The Trypanosome Flagellar Pocket Collar and Its Ring Forming Protein—TbBILBO1
Abstract
:1. Introduction
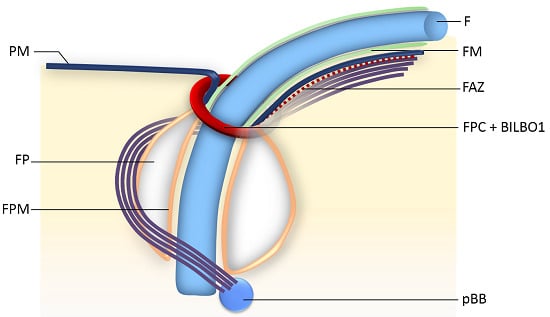
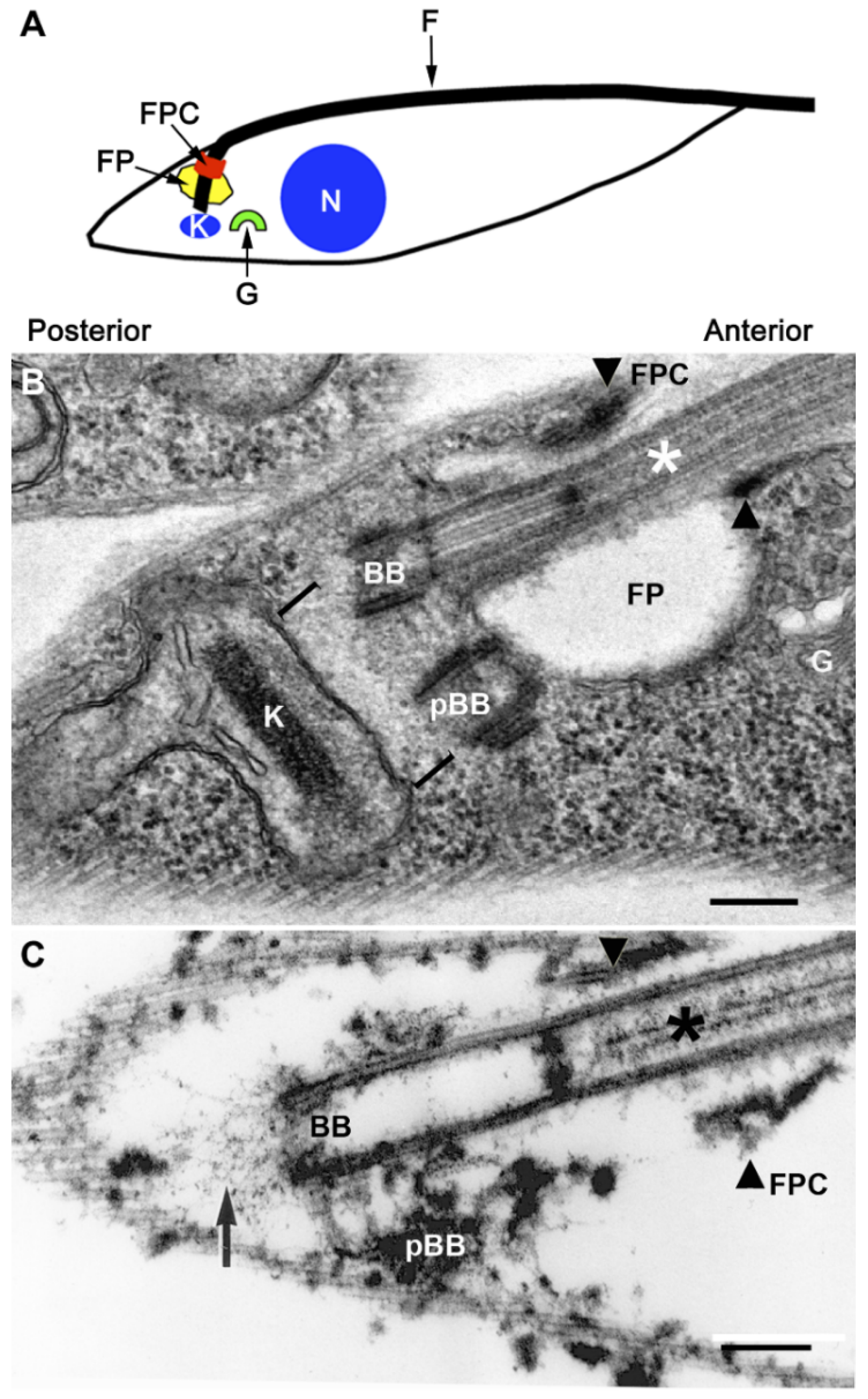
2. The Flagellar Pocket
3. The Flagellum and Its FP Associated Cytoskeleton
4. The Flagellar Pocket Collar
5. BILBO1 and Flagellar Pocket Collar
6. BILBO1, the Cytoskeletal Ring of Power
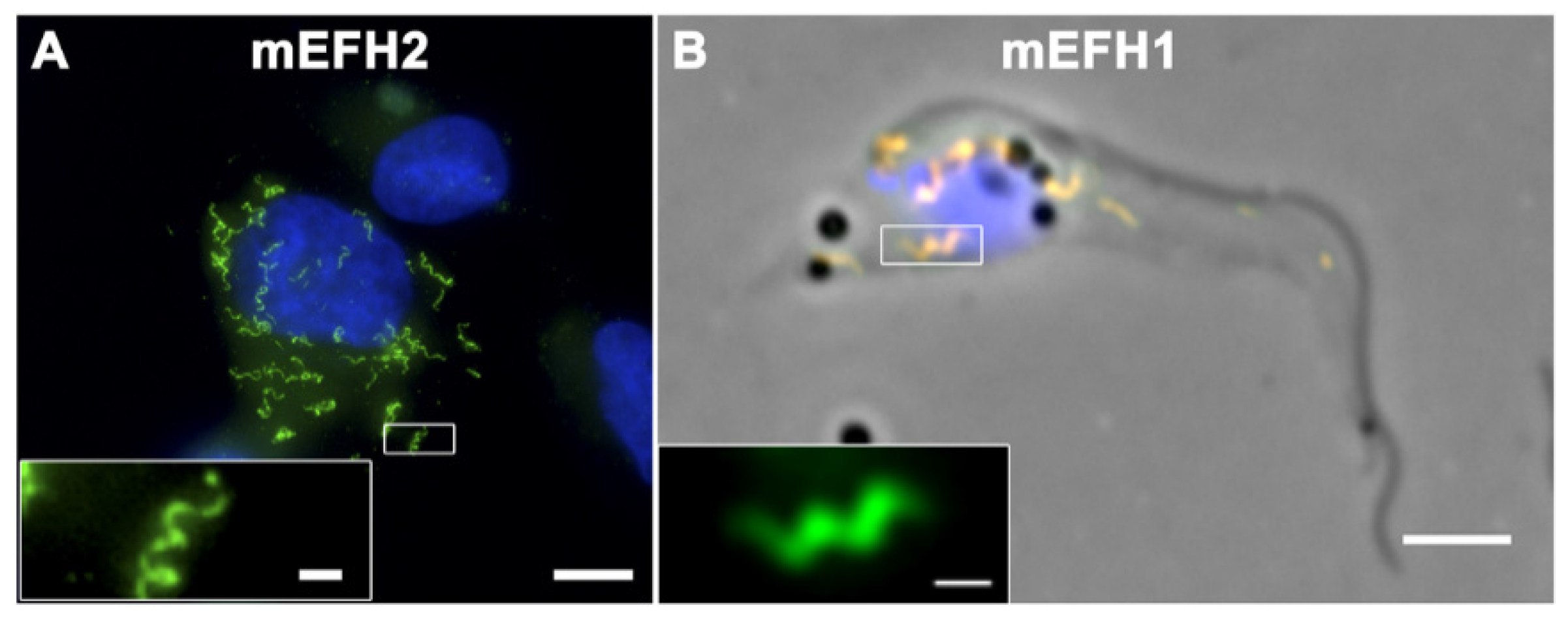
7. TbBILBO1 Forms Polymers in Mammalian Cells
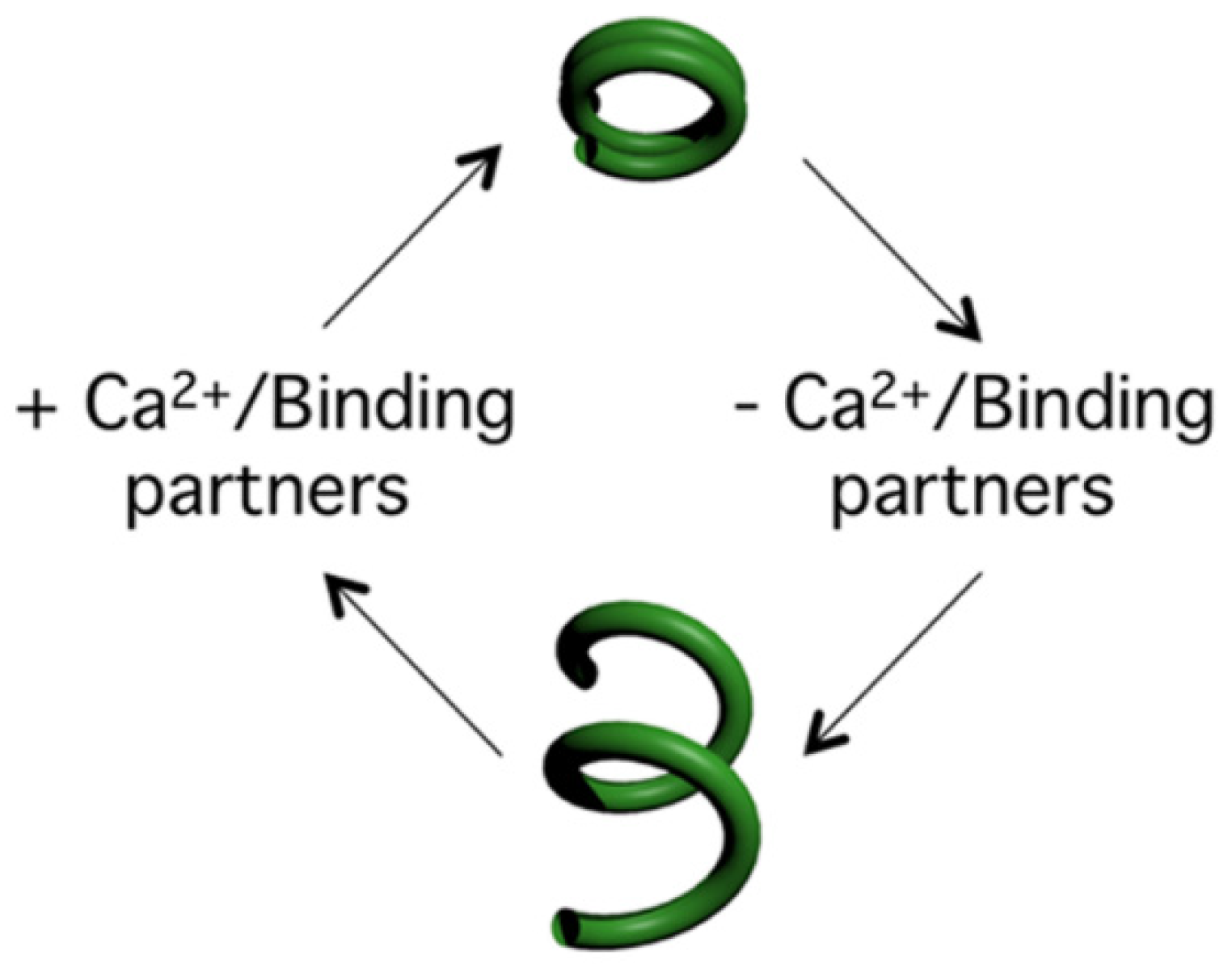
8. TbBILBO1 Binds Calcium
9. TbBILBO1 Calcium Binding and Polymer Shape
10. Other Issues
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HAT | Human Africa Trypanosomiasis |
| WHO | The World Health Organisation |
| FP | Flagellar Pocket |
| FPC | Flagellar Pocket Collar |
| PF | Procyclc Form |
| BSF | Bloodstream Form |
| 4MT | Four microtubule quartet |
| MTQ | Four microtubule quartet |
| MTOC | microtubule organising centres |
| FAZ | Flagellum Attachment Zone |
References
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukes, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M. Notes on the polymorphism of Trypanosoma gambiense in the blood and its relation to the exogenous cycle in Glossina palpalis. Proc. R. Soc. 1912, 85, 527–539. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 2, 297–354. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.J. The impact of nagana. Onderstepoort J. Vet. Res. 1994, 61, 379–383. [Google Scholar] [PubMed]
- Muhanguzi, D.; Okello, W.O.; Kabasa, J.D.; Waiswa, C.; Welburn, S.C.; Shaw, A.P. Cost analysis of options for management of African Animal Trypanosomiasis using interventions targeted at cattle in Tororo District; south-eastern Uganda. Parasit Vectors 2015, 8, 387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef]
- Schwede, A.; Carrington, M. Bloodstream form trypanosome plasma membrane proteins: Antigenic variation and invariant antigens. Parasitology 2010, 137, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Glover, L.; Hutchinson, S.; Alsford, S.; McCulloch, R.; Field, M.C.; Horn, D. Antigenic variation in African trypanosomes: The importance of chromosomal and nuclear context in VSG expression control. Cell. Microbiol. 2013, 15, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.T.; Boehm, C.; Leung, K.F.; Natesan, S.K.; Field, M.C. Life and times: Synthesis, trafficking, and evolution of VSG. Trends Parasitol. 2014, 30, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.R.; McCulloch, R.; Morrison, L.J. The within-host dynamics of African trypanosome infections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140288. [Google Scholar] [CrossRef] [PubMed]
- Malvy, D.; Chappuis, F. Sleeping sickness. Clin. Microbiol. Infect. 2011, 17, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Vickerman, K. The fine structure of Trypanosoma congolense in its bloodstream phase. J. Protozool. 1969, 16, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.K.; Black, A.; Matthews, K.R.; Mottram, J.C.; Field, M.C. Trypanosoma brucei brucei: Endocytic recycling is important for mouse infectivity. Exp. Parasitol. 2011, 127, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Gull, K. The cell biology of parasitism in Trypanosoma brucei: Insights and drug targets from genomic approaches? Curr. Pharm. Des. 2002, 8, 241–256. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Pypaert, M.; Warren, G. Golgi duplication in Trypanosoma brucei requires Centrin2. Science 2005, 310, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- He, C.Y.; Ho, H.H.; Malsam, J.; Chalouni, C.; West, C.M.; Ullu, E.; Toomre, D.; Warren, G. Golgi duplication in Trypanosoma brucei. J. Cell. Biol. 2004, 165, 313–321. [Google Scholar] [CrossRef] [PubMed]
- De Graffenried, C.L.; Anrather, D.; Von Raussendorf, F.; Warren, G. Polo-like kinase phosphorylation of bilobe-resident TbCentrin2 facilitates flagellar inheritance in Trypanosoma brucei. Mol. Biol. Cell 2013, 24, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.N.; de Graffenried, C.L. Polo-like kinase is necessary for flagellum inheritance in Trypanosoma brucei. J. Cell Sci. 2012, 125 Pt 13, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, I.B.; de Graffenried, C.L.; Ebersberger, I.; Yelinek, J.; He, C.Y.; Price, A.; Warren, G. TbG63, a golgin involved in Golgi architecture in Trypanosoma brucei. J. Cell Sci. 2008, 121 Pt 9, 1538–1546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Graffenried, C.L.; Ho, H.H.; Warren, G. Polo-like kinase is required for Golgi and bilobe biogenesis in Trypanosoma brucei. J. Cell Biol. 2008, 181, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.H.; He, C.Y.; de Graffenried, C.L.; Murrells, L.J.; Warren, G. Ordered assembly of the duplicating Golgi in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2006, 103, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Gull, K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature 1991, 352, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Plessmann, U.; Weber, K. Subpellicular and flagellar microtubules of Trypanosoma brucei are extensively glutamylated. J. Cell Sci. 1997, 110, 431–437. [Google Scholar] [PubMed]
- Sasse, R.; Gull, K. Tubulin post-translational modifications and the construction of microtubular organelles in Trypanosoma brucei. J. Cell Sci. 1988, 90, 577–589. [Google Scholar] [PubMed]
- Sherwin, T.; Schneider, A.; Sasse, R.; Seebeck, T.; Gull, K. Distinct localization and cell cycle dependence of COOH terminally tyrosinolated alpha-tubulin in the microtubules of Trypanosoma brucei brucei. J. Cell Biol. 1987, 104, 439–446. [Google Scholar] [CrossRef] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [PubMed]
- Schneider, A.; Sherwin, T.; Sasse, R.; Russell, D.G.; Gull, K.; Seebeck, T. Subpellicular and flagellar microtubules of Trypanosoma brucei brucei contain the same alpha-tubulin isoforms. J. Cell Biol. 1987, 104, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, T.; Gull, K. Visualization of detyrosination along single microtubules reveals novel mechanisms of assembly during cytoskeletal duplication in trypanosomes. Cell 1989, 57, 211–221. [Google Scholar] [CrossRef]
- Robinson, D.; Beattie, P.; Sherwin, T.; Gull, K. Microtubules, tubulin, and microtubule-associated proteins of trypanosomes. Methods Enzymol. 1991, 196, 285–299. [Google Scholar] [PubMed]
- Robinson, D.R.; Sherwin, T.; Ploubidou, A.; Byard, E.H.; Gull, K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 1995, 128, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Gull, K.; Birkett, C.; Gerke-Bonet, R.; Parma, A.; Robinson, D.; Sherwin, T.; Woodward, R. The cell cycle and cytoskeletal morphogenesis in Trypanosoma brucei. Biochem. Soc. Trans. 1990, 18, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Sherwin, T.; Sasse, R.; MacRae, T.H.; Baines, A.J.; Gull, K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 1989, 93 Pt 3, 491–500. [Google Scholar] [PubMed]
- Sherwin, T.; Gull, K. The cell division cycle of Trypanosoma brucei brucei: Timing of event markers and cytoskeletal modulations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1989, 323, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Bastin, P.; Sherwin, T.; Gull, K. Paraflagellar rod is vital for trypanosome motility. Nature 1998, 391, 548. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.; Sherwin, T.; Gull, K. Gamma-tubulin in trypanosomes: Molecular characterisation and localisation to multiple and diverse microtubule organising centres. J. Cell Sci. 1997, 110, 157–168. [Google Scholar] [PubMed]
- Moreira-Leite, F.F.; Sherwin, T.; Kohl, L.; Gull, K. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science 2001, 294, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.P.; Bastin, P. More than meets the eye: Understanding Trypanosoma brucei morphology in the tsetse. Front. Cell. Infect. Microbiol. 2013, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.D.; Varga, V.; Dean, S.; Gull, K. A dynamic coordination of flagellum and cytoplasmic cytoskeleton assembly specifies cell morphogenesis in trypanosomes. J. Cell Sci. 2015, 128, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Adung’a, V.; Obado, S.; Chait, B.T.; Rout, M.P. Proteomics on the rims: Insights into the biology of the nuclear envelope and flagellar pocket of trypanosomes. Parasitology 2012, 139, 1158–1167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Demmel, L.; Schmidt, K.; Lucast, L.; Havlicek, K.; Zankel, A.; Koestler, T.; Reithofer, V.; de Camilli, P.; Warren, G. The endocytic activity of the flagellar pocket in Trypanosoma brucei is regulated by an adjacent phosphatidylinositol phosphate kinase. J. Cell Sci. 2014, 127 Pt 10, 2351–2364. [Google Scholar] [CrossRef] [PubMed]
- Engstler, M.; Pfohl, T.; Herminghaus, S.; Boshart, M.; Wiegertjes, G.; Heddergott, N.; Overath, P. Hydrodynamic flow-mediated protein sorting on the cell surface of trypanosomes. Cell 2007, 131, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Florimond, C.; Sahin, A.; Vidilaseris, K.; Dong, G.; Landrein, N.; Dacheux, D.; Albisetti, A.; Byard, E.H.; Bonhivers, M.; Robinson, D.R. BILBO1 is a scaffold protein of the flagellar pocket collar in the pathogen Trypanosoma brucei. PLoS Pathog. 2015, 11, e1004654. [Google Scholar]
- Ogbadoyi, E.O.; Robinson, D.R.; Gull, K. A high-order trans-membrane structural linkage is responsible for mitochondrial genome positioning and segregation by flagellar basal bodies in trypanosomes. Mol. Biol. Cell 2003, 14, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Kohl, L.; Sherwin, T.; Gull, K. Assembly of the paraflagellar rod and the flagellum attachment zone complex during the Trypanosoma brucei cell cycle. J. Eukaryot. Microbiol. 1999, 46, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hu, H.; He, C.Y.; Li, Z. Assembly and maintenance of the flagellum attachment zone filament in Trypanosoma brucei. J. Cell Sci. 2015, 128, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.D.; Benz, C.; Andre, J.; Whipple, S.; McKean, P.G.; Gull, K.; Ginger, M.L.; Lukes, J. Modulation of flagellum attachment zone protein FLAM3 and regulation of the cell shape in Trypanosoma brucei life cycle transitions. J. Cell Sci. 2015, 128, 3117–3130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, B.; Sun, Y.; He, C.Y. A coiled-coil- and C2-domain-containing protein is required for FAZ assembly and cell morphology in Trypanosoma brucei. J. Cell Sci. 2011, 124 Pt 22, 3848–3458. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S. Assembly of the flagellum and its role in cell morphogenesis in Trypanosoma brucei. Curr. Opin. Microbiol. 2010, 13, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, C.; Wickstead, B.; Gull, K. Flagellar and ciliary beating in trypanosome motility. Cell Motil. Cytoskelet. 2007, 64, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.D.; Matthews, K.R. Restless gossamers: Antibody clearance by hydrodynamic flow forces generated at the surface of motile trypanosome parasites. Cell Host Microbe 2007, 2, 279–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alizadehrad, D.; Kruger, T.; Engstler, M.; Stark, H. Simulating the complex cell design of Trypanosoma brucei and its motility. PLoS Comput. Biol. 2015, 11, e1003967. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.; Engstler, M. Flagellar motility in eukaryotic human parasites. Semin. Cell Dev. Biol. 2015, 46, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Kohl, L.; Robinson, D.; Bastin, P. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 2003, 22, 5336–5346. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, R.; Dawe, H.R.; Farr, H.; Griffiths, S.; Hart, S.R.; Portman, N.; Shaw, M.K.; Ginger, M.L.; Gaskell, S.J.; McKean, P.G.; et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 2006, 440, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Ralston, K.S.; Lerner, A.G.; Diener, D.R.; Hill, K.L. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot. Cell 2006, 5, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.D.; Gull, K. The Flagellum Attachment Zone: ‘The Cellular Ruler’ of Trypanosome Morphology. Trends Parasitol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.; Gull, K. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J. Cell Sci. 1990, 95, 49–57. [Google Scholar] [PubMed]
- Vaughan, S.; Scott, V.; Ogbadoyi, E.; Robinson, D.; Gull, K. The cell cycle and organelle morphogenesis in Trypanosoma brucei. Mem. Inst. Oswaldo Cruz 1998, 93, 16–18. [Google Scholar]
- Ploubidou, A.; Robinson, D.R.; Docherty, R.C.; Ogbadoyi, E.O.; Gull, K. Evidence for novel cell cycle checkpoints in trypanosomes: Kinetoplast segregation and cytokinesis in the absence of mitosis. J. Cell Sci. 1999, 112, 4641–4650. [Google Scholar] [PubMed]
- Hammarton, T.C. Cell cycle regulation in Trypanosoma brucei. Mol. Biochem. Parasitol. 2007, 153, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lacomble, S.; Vaughan, S.; Gadelha, C.; Morphew, M.K.; Shaw, M.K.; McIntosh, J.R.; Gull, K. Basal body movements orchestrate membrane organelle division and cell morphogenesis in Trypanosoma brucei. J. Cell Sci. 2010, 123, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Briggs, L.J.; McKean, P.G.; Baines, A.; Moreira-Leite, F.; Davidge, J.; Vaughan, S.; Gull, K. The flagella connector of Trypanosoma brucei: An unusual mobile transmembrane junction. J. Cell Sci. 2004, 117 Pt 9, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Towers, K.; Starborg, T.; Gull, K.; Vaughan, S. A cell-body groove housing the new flagellum tip suggests an adaptation of cellular morphogenesis for parasitism in the bloodstream form of Trypanosoma brucei. J. Cell Sci. 2013, 126, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Lacomble, S.; Vaughan, S.; Gadelha, C.; Morphew, M.K.; Shaw, M.K.; McIntosh, J.R.; Gull, K. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J. Cell Sci. 2009, 122, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, C.; Rothery, S.; Morphew, M.; McIntosh, J.R.; Severs, N.J.; Gull, K. Membrane domains and flagellar pocket boundaries are influenced by the cytoskeleton in African trypanosomes. Proc. Natl. Acad. Sci. USA 2009, 106, 17425–17430. [Google Scholar] [CrossRef] [PubMed]
- Bonhivers, M.; Nowacki, S.; Landrein, N.; Robinson, D.R. Biogenesis of the trypanosome endo-exocytotic organelle is cytoskeleton mediated. PLoS Biol. 2008, 6, e105. [Google Scholar] [CrossRef] [PubMed]
- Morriswood, B.; He, C.Y.; Sealey-Cardona, M.; Yelinek, J.; Pypaert, M.; Warren, G. The bilobe structure of Trypanosoma brucei contains a MORN-repeat protein. Mol. Biochem. Parasitol. 2009, 167, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Esson, H.J.; Morriswood, B.; Yavuz, S.; Vidilaseris, K.; Dong, G.; Warren, G. Morphology of the trypanosome bilobe, a novel cytoskeletal structure. Eukaryot. Cell 2012, 11, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The genome of the African trypanosome Trypanosoma brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Baudhuin, P.; Opperdoes, F.R.; Courtoy, P.J. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: Purification and involvement in the growth of the parasite. Proc. Natl. Acad. Sci. USA 1988, 85, 6753–6757. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.F. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem. Sci. 2015, 40, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Absalon, S.; Blisnick, T.; Bonhivers, M.; Kohl, L.; Cayet, N.; Toutirais, G.; Buisson, J.; Robinson, D.; Bastin, P. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J. Cell Sci. 2008, 121, 3704–3716. [Google Scholar] [CrossRef] [PubMed]
- Vidilaseris, K.; Morriswood, B.; Kontaxis, G.; Dong, G. Structure of the TbBILBO1 protein N-terminal domain from Trypanosoma brucei reveals an essential requirement for a conserved surface patch. J. Biol. Chem. 2014, 289, 3724–3735. [Google Scholar] [CrossRef] [PubMed]
- Durante, I.M.; Camara Mde, L.; Buscaglia, C.A. A Novel Trypanosoma cruzi Protein Associated to the Flagellar Pocket of Replicative Stages and Involved in Parasite Growth. PLoS ONE 2015, 10, e0130099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gheiratmand, L.; Chen, Y.; Lim, T.K.; Zhang, J.; Li, S.; Xia, N.; Liu, B.; Lin, Q.; He, C.Y. A comparative proteomic analysis reveals a new bi-lobe protein required for bi-lobe duplication and cell division in Trypanosoma brucei. PLoS ONE 2010, 5, e9660. [Google Scholar] [CrossRef] [PubMed]
- Morriswood, B.; Havlicek, K.; Demmel, L.; Yavuz, S.; Sealey-Cardona, M.; Vidilaseris, K.; Anrather, D.; Kostan, J.; Djinovic-Carugo, K.; Roux, K.J.; Warren, G. Novel Bilobe Components in Trypanosoma brucei Identified Using Proximity-Dependent Biotinylation. Eukaryot. Cell 2013, 12, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, A.; Bayat, S.; Chua, X.L.; Zhang, Y.; Zhou, Q.; Low, B.C.; He, C.Y. The bi-lobe-associated LRRP1 regulates Ran activity in Trypanosoma brucei. J. Cell Sci. 2014, 127, 4846–4856. [Google Scholar] [CrossRef] [PubMed]
- Morriswood, B. Form, Fabric, and Function of a Flagellum-Associated Cytoskeletal Structure. Cells 2015, 4, 726–747. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Yuan, Y.A.; He, C.Y. An intra-cellular membrane junction mediated by flagellum adhesion glycoproteins links flagellum biogenesis to cell morphogenesis in Trypanosoma brucei. J. Cell Sci. 2013, 126, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Lacomble, S.; Vaughan, S.; Deghelt, M.; Moreira-Leite, F.F.; Gull, K. A Trypanosoma brucei protein required for maintenance of the flagellum attachment zone and flagellar pocket ER domains. Protist 2012, 163, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Kohl, L.; Ngai, I.; Wheeler, R.J.; Gull, K. A repetitive protein essential for the flagellum attachment zone filament structure and function in Trypanosoma brucei. Protist 2008, 159, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.; Carden, M.J.; Gull, K. Immunological characterization of cytoskeletal proteins associated with the basal body, axoneme and flagellum attachment zone of Trypanosoma brucei. Parasitology 1995, 111, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Portman, N.; Gull, K. The paraflagellar rod of kinetoplastid parasites: From structure to components and function. Int. J. Parasitol. 2010, 40, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Vidilaseris, K.; Dong, G. Expression, purification and preliminary crystallographic analysis of the N-terminal domain of Trypanosoma brucei BILBO1. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70 Pt 5, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Vidilaseris, K.; Shimanovskaya, E.; Esson, H.J.; Morriswood, B.; Dong, G. Assembly mechanism of Trypanosoma brucei BILBO1, a multidomain cytoskeletal protein. J. Biol. Chem. 2014, 289, 23870–23881. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Arndt, K.M. Coiled coil domains: Stability, specificity, and biological implications. Chembiochem 2004, 5, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Weitz, D.A.; Goldman, R.D.; Aebi, U.; Herrmann, H. Intermediate filament mechanics in vitro and in the cell: From coiled coils to filaments, fibers and networks. Curr. Opin. Cell. Biol. 2015, 32, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ryadnov, M.G.; Ceyhan, B.; Niemeyer, C.M.; Woolfson, D.N. “Belt and braces“: A peptide-based linker system of de novo design. J. Am. Chem. Soc. 2003, 125, 9388–9394. [Google Scholar] [CrossRef] [PubMed]
- Langreth, S.G.; Balber, A.E. Protein uptake and digestion in bloodstream and culture forms of Trypanosoma brucei. J. Protozool. 1975, 22, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Natesan, S.K.; Gabernet-Castello, C.; Lila Koumandou, V. Intracellular trafficking in the trypanosomatids. Traffic 2007, 8, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Overath, P.; Engstler, M. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol. Microbiol. 2004, 53, 735–744. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.; Martinez-Palomo, A.; Gonzalez-Robles, A. The cell surface of Trypanosoma cruzi: Cytochemistry and freeze-fracture. J. Cell Sci. 1978, 33, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, C.L.; Vidal, J.C.; de Souza, W.; Cunha-e-Silva, N.L. The three-dimensional structure of the cytostome-cytopharynx complex of Trypanosoma cruzi epimastigotes. J. Cell Sci. 2014, 127, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Kurup, S.P.; Yao, P.Y.; Minning, T.A.; Tarleton, R.L. CRISPR-Cas9-mediated single-gene and gene family disruption in Trypanosoma cruzi. MBio 2015, 6, e02097–e02014. [Google Scholar] [CrossRef] [PubMed]
- Lander, N.; Li, Z.H.; Niyogi, S.; Docampo, R. CRISPR/Cas9-Induced Disruption of Paraflagellar Rod Protein 1 and 2 Genes in Trypanosoma cruzi Reveals Their Role in Flagellar Attachment. MBio 2015, 6, e01012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Serra, R.A.; Yang, S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann. N. Y. Acad. Sci. 2015, 1335, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Nachury, M.V. How do cilia organize signalling cascades? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Molla-Herman, A.; Ghossoub, R.; Blisnick, T.; Meunier, A.; Serres, C.; Silbermann, F.; Emmerson, C.; Romeo, K.; Bourdoncle, P.; Schmitt, A.; Saunier, S.; Spassky, N.; Bastin, P.; Benmerah, A. The ciliary pocket: An endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 2010, 123 Pt 10, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Satir, P.; Christensen, S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007, 69, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K.R. A physiological view of the primary cilium. Annu. Rev. Physiol. 2005, 67, 515–529. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo, D.; Bonhivers, M.; Robinson, D.R. The Trypanosome Flagellar Pocket Collar and Its Ring Forming Protein—TbBILBO1. Cells 2016, 5, 9. https://doi.org/10.3390/cells5010009
Perdomo D, Bonhivers M, Robinson DR. The Trypanosome Flagellar Pocket Collar and Its Ring Forming Protein—TbBILBO1. Cells. 2016; 5(1):9. https://doi.org/10.3390/cells5010009
Chicago/Turabian StylePerdomo, Doranda, Mélanie Bonhivers, and Derrick R. Robinson. 2016. "The Trypanosome Flagellar Pocket Collar and Its Ring Forming Protein—TbBILBO1" Cells 5, no. 1: 9. https://doi.org/10.3390/cells5010009
APA StylePerdomo, D., Bonhivers, M., & Robinson, D. R. (2016). The Trypanosome Flagellar Pocket Collar and Its Ring Forming Protein—TbBILBO1. Cells, 5(1), 9. https://doi.org/10.3390/cells5010009