Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD)
Abstract
:1. Introduction
2. AMD and Bruch’s Membrane Pathology
3. Induced Pluripotent Stem Cells
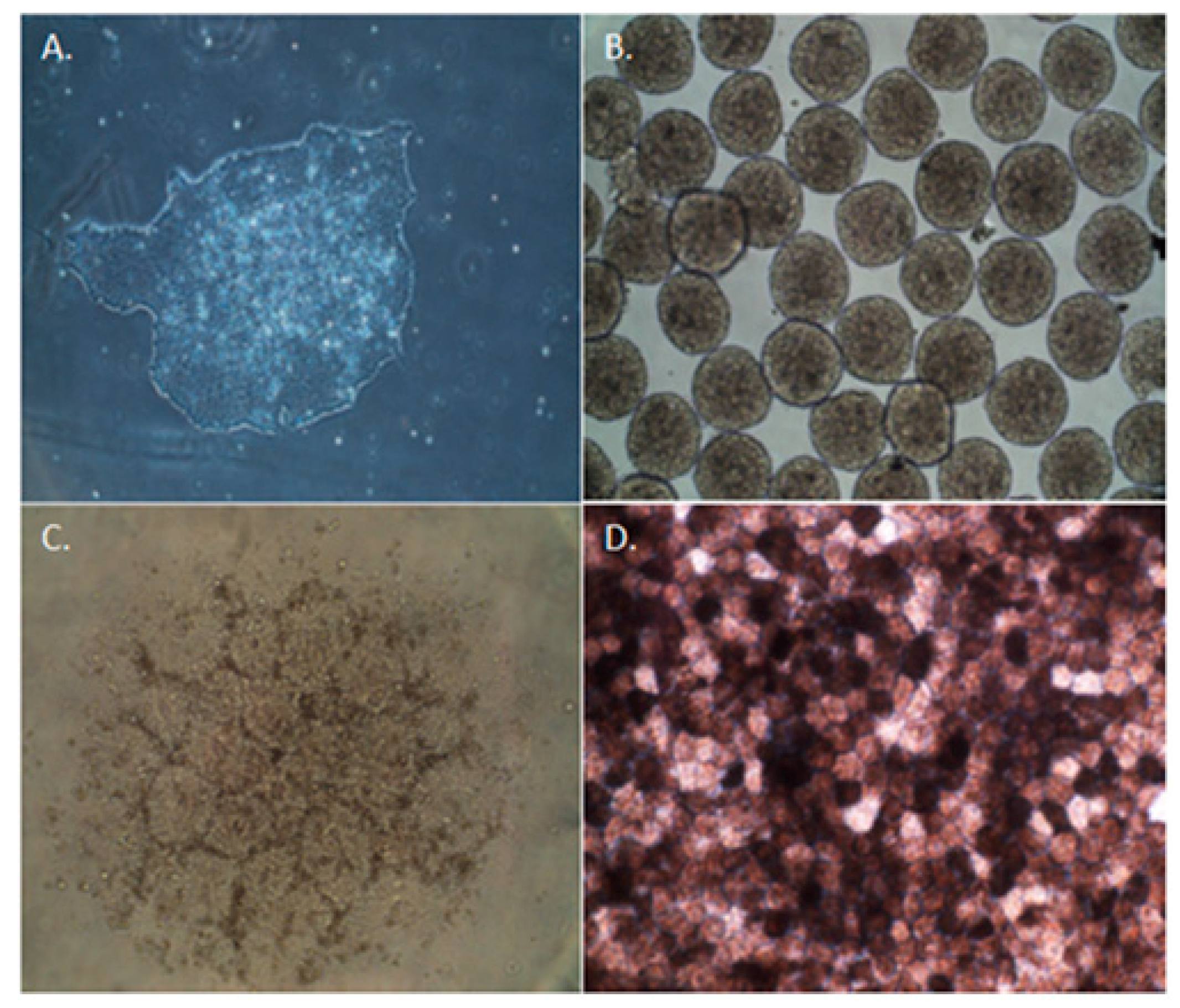
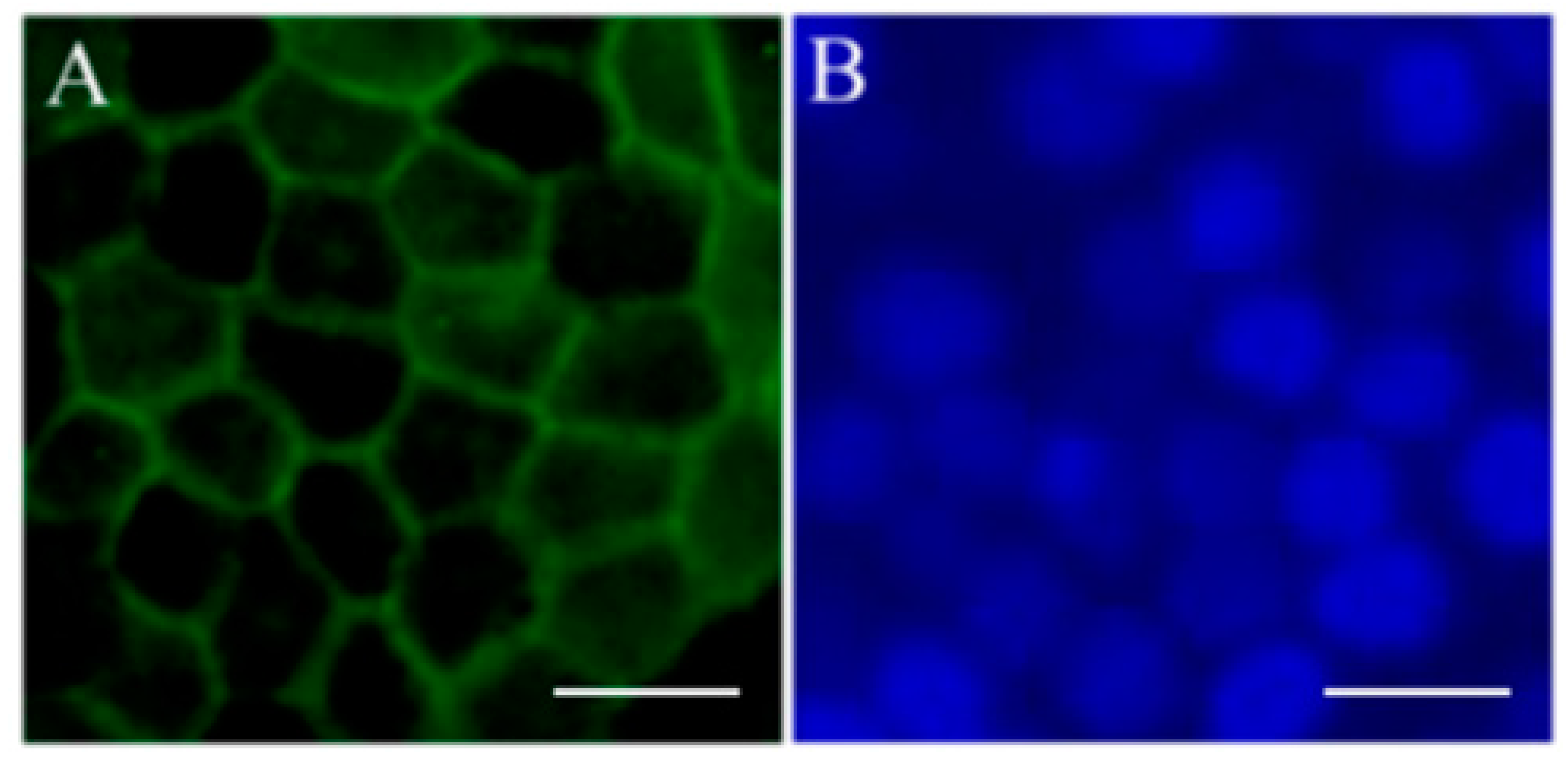
4. Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium
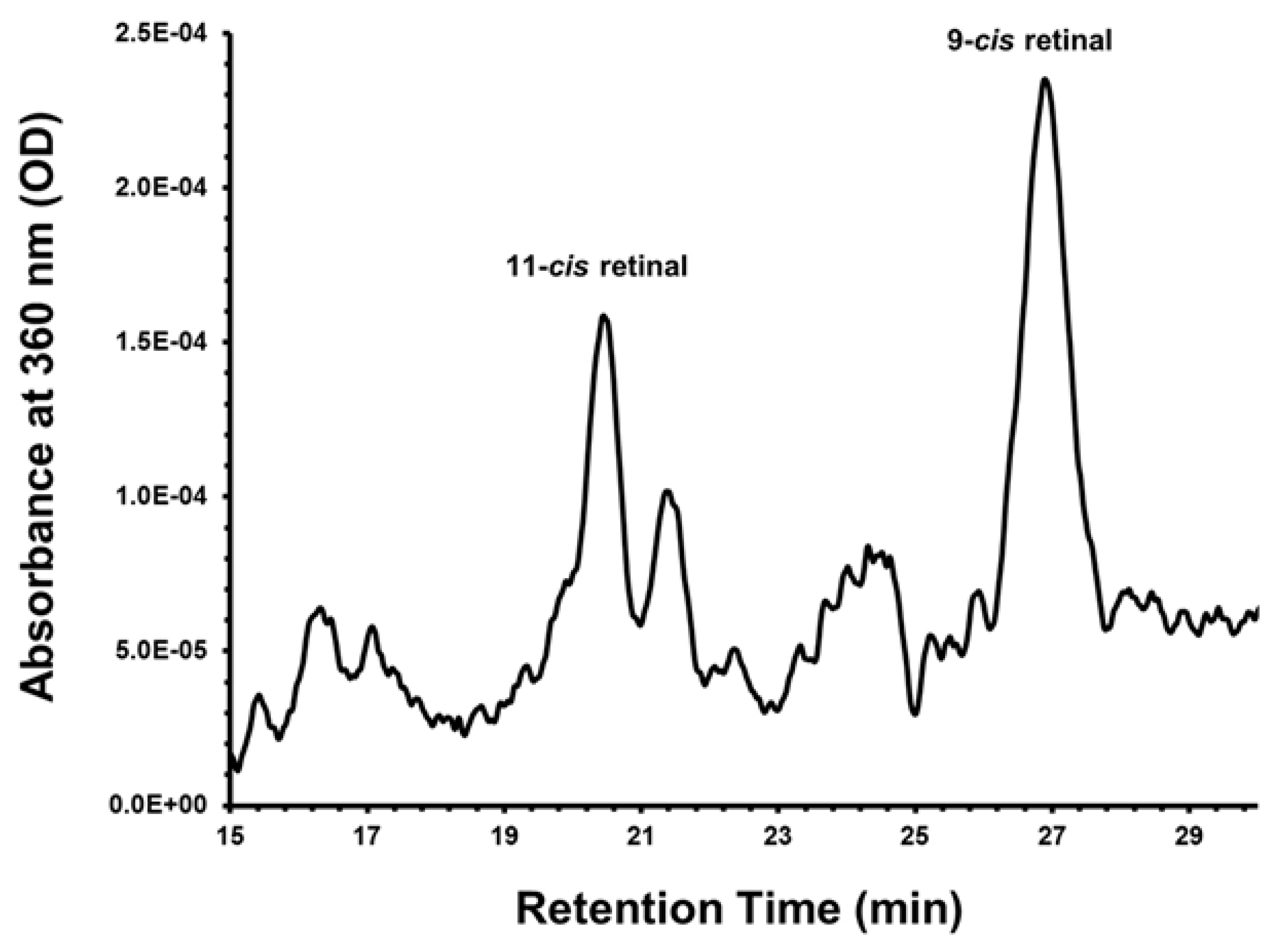
5. Use of iPSC-Derived RPE to Model Age-Related Macular Degeneration
6. Current Status of iPSC Therapies for the Treatment of Retinal Disorders
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| iPSC | Induced pluripotent stem cell |
| AMD | Age-related macular degeneration |
| RPE | Retinal pigment epithelium |
| ESC | Embryonic stem cell |
| BM | Bruch’s membrane |
| ZO-1 | Zonula occludens protein-1 |
| GA | Geographic atrophy |
| HLA | Human leukocyte antigen |
| MHC | Major histocompatibility |
| FDA | U.S. Food and Drug Administration |
| ECM | Extracellular matrix |
| VEGF | Vascular endothelial growth factor |
| SOX2 | (Sex determining region Y)-box 2 |
| OCT3/4 | Octamer-binding transcription factor 3/4 |
| Klf4 | Kruppel-like factor 4 |
| c-MYC | Regulator gene that codes for a transcription factor; Myc |
| BEST1 | RPE-specific protein bestrophin-1 |
References
- Cheng, S.K.; Park, E.Y.; Pehar, A.; Rooney, A.C.; Gallicano, G.I. Current Progress of Human Trials Using Stem Cell Therapy as a Treatment for Diabetes Mellitus. Am. J. Stem Cells 2016, 5, 74–86. [Google Scholar] [PubMed]
- Kumar, A.; Narayanan, K.; Chaudhary, R.K.; Mishra, S.; Kumar, S.; Vinoth, K.J.; Padmanabhan, P.; Gulyas, B. Current Perspective of Stem Cell Therapy in Neurodegenerative and Metabolic Diseases. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-Up of Two Open-Label Phase 1/2 Studies. Lancet 2014, 385, 509–516. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report. Lancet 2012, 379, 713–720. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible Immunogenicity of Terminally Differentiated Cells Derived from Induced Pluripotent or Embryonic Stem Cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Morgan, J.W.; Mostoslavsky, G.; Rodrigues, N.P.; Boyd, A.S. Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell 2013, 12, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nagata, N.; Kurokawa, H.; Yamanaka, S. iPS Cells: A Game Changer for Future Medicine. EMBO J. 2014, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Yamanaka, S. iPS Cell Technologies: Significance and Applications to CNS Regeneration and Disease. Mol. Brain 2014, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Yamanaka, S. The Use of Induced Pluripotent Stem Cells in Drug Development. Clin. Pharmacol. Ther. 2011, 89, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Bharti, K. Looking into the Future: Using Induced Pluripotent Stem Cells to Build Two and Three Dimensional Ocular Tissue for Cell Therapy and Disease Modeling. Brain Res. 2016, 1638 Pt A, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chan, L.; Nguyen, H.V.; Tsang, S.H. Personalized Medicine: Cell and Gene Therapy Based on Patient-Specific iPSC-Derived Retinal Pigment Epithelium Cells. Adv. Exp. Med. Biol. 2016, 854, 549–555. [Google Scholar] [PubMed]
- Cai, H.; Del Priore, L.V. Bruch Membrane Aging Alters the Gene Expression Profile of Human Retinal Pigment Epithelium. Curr. Eye Res. 2006, 31, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-Related Macular Degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Bressler, S.B.; Fine, S.L. Age-Related Macular Degeneration. Surv. Ophthalmol. 1988, 32, 375–413. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B.; Seddon, J.M.; Gragoudas, E.S.; Jacobson, L.P. Drusen Characteristics in Patients with Exudative Versus Non-Exudative Age-Related Macular Degeneration. Retina 1988, 8, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.M.; Marmor, M.F. The Retinal Pigment Epithelium; Harvard University Press: Cambridge, UK, 1979. [Google Scholar]
- Miller, F.S., III; Bunt-Milam, A.H.; Kalina, R.E. Clinical-Ultrastructural Study of Thioridazine Retinopathy. Ophthalmology 1982, 89, 1478–1488. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, Y.; Kaiser-Kupfer, M.I. Experimental Model of Gyrate Atrophy in Animals. Ophthalmology 1981, 88, 331–335. [Google Scholar] [CrossRef]
- Henkind, P.; Gartner, S. The Relationship between Retinal Pigment Epithelium and the Choriocapillaris. Trans. Ophthalmol. Soc. U. K. 1983, 103 Pt 4, 444–447. [Google Scholar] [PubMed]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE Destruction Causes Choriocapillary Atrophy. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar]
- Takeuchi, M.; Itagaki, T.; Okuma, H.; Takahashi, K.; Uyama, M. Retinal Degeneration after Intravitreal Injection of Ornithine. 2. Late Change after Administration. Nippon Ganka Gakkai Zasshi 1992, 96, 161–168. [Google Scholar] [PubMed]
- Takeuchi, M.; Itagaki, T.; Takahashi, K.; Ohkuma, H.; Uyama, M. Changes in the Intermediate Stage of Retinal Degeneration after Intravitreal Injection of Ornithine. Nippon Ganka Gakkai Zasshi 1993, 97, 17–28. [Google Scholar] [PubMed]
- Nasir, M.A.; Sugino, I.; Zarbin, M.A. Decreased Choriocapillaris Perfusion Following Surgical Excision of Choroidal Neovascular Membranes in Age-Related Macular Degeneration. Br. J. Ophthalmol. 1997, 81, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.S.; Del Priore, L.V.; Smith, M.E.; Feiner, M.A.; Kaplan, H.J. Postoperative Abnormalities of the Choriocapillaris in Exudative Age-Related Macular Degeneration. Br. J. Ophthalmol. 1996, 80, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.N.; Del Priore, L.V.; Kaplan, H.J. Choriocapillaris Atrophy after Submacular Surgery in Presumed Ocular Histoplasmosis Syndrome. Arch. Ophthalmol. 1995, 113, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.S.; Zhang, X.G.; Panozzo, G.; Sugino, I.K.; Zarbin, M.A. Clinicopathologic Correlation of Localized Retinal Pigment Epithelium Debridement. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1094–1109. [Google Scholar]
- Del Priore, L.V.; Hornbeck, R.; Kaplan, H.J.; Jones, Z.; Valentino, T.L.; Mosinger-Ogilvie, J.; Swinn, M. Debridement of the Pig Retinal Pigment Epithelium In Vivo. Arch. Ophthalmol. 1995, 113, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Valentino, T.L.; Kaplan, H.J.; Del Priore, L.V.; Fang, S.R.; Berger, A.; Silverman, M.S. Retinal Pigment Epithelial Repopulation in Monkeys after Submacular Surgery. Arch. Ophthalmol. 1995, 113, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, L.V.; Kaplan, H.J.; Hornbeck, R.; Jones, Z.; Swinn, M. Retinal Pigment Epithelial Debridement as a Model for the Pathogenesis and Treatment of Macular Degeneration. Am. J. Ophthalmol. 1996, 122, 629–643. [Google Scholar] [CrossRef]
- Fields, M.A.; Hwang, J.; Gong, J.; Cai, H.; Del Priore, L.V. The Eye as a Target Organ for Stem Cell Therapy. In Stem Cell Biology and Regenerative Medicine in Ophthalmology; Stephen, H.T., Ed.; Springer: New York, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Amadio, M.; Govoni, S.; Pascale, A. Targeting VEGF in Eye Neovascularization: What’s New?: A Comprehensive Review on Current Therapies and Oligonucleotide-Based Interventions under Development. Pharmacol. Res. 2016, 103, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Pauleikhoff, D.; Harper, C.A.; Marshall, J.; Bird, A.C. Aging Changes in Bruch’s Membrane. A Histochemical and Morphologic Study. Ophthalmology 1990, 97, 171–178. [Google Scholar] [CrossRef]
- Sarks, S.H.; Arnold, J.J.; Killingsworth, M.C.; Sarks, J.P. Early Drusen Formation in the Normal and Aging Eye and Their Relation to Age Related Maculopathy: A Clinicopathological Study. Br. J. Ophthalmol. 1999, 83, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Spraul, C.W.; Lang, G.E.; Grossniklaus, H.E.; Lang, G.K. Histologic and Morphometric Analysis of the Choroid, Bruch’s Membrane, and Retinal Pigment Epithelium in Postmortem Eyes with Age-Related Macular Degeneration and Histologic Examination of Surgically Excised Choroidal Neovascular Membranes. Surv. Ophthalmol. 1999, 44 (Suppl. S1), S10–S32. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Del Priore, L.; Zarbin, M.A. Drusen in Age-Related Macular Degeneration: Pathogenesis, Natural Course, and Laser Photocoagulation-Induced Regression. Surv. Ophthalmol. 1999, 44, 1–29. [Google Scholar] [CrossRef]
- Mullins, R.F.; Aptsiauri, N.; Hageman, G.S. Structure and Composition of Drusen Associated with Glomerulonephritis: Implications for the Role of Complement Activation in Drusen Biogenesis. Eye 2001, 15 Pt 3, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Johnson, M.; Rudolf, M.; Huang, J.D. The Oil Spill in Ageing Bruch Membrane. Br. J. Ophthalmol. 2011, 95, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.E.; Konstas, A.G.; Reid, G.G.; Edwards, J.G.; Lee, W.R. Type IV Collagen and Laminin in Bruch’s Membrane and Basal Linear Deposit in the Human Macula. Br. J. Ophthalmol. 1992, 76, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.B. Extracellular Matrix Alterations Precede Vascularization of the Retinal Pigment Epithelium in Dystrophic Rats. Curr. Eye Res. 1989, 8, 907–921. [Google Scholar] [PubMed]
- Del Priore, L.V.; Tezel, T.H. Reattachment Rate of Human Retinal Pigment Epithelium to Layers of Human Bruch’s Membrane. Arch. Ophthalmol. 1998, 116, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Del Priore, L.V. Reattachment of Cultured Human Retinal Pigment Epithelium to Extracellular Matrix and Human Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1110–1118. [Google Scholar]
- Tezel, T.H.; Del Priore, L.V. Reattachment to a Substrate Prevents Apoptosis of Human Retinal Pigment Epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tezel, T.H.; Del Priore, L.V. TGF Beta Secretion Modulates the Density-Dependent Growth of Pig Retinal Pigment Epithelium In Vitro. Ophthalmic. Res. 1999, 31, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, V.K.; Sugino, I.K.; van Patten, Y.; Shah, S.; Zarbin, M.A. Impaired RPE Survival on Aged Submacular Human Bruch’s Membrane. Exp. Eye Res. 2005, 80, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yagi, F.; Cheewatrakoolpong, N.; Sugino, I.K.; Zarbin, M.A. Short-Term Study of Retinal Pigment Epithelium Sheet Transplants onto Bruch’s Membrane. Exp. Eye Res. 2004, 78, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Tezel, T.H.; Del Priore, L.V. Repopulation of Different Layers of Host Human Bruch’s Membrane by Retinal Pigment Epithelial Cell Grafts. Investig. Ophthalmol. Vis. Sci. 1999, 40, 767–774. [Google Scholar]
- Castellarin, A.A.; Sugino, I.K.; Vargas, J.A.; Parolini, B.; Lui, G.M.; Zarbin, M.A. In Vitro Transplantation of Fetal Human Retinal Pigment Epithelial Cells onto Human Cadaver Bruch’s Membrane. Exp. Eye Res. 1998, 66, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.F.; Cai, H.; Tezel, T.H.; Fields, M.A.; Del Priore, L.V. Reengineering Human Bruch’s Membrane Increases Rod Outer Segment Phagocytosis by Human Retinal Pigment Epithelium. Transl. Vis. Sci. Technol. 2015, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Paik, D.C.; Del Priore, L.V.; Burch, R.L.; Gaillard, E.R. Nitrite-Modified Extracellular Matrix Proteins Deleteriously Affect Retinal Pigment Epithelial Cell Function and Viability: A Comparison Study with Nonenzymatic Glycation Mechanisms. Curr. Eye Res. 2005, 30, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.A.; Cai, H.; Bowrey, H.E.; Moreira, E.F.; Gooz, M.B.; Kunchithapautham, K.; Gong, J.; Vought, E.; Del Priore, L.V. Nitrite Modification of Extracellular Matrix Alters CD46 Expression and VEGF Release in Human Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, B.J.; Fan, W.; Zheng, J.J.; Cai, H.; Del Priore, L.V.; Bora, N.S.; Kaplan, H.J. Novel Role for a Complement Regulatory Protein (CD46) in Retinal Pigment Epithelial Adhesion. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3669–3674. [Google Scholar] [CrossRef]
- Tezel, T.H.; Del Priore, L.V.; Kaplan, H.J. Reengineering of Aged Bruch’s Membrane to Enhance Retinal Pigment Epithelium Repopulation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Alamein, M.; Skabo, S.; Stephens, S.; Bourke, R.; Heiner, P.; Liu, Q. Primordium of an Artificial Bruch’s Membrane Made of Nanofibers for Engineering of Retinal Pigment Epithelium Cell Monolayers. Acta Biomater. 2013, 9, 9414–9422. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.; Thomas, P.; Thomas, B.; Ribeiro, R.; Hu, Y.; Brant, R.; Ahuja, A.; Zhu, D.; Liu, L.; Koss, M.; et al. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5087–5096. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.P.; Petrus-Reurer, S.; Antonsson, L.; Stenfelt, S.; Bartuma, H.; Panula, S.; Mader, T.; Douagi, I.; Andre, H.; Hovatta, O.; et al. Xeno-Free and Defined Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Functionally Integrate in a Large-Eyed Preclinical Model. Stem Cell Reports 2016, 6, 9–17. [Google Scholar]
- Liao, J.; Cui, C.; Chen, S.; Ren, J.; Chen, J.; Gao, Y.; Li, H.; Jia, N.; Cheng, L.; Xiao, H.; Xiao, L. Generation of Induced Pluripotent Stem Cell Lines from Adult Rat Cells. Cell Stem Cell 2009, 4, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nakada, A.; Hashimoto, Y.; Shigeno, K.; Shionoya, Y.; Nakamura, T. Generation of Canine Induced Pluripotent Stem Cells by Retroviral Transduction and Chemical Inhibitors. Mol. Reprod. Dev. 2010, 77, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, F.; Yong, J.; Zhang, P.; Hou, P.; Li, H.; Jiang, W.; Cai, J.; Liu, M.; Cui, K.; et al. Generation of Induced Pluripotent Stem Cells from Adult Rhesus Monkey Fibroblasts. Cell Stem Cell 2008, 3, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, W.; Pan, G.; Zhou, J. Advances in Understanding the Cell Types and Approaches Used for Generating Induced Pluripotent Stem Cells. J. Hematol. Oncol. 2014, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of Mouse Induced Pluripotent Stem Cells without Viral Vectors. Science 2008, 322, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. A Fresh Look at iPS Cells. Cell 2009, 137, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Doorenbos, M.; Chen, D.F. Induced Pluripotent Stem Cells: Development in the Ophthalmologic Field. Stem Cells Int. 2016, 2016, 2361763. [Google Scholar] [CrossRef] [PubMed]
- Tezel, T.H.; Del Priore, L.V.; Berger, A.S.; Kaplan, H.J. Adult Retinal Pigment Epithelial Transplantation in Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2007, 143, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, L.V.; Kaplan, H.J.; Tezel, T.H.; Hayashi, N.; Berger, A.S.; Green, W.R. Retinal Pigment Epithelial Cell Transplantation after Subfoveal Membranectomy in Age-Related Macular Degeneration: Clinicopathologic Correlation. Am. J. Ophthalmol. 2001, 131, 472–480. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1-9. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Fields, M.A.; Moreira, E.F.; Bowrey, H.E.; Gooz, M.; Ablonczy, Z.; Del Priore, L.V. Differentiation of Human Protein-Induced Pluripotent Stem Cells toward a Retinal Pigment Epithelial Cell Fate. PLoS ONE 2015, 10, e0143272. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.E.; Hikita, S.T.; Rowland, T.J.; Friedrich, A.M.; Hinman, C.R.; Johnson, L.V.; Clegg, D.O. Derivation of Functional Retinal Pigmented Epithelium from Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Vugler, A.A.; Hikita, S.T.; Lawrence, J.M.; Gias, C.; Chen, L.L.; Buchholz, D.E.; Ahmado, A.; Semo, M.; Smart, M.J.; et al. Protective Effects of Human iPS-Derived Retinal Pigment Epithelium Cell Transplantation in the Retinal Dystrophic Rat. PLoS ONE 2009, 4, e8152. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Phillips, M.J.; Kuai, D.; Meyer, J.; Martin, J.M.; Smith, M.A.; Perez, E.T.; Shen, W.; Wallace, K.A.; Capowski, E.E.; et al. Functional Analysis of Serially Expanded Human iPS Cell-Derived RPE Cultures. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6767–6778. [Google Scholar] [CrossRef] [PubMed]
- Westenskow, P.; Sedillo, Z.; Barnett, A.; Friedlander, M. Efficient Derivation of Retinal Pigment Epithelium Cells from Stem Cells. J. Vis. Exp. 2015, 97, e52214. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Takahashi, M. Induction of Retinal Pigment Epithelial Cells from Monkey iPS Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8785–8790. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Sheets Aiming for Clinical Application. Stem Cell Reports 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.A.; Bowrey, H.E.; Gong, J.; Ablonczy, Z.; Del Priore, L.V. Retinoid Processing in Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cultures. Prog. Mol. Biol. Transl. Sci. 2015, 134, 477–490. [Google Scholar] [PubMed]
- Li, Y.; Tsai, Y.T.; Hsu, C.W.; Erol, D.; Yang, J.; Wu, W.H.; Davis, R.J.; Egli, D.; Tsang, S.H. Long-Term Safety and Efficacy of Human-Induced Pluripotent Stem Cell (iPS) Grafts in a Preclinical Model of Retinitis Pigmentosa. Mol. Med. 2012, 18, 1312–1319. [Google Scholar] [PubMed]
- Westenskow, P.D.; Bucher, F.; Bravo, S.; Kurihara, T.; Feitelberg, D.; Paris, L.P.; Aguilar, E.; Lin, J.H.; Friedlander, M. iPSC-Derived Retinal Pigment Epithelium Allografts Do Not Elicit Detrimental Effects in Rats: A Follow-Up Study. Stem Cells Int. 2016, 2016, 8470263. [Google Scholar] [CrossRef] [PubMed]
- Yvon, C.; Ramsden, C.M.; Lane, A.; Powner, M.B.; da Cruz, L.; Coffey, P.J.; Carr, A.J. Using Stem Cells to Model Diseases of the Outer Retina. Comput. Struct. Biotechnol. J. 2015, 13, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shen, W.; Kuai, D.; Martin, J.M.; Guo, X.; Smith, M.A.; Perez, E.T.; Phillips, M.J.; Simonett, J.M.; Wallace, K.A.; et al. iPS Cell Modeling of Best Disease: Insights into the Pathophysiology of an Inherited Macular Degeneration. Hum. Mol. Genet. 2013, 22, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.A.; Solivan-Timpe, F.; Roos, B.R.; Anfinson, K.R.; Robin, A.L.; Wiley, L.A.; Mullins, R.F.; Fingert, J.H. Duplication of TBK1 Stimulates Autophagy in iPSC-Derived Retinal Cells from a Patient with Normal Tension Glaucoma. J. Stem Cell Res. Ther. 2014, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Li, Y.; Tsang, S.H. Patient-Specific iPSC-Derived RPE for Modeling of Retinal Diseases. J. Clin. Med. 2015, 4, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, W.H.; Hsu, C.W.; Nguyen, H.V.; Tsai, Y.T.; Chan, L.; Nagasaki, T.; Maumenee, I.H.; Yannuzzi, L.A.; Hoang, Q.V.; et al. Gene Therapy in Patient-Specific Stem Cell Lines and a Preclinical Model of Retinitis Pigmentosa with Membrane Frizzled-Related Protein Defects. Mol. Ther. 2014, 22, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The Epidemiology of Age-Related Macular Degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.C.; Dillon, J.; Galicia, E.; Tilson, M.D. The Nitrite/Collagen Reaction: Non-Enzymatic Nitration as a Model System for Age-Related Damage. Connect. Tissue Res. 2001, 42, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, A.; Chew, E.Y.; Rickman, C.B.; Abecasis, G.R. Unraveling a Multifactorial Late-Onset Disease: From Genetic Susceptibility to Disease Mechanisms for Age-Related Macular Degeneration. Annu. Rev. Genom. Hum. Genet. 2009, 10, 19–43. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.; Bouckenheimer, J.; Sansac, C.; Lemaitre, J.M.; Assou, S. Human Induced Pluripotent Stem Cells: A Disruptive Innovation. Curr. Res. Transl. Med. 2016, 64, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, E.A.; Lanza, R. Current Status of Pluripotent Stem Cells: Moving the First Therapies to the Clinic. Nat. Rev. Drug Discov. 2015, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H. Stem Cells in Clinical Trials for Treatment of Retinal Degeneration. Expert Opin. Biol. Ther. 2016, 16, 7–14. [Google Scholar] [CrossRef] [PubMed]
- McGill, T.J.; Cottam, B.; Lu, B.; Wang, S.; Girman, S.; Tian, C.; Huhn, S.L.; Lund, R.D.; Capela, A. Transplantation of Human Central Nervous System Stem Cells - Neuroprotection in Retinal Degeneration. Eur. J. Neurosci. 2012, 35, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, S.; Kanemura, H.; Sakai, N.; Takahashi, M.; Go, M.J. Design of a Tumorigenicity Test for Induced Pluripotent Stem Cell (iPSC)-Derived Cell Products. J. Clin. Med. 2015, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S.; Cyranoski, D. Japan Stem-Cell Trial Stirs Envy. Nature 2014, 513, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Hayashizaki, Y.; Murakawa, Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, S. An Eye to the Future: Researchers Debate Best Path for Stem Cell-Derived Therapies. Nat. Med. 2016, 22, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Riken Suspends First Clinical Trial Involving Induced Pluripotent Stem Cells. Nat. Biotechnol. 2015, 33, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in Mhc-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kimura, T.; Futagami, T.; Suegami, S.; Takahashi, M. Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors. Stem Cell Rep. 2016, 7, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Riken to Resume Retinal iPS Transplant Study in Cooperation with Kyoto University. The Japan Times. 7 June 2016. Available online: http://www.japantimes.co.jp/news/2016/06/07/national/science-health/riken-resume-retinal-iPS-transplantation-cooperation-kyoto-university/#.WEdkwH_YWpo (accessed on 26 October 2016).
- Kanemura, H.; Go, M.J.; Shikamura, M.; Nishishita, N.; Sakai, N.; Kamao, H.; Mandai, M.; Morinaga, C.; Takahashi, M.; Kawamata, S. Tumorigenicity Studies of Induced Pluripotent Stem Cell (iPSC)-Derived Retinal Pigment Epithelium (RPE) for the Treatment of Age-Related Macular Degeneration. PLoS ONE 2014, 9, e85336. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; DeWitt, N.D. Pluripotent Stem Cells Progressing to the Clinic. Nat. Rev. Mol. Cell Biol. 2016, 17, 194–200. [Google Scholar] [CrossRef] [PubMed]
| Sponsor | Cell Type or Intervention | Condition | Phase of Trial | Type of Delivery (Intervention) | ClinicalTrials.gov Identifier | Status |
|---|---|---|---|---|---|---|
| Regenerative Patch Technologies, LLC | CPCB-RPE1; human ESC-derived RPE seeded on polymeric substrate | Advanced, dry age-related macular degeneration (AMD) | Phase I and II | Subretinal implantation | NCT02590692 | Recruiting |
| Astellas Institute for Regenerative Medicine | MA09-hRPE; human ESC-derived RPE | Advanced, dry age-related macular degeneration (AMD) | Phase I and II | Subretinal implantation | NCT01344993 | Completed |
| Astellas Institute for Regenerative Medicine | MA09-hRPE; human ESC-derived RPE | Stargardt macular dystrophy (SMD) | Phase I and II | Subretinal implantation | NCT01469832 | Completed |
| CHABiotech Co., Ltd. | MA09-hRPE; human ESC-derived RPE | Advanced, dry age-related macular degeneration (AMD) | Phase I and II | Subretinal implantation | NCT01674829 | Unknown |
| Astellas Institute for Regenerative Medicine | MA09-hRPE; human ESC-derived RPE | Stargardt macular dystrophy (SMD) | Phase I and II | Subretinal implantation | NCT01345006 | Completed |
| University of California, Los Angeles | MA09-hRPE; human ESC-derived RPE | Myopic macular degeneration (MMD) | Phase I and II | Subretinal implantation | NCT02122159 | Withdrawn |
| Cell Cure Neurosciences, Ltd. | OpRegen: human ESC-derived RPE | Advanced, dry-form age-related macular degeneration (geographic atrophy, GA) | Phase I and II | Subretinal implantation | NCT02286089 | Recruiting |
| CHABiotech Co., Ltd. | MA09-hRPE; human ESC-derived RPE | Stargardt macular dystrophy (SMD) | Phase I | Subretinal implantation | NCT01625559 | Unknown |
| Federal University of São Paulo | Human ESC-derived RPE in suspension; human ESC-derived RPE seeded in a substrate | Age-related macular degeneration | Phase I and II | Subretinal implantation | NCT02903576 | Recruiting |
| Exudative, age-related macular degeneration | ||||||
| Pfizer | PF-05206388; human ESC-derived RPE | Acute, wet age-related macular degeneration | Phase I | Intraocular implantation | NCT01691261 | Active, not recruiting |
| Rapid vision decline | ||||||
| Southwest Hospital, China | Human ESC-derived RPE | Macular degeneration, Stargardt macular dystrophy | Phase I | Subretinal transplantation | NCT02749734 | Recruiting |
| Sponsor | Cell Type or Intervention | Condition | Phase of Trial | Type of Delivery (Intervention) | ClinicalTrials.gov Identifier | Status |
|---|---|---|---|---|---|---|
| StemCells, Inc. | Human central nervous system stem cells (HuCNS-SC) | Geographic atrophy (GA) of age-related macular degeneration (AMD) | Phase I and II | Subretinal transplantation | NCT01632527 | Completed |
| University of São Paulo | Autologous bone marrow stem cells | Macular degeneration | Phase I and II | Intravitreal injection | NCT01518127 | Recruiting |
| Al-Azhar University | Autologous bone marrow stem cells | Dry, age-related macular degeneration (AMD) | Phase I and II | Intravitreal injection | NCT02016508 | Unknown |
| Bioheart, Inc. | Adipose-derived stem cells | Dry, macular degeneration | Not reported | Intravitreal injection | NCT02024269 | Withdrawn |
| Retina Association of South Florida | Bone-marrow delivered stem cells (BMSC) | Retinal disease | Not reported | Retrobulbar | NCT01920867 | Recruiting |
| Macular degeneration | Subtenon | |||||
| Hereditary retinal dystrophy | Intravenous | |||||
| Optic nerve disease | Intravitreal | |||||
| Glaucoma | Intraocular | |||||
| University of California, Davis | CD34 + bone marrow stem cells | Non-exudative, age-related macular degeneration | Phase I | Intravitreal injection | NCT01736059 | Enrolled by invitation |
| Diabetic retinopathy | ||||||
| Retinal vein occlusion | ||||||
| Retinitis pigmentosa | ||||||
| Hereditary macular degeneration | ||||||
| Red de Terapia Celular | Autologous bone marrow stem cells | Retinitis pigmentosa | Phase I | Intravitreal injection; subconjunctival injection of saline | NCT02280135 | Recruiting |
| StemCells, Inc. | Human central nervous system stem cells (HuCNS-SC) | Age-related macular degeneration | Phase II | Subretinal transplantation | NCT02467634 | Terminated; based on a business decision unrelated to any safety concerns |
| Sponsor | Cell Type | Condition | ClinicalTrials.gov Identifier | Status | Objective |
|---|---|---|---|---|---|
| Moorfields Eye Hospital | Human iPSC-derived RPE | Age-related macular degeneration | NCT02464956 | Not yet recruiting | Successful production of a retinal epithelial layer of cells that fulfills Regulatory Regulation for Transplantation. |
| NHS Foundation Trust | |||||
| Mayo Clinic | Human iPSC-derived RPE | Autosomal recessive bestrophinopathy (ARB) | NCT02162953 | Recruiting | To collect DNA, RNA, and skin samples from individuals with ARB or other diseases due to mutations in the gene BEST1. These models will be used to identify and test therapeutic approaches to treating these diseases. |
| Best vitelliform macular dystrophy (BVMD) | |||||
| Adult-onset vitelliform dystrophy (AVMD) | |||||
| Autosomal dominant vitreoretinalchoroidopathy (ADVIRC) | |||||
| Retinitis pigmentosa (RP) | |||||
| National Eye Institute (NEI) | Human iPSC-derived RPE | NCT01432847 | Recruiting | To collect hair, skin, and blood samples to study three eye diseases that affect the retina (Best disease, L-ORD, and AMD) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fields, M.; Cai, H.; Gong, J.; Del Priore, L. Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD). Cells 2016, 5, 44. https://doi.org/10.3390/cells5040044
Fields M, Cai H, Gong J, Del Priore L. Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD). Cells. 2016; 5(4):44. https://doi.org/10.3390/cells5040044
Chicago/Turabian StyleFields, Mark, Hui Cai, Jie Gong, and Lucian Del Priore. 2016. "Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD)" Cells 5, no. 4: 44. https://doi.org/10.3390/cells5040044
APA StyleFields, M., Cai, H., Gong, J., & Del Priore, L. (2016). Potential of Induced Pluripotent Stem Cells (iPSCs) for Treating Age-Related Macular Degeneration (AMD). Cells, 5(4), 44. https://doi.org/10.3390/cells5040044





