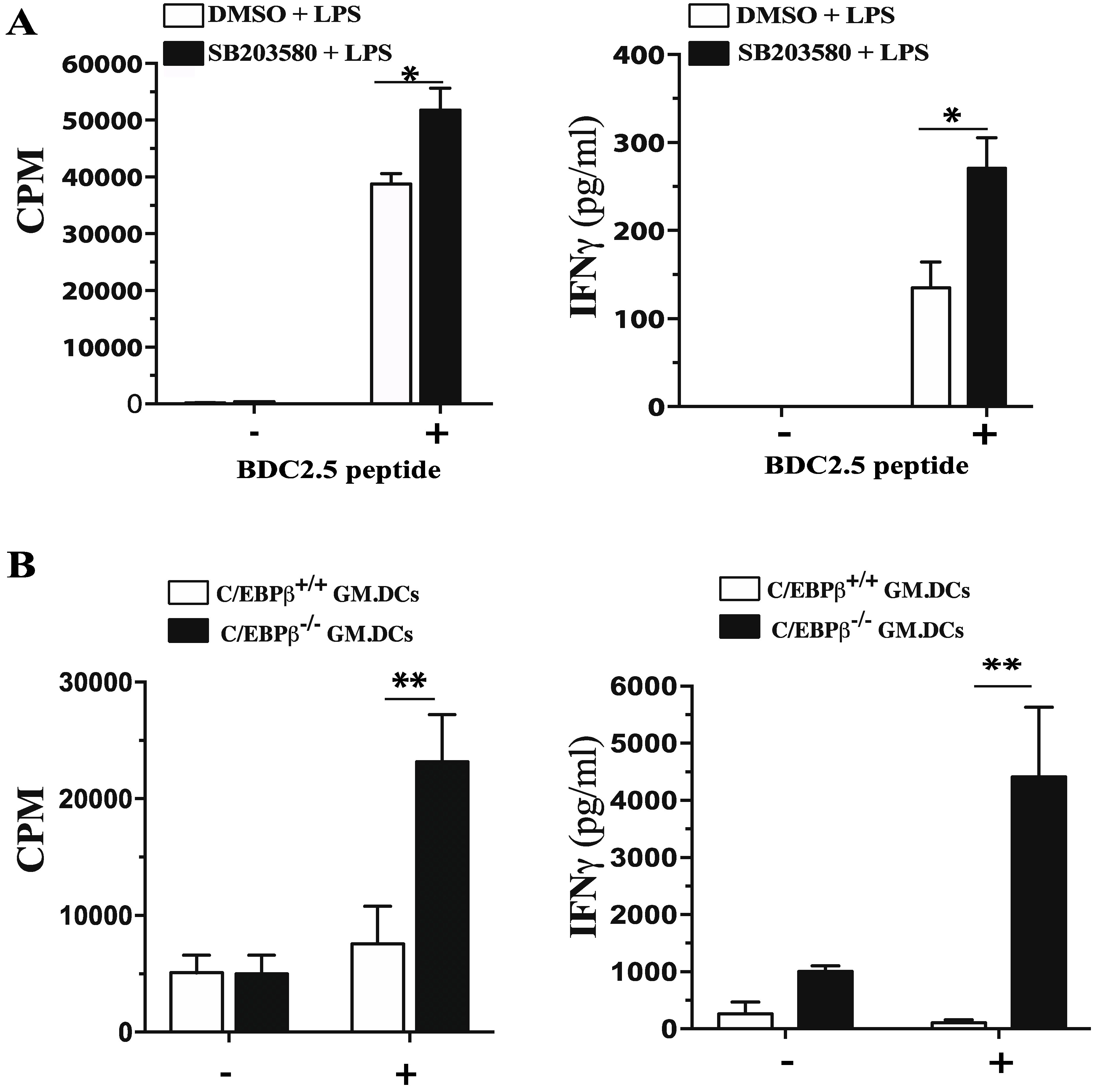
3.3. Functional Impact of Inhibition of C/EBPβ and CREB DNA Binding on Cytokine Production and Phenotype of Tolerogenic GM/DCs
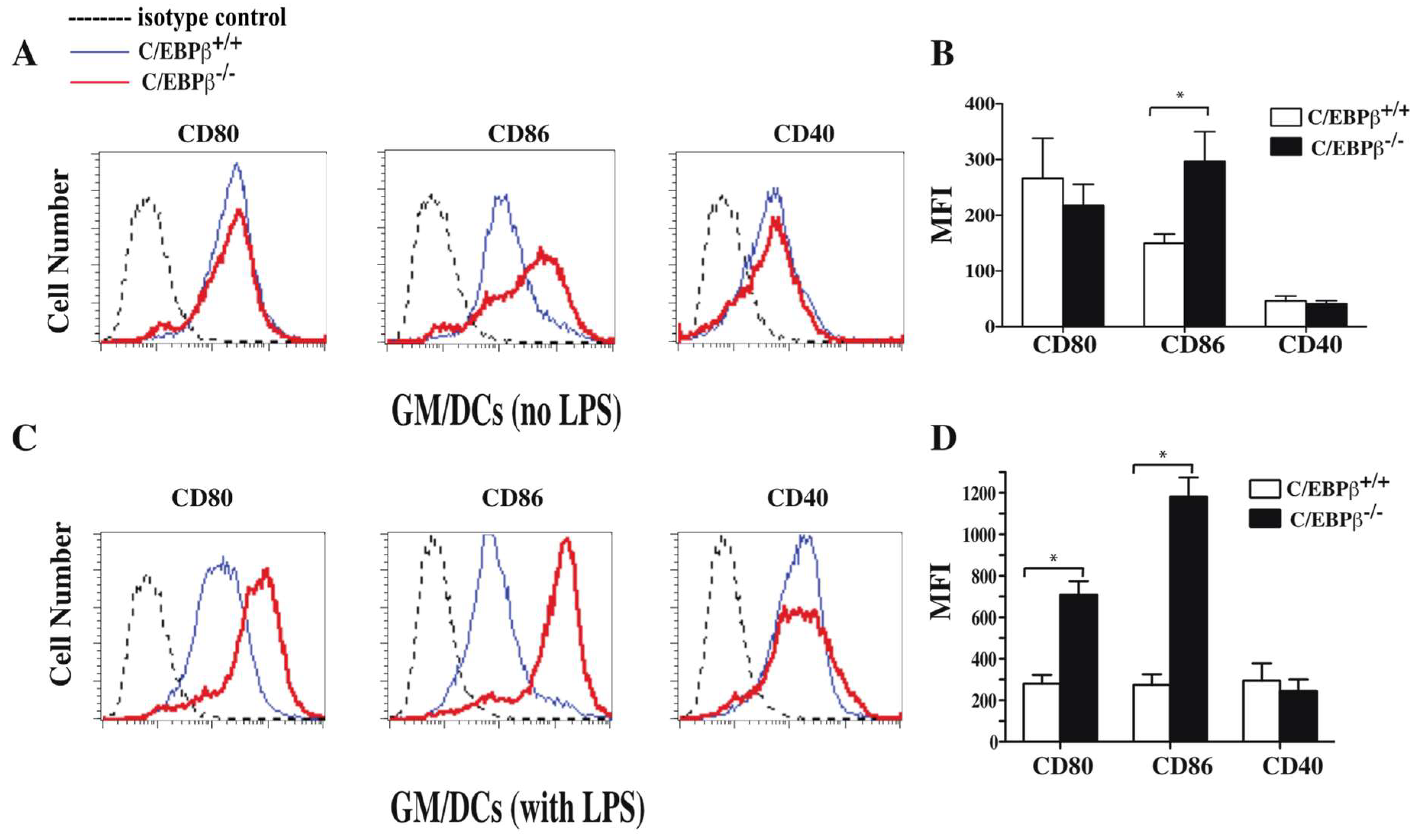
3.4. Effect of C/EBPβ Deficiency on the Phenotype of GM/DCs
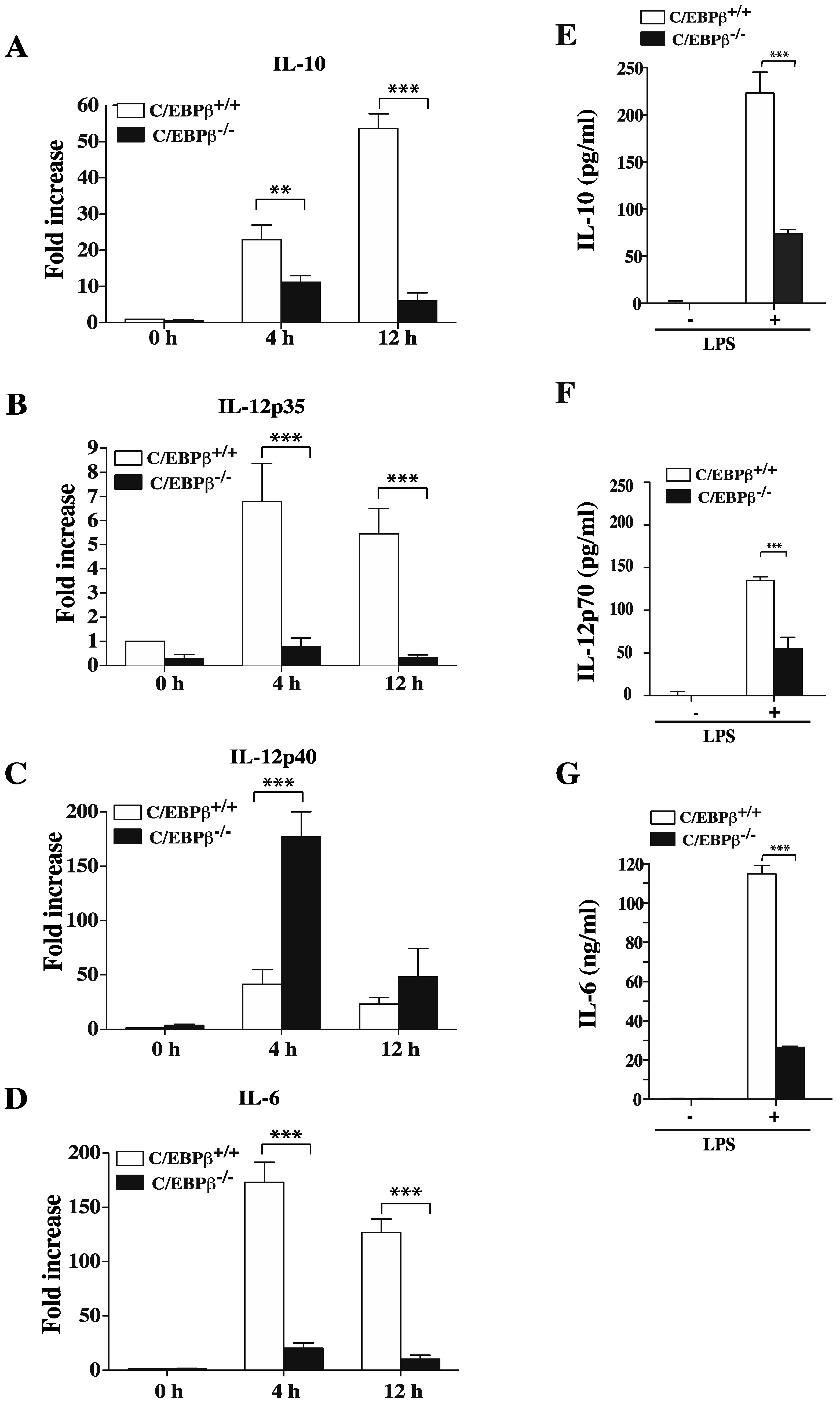
3.5. Role of C/EBPβ in Regulating GM/DCs Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Vliet, S.J.; den Dunnen, J.; Gringhuis, S.I.; Geijtenbeek, T.B.; van Kooyk, Y. Innate signaling and regulation of dendritic cell immunity. Curr. Opin. Immunol. 2007, 19, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- McColl, S.R. Chemokines and dendritic cells: A crucial alliance. Immunol. Cell Biol. 2002, 80, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Besin, G.; Gaudreau, S.; Menard, M.; Guindi, C.; Dupuis, G.; Amrani, A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes 2008, 57, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, S.; Guindi, C.; Menard, M.; Besin, G.; Dupuis, G.; Amrani, A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in nod mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 2007, 179, 3638–3647. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, S.; Guindi, C.; Menard, M.; Benabdallah, A.; Dupuis, G.; Amrani, A. GM-CSF induces bone marrow precursors of NOD mice to skew into tolerogenic dendritic cells that protect against diabetes. Cell Immunol. 2010, 265, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Guindi, C.; Menard, M.; Cloutier, A.; Gaudreau, S.; Besin, G.; Larivee, P.; McDonald, P.P.; Dupuis, G.; Amrani, A. Differential role of NF-kappaB, ERK1/2 and AP-1 in modulating the immunoregulatory functions of bone marrow-derived dendritic cells from nod mice. Cell. Immunol. 2012, 272, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef]
- Rutella, S.; Bonanno, G.; Pierelli, L.; Mariotti, A.; Capoluongo, E.; Contemi, A.M.; Ameglio, F.; Curti, A.; De Ritis, D.G.; Voso, M.T.; et al. Granulocyte colony-stimulating factor promotes the generation of regulatory dc through induction of IL-10 and IFN-Alpha. Eur. J. Immunol. 2004, 34, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Chorny, A.; Gonzalez-Rey, E.; Fernandez-Martin, A.; Pozo, D.; Ganea, D.; Delgado, M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 13562–13567. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.B.; Cheatem, D.M.; Sheng, J.R.; Vasu, C.; Prabhakar, B.S. GM-CSF-induced CD11C+CD8A—Dendritic cells facilitate FOXP3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 2009, 21, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Burkly, L.; Hession, C.; Ogata, L.; Reilly, C.; Marconi, L.A.; Olson, D.; Tizard, R.; Cate, R.; Lo, D. Expression of relb is required for the development of thymic medulla and dendritic cells. Nature 1995, 373, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Ouaaz, F.; Arron, J.; Zheng, Y.; Choi, Y.; Beg, A.A. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 2002, 16, 257–270. [Google Scholar] [CrossRef]
- Rescigno, M.; Martino, M.; Sutherland, C.L.; Gold, M.R.; Ricciardi-Castagnoli, P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1998, 188, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; D’Amico, A.; Winkel, K.D.; Suter, M.; Lo, D.; Shortman, K. Relb is essential for the development of myeloid-related CD8alpha- dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity 1998, 9, 839–847. [Google Scholar] [CrossRef]
- Loscher, C.E.; Draper, E.; Leavy, O.; Kelleher, D.; Mills, K.H.; Roche, H.M. Conjugated linoleic acid suppresses NF-kappaB activation and IL-12 production in dendritic cells through ERK-mediated IL-10 induction. J. Immunol. 2005, 175, 4990–4998. [Google Scholar] [CrossRef] [PubMed]
- Puig-Kroger, A.; Relloso, M.; Fernandez-Capetillo, O.; Zubiaga, A.; Silva, A.; Bernabeu, C.; Corbi, A.L. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood 2001, 98, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, D.; Majewski, P.; Schulte, L.C.; Korn, J.M.; Young, R.A.; Lander, E.S.; Hacohen, N. The plasticity of dendritic cell responses to pathogens and their components. Science 2001, 294, 870–875. [Google Scholar] [CrossRef]
- Langenkamp, A.; Messi, M.; Lanzavecchia, A.; Sallusto, F. Kinetics of dendritic cell activation: Impact on priming of Th1, Th2 and nonpolarized T cells. Nat. Immunol. 2000, 1, 311–316. [Google Scholar] [CrossRef]
- Yi, A.K.; Yoon, J.G.; Yeo, S.J.; Hong, S.C.; English, B.K.; Krieg, A.M. Role of mitogen-activated protein kinases in CPG DNA-mediated IL-10 and IL-12 production: Central role of extracellular signal-regulated kinase in the negative feedback loop of the CPG DNA-mediated Th1 response. J. Immunol. 2002, 168, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.; Agrawal, A.; Van Dyke, T.; Landreth, G.; McCauley, L.; Koh, A.; Maliszewski, C.; Akira, S.; Pulendran, B. A toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and C-FOS in dendritic cells. J. Immunol. 2004, 172, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Escors, D.; Lopes, L.; Lin, R.; Hiscott, J.; Akira, S.; Davis, R.J.; Collins, M.K. Targeting dendritic cell signaling to regulate the response to immunization. Blood 2008, 111, 3050–3061. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Dillon, S.; Denning, T.L.; Pulendran, B. ERK1-/- mice exhibit th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 2006, 176, 5788–5796. [Google Scholar] [CrossRef] [PubMed]
- Poli, V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998, 273, 29279–29282. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Foka, P. Ccaat/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Prosch, S.; Schenke-Layland, K.; Riese, U.; Gausmann, U.; Platzer, C. Camp-induced interleukin-10 promoter activation depends on ccaat/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 2003, 278, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Tseng, H.P.; Chen, L.C.; Chen, B.K.; Chang, W.C. Functional cooperation of simian virus 40 promoter factor 1 and ccaat/enhancer-binding protein beta and delta in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages. J. Immunol. 2003, 171, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, B.; Maritano, D.; Marthyn, P.; Righi, M.; Poli, V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mrnas in macrophages. J. Immunol. 2002, 168, 4055–4062. [Google Scholar] [CrossRef]
- Screpanti, I.; Romani, L.; Musiani, P.; Modesti, A.; Fattori, E.; Lazzaro, D.; Sellitto, C.; Scarpa, S.; Bellavia, D.; Lattanzio, G.; et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. Embo J. 1995, 14, 1932–1941. [Google Scholar] [CrossRef]
- Tanaka, T.; Akira, S.; Yoshida, K.; Umemoto, M.; Yoneda, Y.; Shirafuji, N.; Fujiwara, H.; Suematsu, S.; Yoshida, N.; Kishimoto, T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 1995, 80, 353–361. [Google Scholar] [CrossRef]
- Bradley, M.N.; Zhou, L.; Smale, S.T. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol. Cell. Biol. 2003, 23, 4841–4858. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Smeal, T.; Defize, L.H.; Angel, P.; Woodgett, J.R.; Karin, M.; Hunter, T. Activation of protein kinase c decreases phosphorylation of C-JUN at sites that negatively regulate its DNA-binding activity. Cell 1991, 64, 573–584. [Google Scholar] [CrossRef]
- Bullock, B.P.; Habener, J.F. Phosphorylation of the camp response element binding protein creb by camp-dependent protein kinase a and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry 1998, 37, 3795–3809. [Google Scholar] [CrossRef] [PubMed]
- Fiol, C.J.; Williams, J.S.; Chou, C.H.; Wang, Q.M.; Roach, P.J.; Andrisani, O.M. A secondary phosphorylation of CREB341 at SER129 is required for the camp-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J. Biol. Chem. 1994, 269, 32187–32193. [Google Scholar]
- Grimes, C.A.; Jope, R.S. Creb DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J. Neurochem. 2001, 78, 1219–1232. [Google Scholar] [CrossRef]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A creb-C/EBPbeta cascade induces m2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef]
- Park, J.M.; Greten, F.R.; Wong, A.; Westrick, R.J.; Arthur, J.S.; Otsu, K.; Hoffmann, A.; Montminy, M.; Karin, M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–creb and NF-kappaB as key regulators. Immunity 2005, 23, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef]
- Kim, J.W.; Tang, Q.Q.; Li, X.; Lane, M.D. Effect of phosphorylation and s-s bond-induced dimerization on DNA binding and transcriptional activation by C/EBPbeta. Proc. Natl. Acad. Sci. USA 2007, 104, 1800–1804. [Google Scholar] [CrossRef]
- Lobo, P.I.; Schlegel, K.H.; Bajwa, A.; Huang, L.; Okusa, M.D. Natural igm and tlr agonists switch murine splenic pan-b to “regulatory” cells that suppress ischemia-induced innate inflammation via regulating NKT-1 cells. Front. Immunol. 2017, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, A.; Guindi, C.; Larivee, P.; Dubois, C.M.; Amrani, A.; McDonald, P.P. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J. Immunol. 2009, 182, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Csoka, B.; Nemeth, Z.H.; Virag, L.; Gergely, P.; Leibovich, S.J.; Pacher, P.; Sun, C.X.; Blackburn, M.R.; Vizi, E.S.; Deitch, E.A.; et al. A2a adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 2007, 110, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamashita, N.; Matsuyama, T. Human peripheral blood monocyte-derived interleukin-10-induced semi-mature dendritic cells induce anergic CD4(+) and CD8(+) T cells via presentation of the internalized soluble antigen and cross-presentation of the phagocytosed necrotic cellular fragments. Cell. Immunol. 2002, 215, 186–194. [Google Scholar] [CrossRef]
- Steinbrink, K.; Graulich, E.; Kubsch, S.; Knop, J.; Enk, A.H. CD4(+) and CD8(+) anergic t cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 2002, 99, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, K.; Wolfl, M.; Jonuleit, H.; Knop, J.; Enk, A.H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997, 159, 4772–4780. [Google Scholar] [PubMed]
- Wakkach, A.; Fournier, N.; Brun, V.; Breittmayer, J.P.; Cottrez, F.; Groux, H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003, 18, 605–617. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Gronborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of ccaat enhancer-binding protein beta by mapk and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef]
- Dai, J.; Kumbhare, A.; Youssef, D.; Yao, Z.Q.; McCall, C.E.; El Gazzar, M. Expression of C/EBPbeta in myeloid progenitors during sepsis promotes immunosuppression. Mol. Immunol. 2017, 91, 165–172. [Google Scholar] [CrossRef]
- Rehm, A.; Gatjen, M.; Gerlach, K.; Scholz, F.; Mensen, A.; Gloger, M.; Heinig, K.; Lamprecht, B.; Mathas, S.; Begay, V.; et al. Dendritic cell-mediated survival signals in EMU-MYC B-cell lymphoma depend on the transcription factor C/EBPbeta. Nat. Commun. 2014, 5, 5057. [Google Scholar] [CrossRef]
- Poligone, B.; Weaver, D.J., Jr.; Sen, P.; Baldwin, A.S., Jr.; Tisch, R. Elevated NF-kappab activation in nonobese diabetic mouse dendritic cells results in enhanced apc function. J. Immunol. 2002, 168, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Feili-Hariri, M.; Morel, P.A. Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin. Immunol. 2001, 98, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Piwien-Pilipuk, G.; MacDougald, O.; Schwartz, J. Dual regulation of phosphorylation and dephosphorylation of C/EBPbeta modulate its transcriptional activation and DNA binding in response to growth hormone. J. Biol. Chem. 2002, 277, 44557–44565. [Google Scholar] [CrossRef] [PubMed]
- Niehof, M.; Manns, M.P.; Trautwein, C. Creb controls LAP/C/EBP beta transcription. Mol. Cell. Biol. 1997, 17, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Gotschel, F.; Kern, C.; Lang, S.; Sparna, T.; Markmann, C.; Schwager, J.; McNelly, S.; von Weizsacker, F.; Laufer, S.; Hecht, A.; et al. Inhibition of GSK3 differentially modulates NF-kappaB, creb, AP-1 and beta-catenin signaling in hepatocytes, but fails to promote TNF-alpha-induced apoptosis. Exp. Cell Res. 2008, 314, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Jope, R.S. Differential regulation of stat family members by glycogen synthase kinase-3. J. Biol. Chem. 2008, 283, 21934–21944. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; De Haro, S.A.; Master, S.S.; Keane, J.; Roberts, E.A.; Delgado, M.; Deretic, V. T helper 2 cytokines inhibit autophagic control of intracellular mycobacterium tuberculosis. Immunity 2007, 27, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Nagai, S.; Kondo, S.; Mizuno, S.; Nakamura, K.; Tanabe, M.; Takeuchi, T.; Matsuda, S.; Koyasu, S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 2008, 112, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brown, J.; Garcia, C.A.; Tang, Y.; Benakanakere, M.R.; Greenway, T.; Alard, P.; Kinane, D.F.; Martin, M. The role of glycogen synthase kinase 3 in regulating ifn-beta-mediated IL-10 production. J. Immunol. 2011, 186, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, E.; Conzelmann, M.; Maraskovsky, E.; Hess, M.; Kirsch, M.; Giese, T.; Ho, A.D.; Zoller, M.; Dreger, P.; Luft, T. GSK-3 mediates differentiation and activation of proinflammatory dendritic cells. Blood 2007, 109, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Lee, Y.L.; Yang, Y.Y.; Shih, N.Y.; Ho, C.C.; Wu, Y.C.; Huang, T.S.; Huang, M.C.; Liu, H.C.; Shen, W.W.; et al. Modulation of the development of human monocyte-derived dendritic cells by lithium chloride. J. Cell. Physiol. 2011, 226, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Zheng, X.; Lian, D.; Zhang, Z.X.; Ge, W.; Yang, J.; Vladau, C.; Suzuki, M.; Chen, D.; et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J. Immunol. 2007, 178, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Shih, V.F.; Davis-Turak, J.; Macal, M.; Huang, J.Q.; Ponomarenko, J.; Kearns, J.D.; Yu, T.; Fagerlund, R.; Asagiri, M.; Zuniga, E.I.; et al. Control of relb during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat. Immunol. 2012, 13, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Ardeshna, K.M.; Pizzey, A.R.; Devereux, S.; Khwaja, A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 2000, 96, 1039–1046. [Google Scholar] [PubMed]
- Zaal, A.; Dieker, M.; Oudenampsen, M.; Turksma, A.W.; Lissenberg-Thunnissen, S.N.; Wouters, D.; van Ham, S.M.; Ten Brinke, A. Anaphylatoxin c5a regulates 6-sulfo-lacnac dendritic cell function in human through crosstalk with toll-like receptor-induced creb signaling. Front. Immunol. 2017, 8, 818. [Google Scholar] [CrossRef]
- Armbruster, N.S.; Richardson, J.R.; Schreiner, J.; Klenk, J.; Gunter, M.; Kretschmer, D.; Poschel, S.; Schenke-Layland, K.; Kalbacher, H.; Clark, K.; et al. PSM peptides of staphylococcus aureus activate the p38-creb pathway in dendritic cells, thereby modulating cytokine production and t cell priming. J. Immunol. 2016, 196, 1284–1292. [Google Scholar] [CrossRef]
- Marigo, I.; Bosio, E.; Solito, S.; Mesa, C.; Fernandez, A.; Dolcetti, L.; Ugel, S.; Sonda, N.; Bicciato, S.; Falisi, E.; et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 2010, 32, 790–802. [Google Scholar] [CrossRef]
- Diana, J.; Simoni, Y.; Furio, L.; Beaudoin, L.; Agerberth, B.; Barrat, F.; Lehuen, A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 2013, 19, 65–73. [Google Scholar] [CrossRef]
- Li, Q.; Xu, B.; Michie, S.A.; Rubins, K.H.; Schreriber, R.D.; McDevitt, H.O. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12439–12444. [Google Scholar] [CrossRef] [PubMed]
- Zerif, E.; Maalem, A.; Gaudreau, S.; Guindi, C.; Ramzan, M.; Veroneau, S.; Gris, D.; Stankova, J.; Rola-Pleszczynski, M.; Mourad, W.; et al. Constitutively active stat5b signaling confers tolerogenic functions to dendritic cells of nod mice and halts diabetes progression. J. Autoimmun. 2017, 76, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Anguita, J.; Hoffmeyer, A.; Zapton, T.; Ihle, J.N.; Fikrig, E.; Rincon, M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 2000, 13, 805–815. [Google Scholar] [CrossRef]
- Jankovic, D.; Kullberg, M.C.; Hieny, S.; Caspar, P.; Collazo, C.M.; Sher, A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity 2002, 16, 429–439. [Google Scholar] [CrossRef]
- Rincon, M.; Anguita, J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 1997, 185, 461–469. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guindi, C.; Cloutier, A.; Gaudreau, S.; Zerif, E.; McDonald, P.P.; Tatsiy, O.; Asselin, C.; Dupuis, G.; Gris, D.; Amrani, A. Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells. Cells 2018, 7, 256. https://doi.org/10.3390/cells7120256
Guindi C, Cloutier A, Gaudreau S, Zerif E, McDonald PP, Tatsiy O, Asselin C, Dupuis G, Gris D, Amrani A. Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells. Cells. 2018; 7(12):256. https://doi.org/10.3390/cells7120256
Chicago/Turabian StyleGuindi, Chantal, Alexandre Cloutier, Simon Gaudreau, Echarki Zerif, Patrick P. McDonald, Olga Tatsiy, Claude Asselin, Gilles Dupuis, Denis Gris, and Abdelaziz Amrani. 2018. "Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells" Cells 7, no. 12: 256. https://doi.org/10.3390/cells7120256
APA StyleGuindi, C., Cloutier, A., Gaudreau, S., Zerif, E., McDonald, P. P., Tatsiy, O., Asselin, C., Dupuis, G., Gris, D., & Amrani, A. (2018). Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells. Cells, 7(12), 256. https://doi.org/10.3390/cells7120256





