Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction?
Abstract
:1. Introduction
2. The Origin of Myofibroblasts in the Fibrotic Kidney
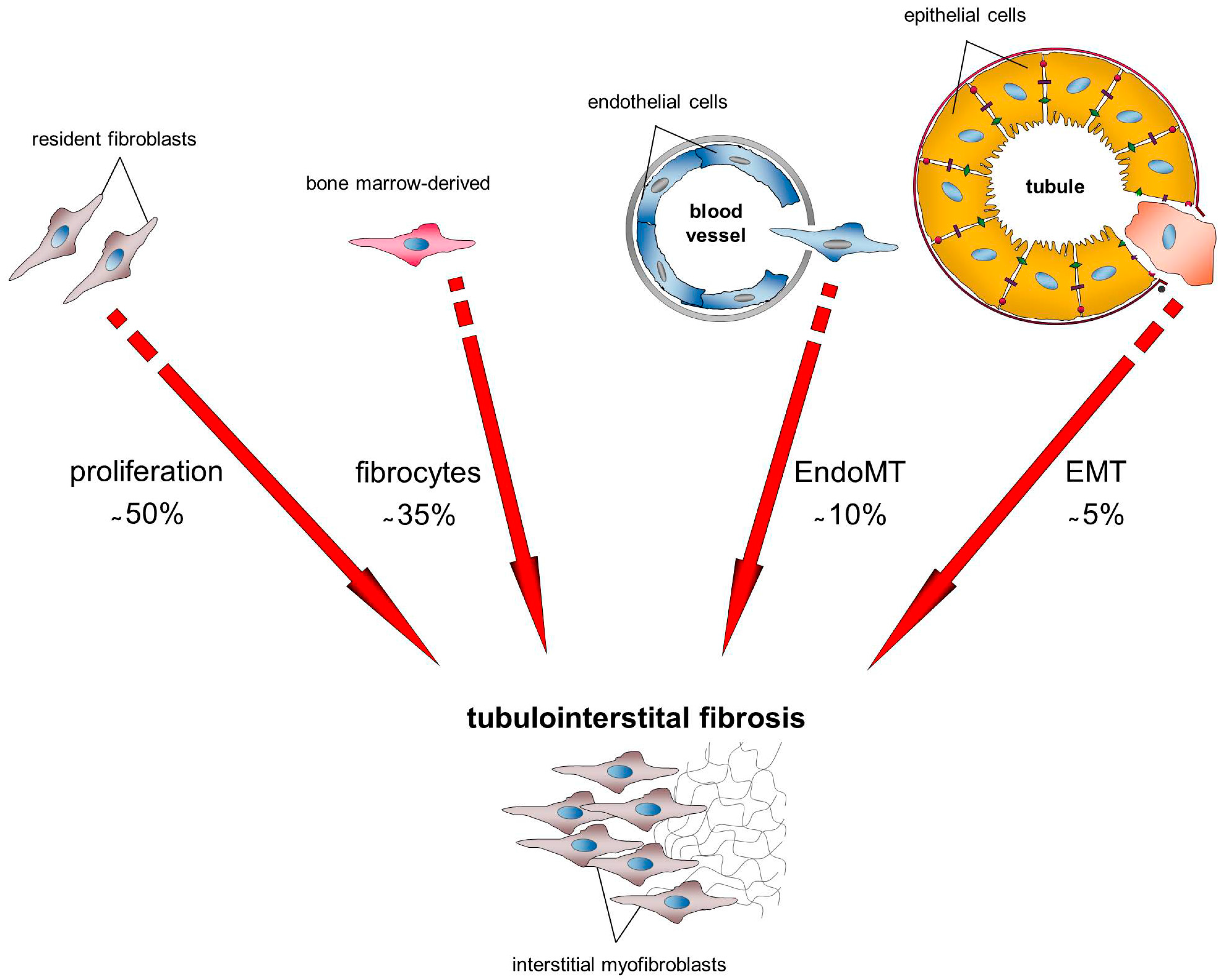
3. Transition of Renal Cells into Activated Myofibroblasts: The Mechanism
4. In Vitro Evidence for EMT and EndoMT of Renal Cells in the Context of Diabetic Conditions
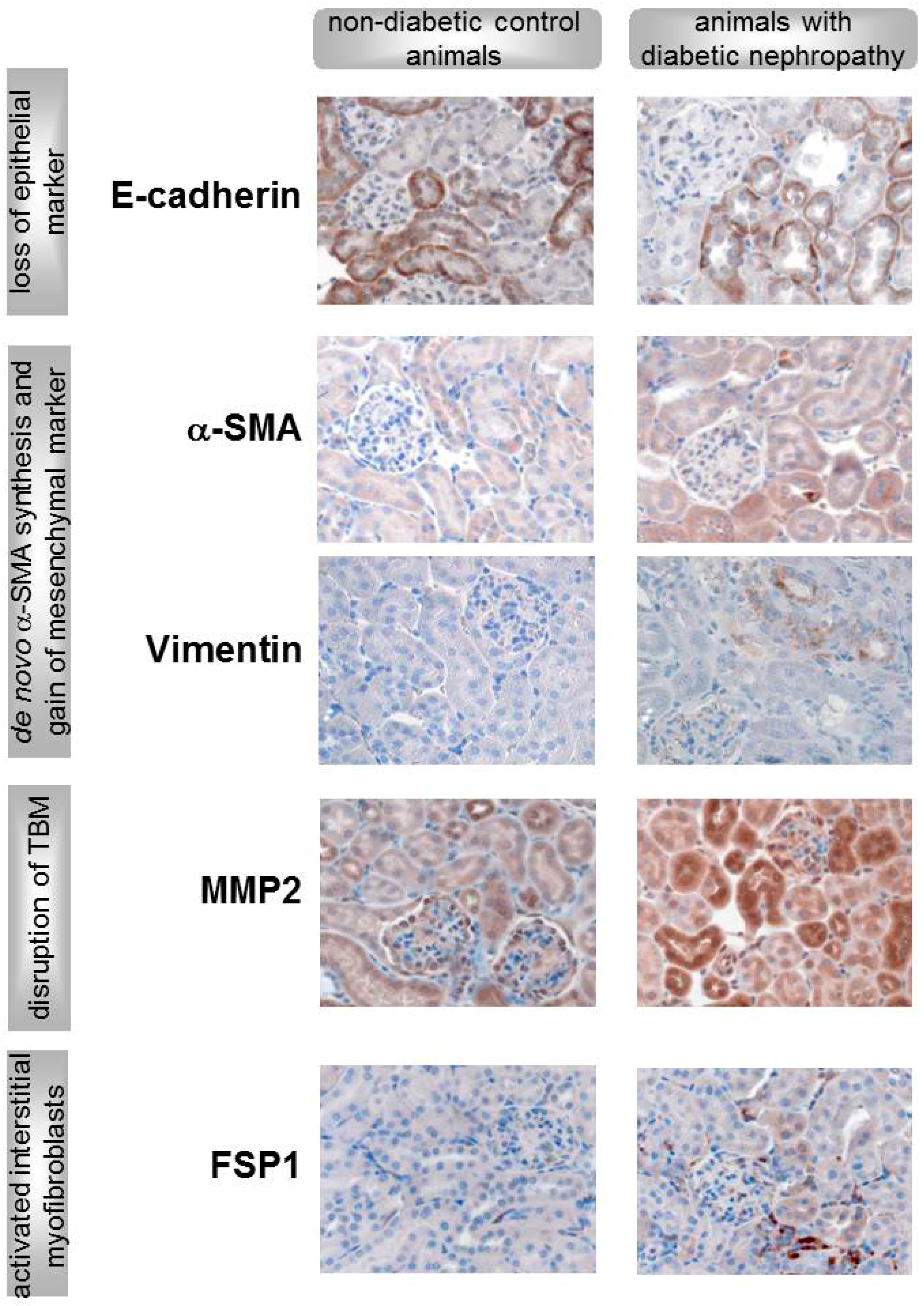
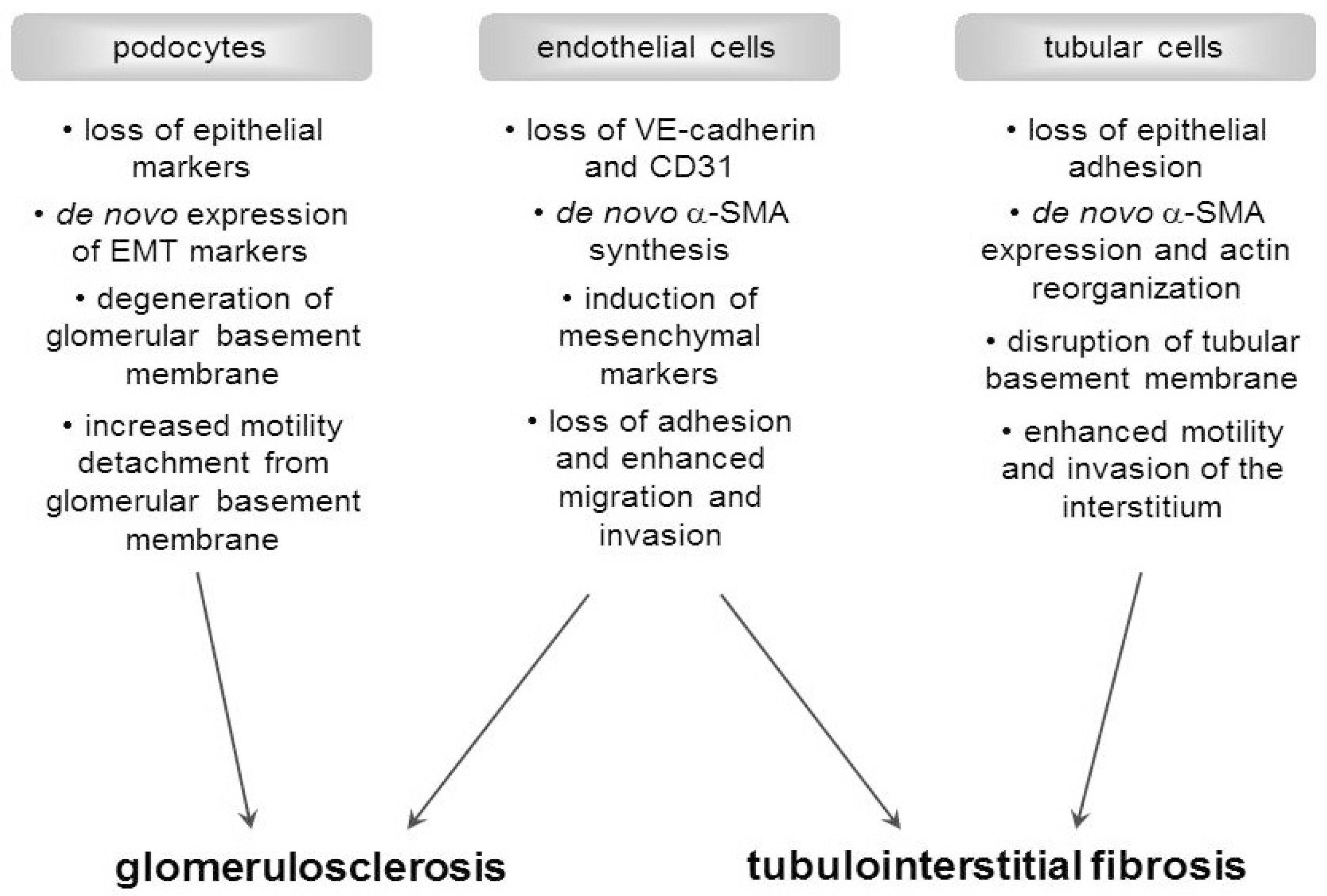
5. In Vivo Evidence for EMT or EndoMT in DN
| Model | EMT Inducer Used | EMT Marker Investigated | Key Events | Study |
|---|---|---|---|---|
| renal epithelial cells | TGF-β1 (3 ng/mL; 48h) | ZO-1, cytokeratines, syndecan-1, α-SMA, vimentin, FSP-1 | 1, 2, (4) | [60] |
| human tubular epithelial cells | TGF-β1 (4 ng/mL; 72h) | E-cadherin, α-SMA, MMPs, disruption of TBM, migration and invasion | 1, 2, 3, 4 | [40] |
| mouse tubular epithelial cells | TGF-β1 (3 ng/mL; 48h) | E-cadherin, ZO-1 | 1 | [12] |
| renal epithelial cells | TGF-β1 (10 ng/mL; 72h) | E-cadherin, α-SMA | 1, 2 | [52] |
| renal epithelial cells | TGF-β1 (5 ng/mL; 48h) | E-cadherin, ZO-1, α-SMA, vimentin | 1, 2 | [53] |
| tubular epithelial cells | TGF-β1 (5 ng/mL; 72h) | E-cadherin, β-catenin, α-SMA, HSP47 | 1, 2, 4 | [58] |
| human tubular epithelial cells | TGF-β1 (10 ng/mL; 72h) | E-cadherin, vimentin | 1, (2) | [28] |
| rat tubular epithelial cells | TGF-β1, TGF-β2 (10 ng/mL; 72h) | E-cadherin, α-SMA, vimentin, collagens | 1, 2 | [43] |
| tubular epithelial cells | TGF-β1 (10 ng/mL; 3 and 6 days) | E-cadherin, α-SMA | 1, 2 | [54] |
| rat tubular epithelial cells | high glucose (20 mmol/l; 12–72h) | E-cadherin, CK-18, α-SMA, fibronectin | 1, 2 | [29] |
| human and rat tubular epithelial cells | high glucose (60 mmol/l; 72h) | E-cadherin, Snail, twist, α-SMA | 1, 2 | [27] |
| rat renal epithelial cells | AGE-BSA (6 days) | E-cadherin, α-SMA | 1, 2 | [25] |
| rat tubular epithelial cells | AGE-BSA (3 and 6 days) | E-cadherin, α-SMA | 1, 2 | [54] |
| rat tubular epithelial cells | albumin (2–6 days) | E-cadherin, α-SMA, FSP-1 | 1, 2, (4) | [57] |
| human tubular epithelial cells | TGF-β1, high glucose, albumin, angiotensin II, aldosterone | E-cadherin, α-SMA | 1, 2 | [55] |
| mouse podocytes | TGF-β1 (0.5–5 ng/mL; 6–72h) | P-cadherin, ZO-1, nephrin, desmin, collagen I, fibronectin, MMP9 | 1, 2, 3 | [35] |
| mouse podocytes | high glucose (30 mmol/l) | desmin | 2 | [46] |
| mouse podocytes | high glucose (25 mmol/l) | synaptopodin, desmin | 1, 2 | [56] |
| Model | EMT/EndoMT | EMT Marker Investigated | Key Events | Study |
|---|---|---|---|---|
| STZ-induced rats | tubular EMT | α-SMA | 2 | [25] |
| human DN | tubular EMT | ZO-1, α-SMA, vimentin, collagens | 1, 2, (4) | [26] |
| STZ-induced rats | tubular EMT | α-SMA, collagen IV | 2 | [54] |
| STZ-induced mice | tubular EMT | α-SMA, collagen IV, fibronectin | 2 | [43] |
| human DN | tubular EMT | bunches in tubular epithelial cells, interstitial myofibroblasts | 2, (4) | [24] |
| STZ-induced mice | tubular EMT | E-cadherin, ZO-1, α-SMA, vimentin, MMP2, FSP1, collagens, fibronectin, integrins, Twist, ZEB1 | 1, 2, 3, (4) | [2] |
| STZ-induced rats | tubular EMT | E-cadherin, α-SMA, fibronectin | 1, 2 | [29] |
| human DN | tubular EMT | α-SMA, fibronectin, MMP9 | 2, 3 | [66] |
| STZ-induced rats | tubular EMT | E-cadherin, Snail, twist, α-SMA | 1, 2 | [27] |
| human DN | glomerular EMT | Nephrin | 1 | [67] |
| STZ-induced mice | glomerular EMT | nephrin, desmin, MMP9 | 1, 2, 3 | [35] |
| human DN | glomerular EMT | ZO-1, nephrin, desmin, MMP9, FSP1 | 1, 2, 3, (4) | [35] |
| human DN | glomerular EMT | ZO-1, Snail, FSP1 | 1, (4) | [44] |
| STZ-induced mice | glomerular EMT | nephrin, synaptopodin | 1 | [68] |
| STZ-induced rats | glomerular EMT | nephrin, desmin | 1, 2 | [46] |
| STZ-induced mice | EndoMT | endothelial lineage tracing | 1, 2, 3, 4 | [19] |
| STZ-induced mice | EndoMT | endothelial lineage tracing | 1, 2, 3, 4 | [17] |
| STZ-induced mice | EndoMT | endothelial lineage tracing | 1, 2, 3, 4 | [47] |
6. Evidence against the EMT Theory as One Source for Matrix-Producing Myofibroblasts
7. Discussion
8. Conclusion
Author Contributions
Conflict of Interest
References
- Hu, C.; Sun, L.; Han, Y.; Fu, X.; Xiong, X.; Xu, X.; Liu, Y.; Yang, S.; Liu, F.; Kanwar, Y.S. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr. Med. Chem. 2015, 22, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, I.; Liebisch, M.; Wolf, G. Collagen viii influences epithelial phenotypic changes in experimental diabetic nephropathy. American journal of physiology. Renal Physiol. 2012, 303, F733–F745. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Amer. Soc. Nephrol. 2010, 21, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Squires, P.E. The role of tgf-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar] [PubMed]
- Li, J.; Bertram, J.F. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology 2010, 15, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Pedagogos, E.; Hewitson, T.; Fraser, I.; Nicholls, K.; Becker, G. Myofibroblasts and arteriolar sclerosis in human diabetic nephropathy. Amer J. Kidney Dis. 1997, 29, 912–918. [Google Scholar] [CrossRef]
- Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J. Amer. Soc. Nephrol. 2004, 15, 1–12. [Google Scholar] [CrossRef]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: Fsp1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Galichon, P.; Hertig, A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: Are we ready for the bedside? Fibrogenesis Tissue Repair 2011, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 2002, 110, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Fragiadaki, M.; Mason, R.M. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int. J. Exp. Pathol. 2011, 92, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Hanai, J.; Sugimoto, H.; Mammoto, T.; Charytan, D.; Strutz, F.; Kalluri, R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 2003, 9, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Simonson, M.S. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007, 71, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Squires, P.E. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Amer. J. Nephrol. 2010, 31, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Susztak, K. Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Amer J. Kidney Dis. 2009, 54, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Kizu, A.; Medici, D.; Kalluri, R. Endothelial-mesenchymal transition as a novel mechanism for generating myofibroblasts during diabetic nephropathy. Am. J. Pathol. 2009, 175, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Bertram, J.F. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 2009, 175, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Koya, D.; Kanasaki, K. Micrornas in kidney fibrosis and diabetic nephropathy: Roles on emt and endmt. BioMed Res. Int. 2013, 2013, 125469. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.E.; Sugimoto, H.; Zeisberg, M.; Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Amer. Soc. Nephrol. 2008, 19, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Kriz, W.; Kaissling, B.; Le Hir, M. Epithelial-mesenchymal transition (emt) in kidney fibrosis: Fact or fantasy? J. Clin. Invest. 2011, 121, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Louis, K.; Hertig, A. How tubular epithelial cells dictate the rate of renal fibrogenesis? World J. Nephrol. 2015, 4, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Dai, H.P. Type 2 epithelial mesenchymal transition in vivo: Truth or pitfalls? Chin. Med. J. 2012, 125, 3312–3317. [Google Scholar] [PubMed]
- Zeisberg, M.; Duffield, J.S. Resolved: Emt produces fibroblasts in the kidney. J. Amer. Soc. Nephrol. 2010, 21, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Mandache, E.; Gherghiceanu, M.; Serafinceanu, C.; Penescu, M.; Mircescu, G. Myofibroblast involvement in tubular basement membrane remodeling in type ii diabetic nephropathy. Rom. J. Morphol. Embryol. 2011, 52, 75–79. [Google Scholar] [PubMed]
- Oldfield, M.D.; Bach, L.A.; Forbes, J.M.; Nikolic-Paterson, D.; McRobert, A.; Thallas, V.; Atkins, R.C.; Osicka, T.; Jerums, G.; Cooper, M.E. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (rage). J. Clin. Invest. 2001, 108, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Rastaldi, M.P.; Ferrario, F.; Giardino, L.; Dell’Antonio, G.; Grillo, C.; Grillo, P.; Strutz, F.; Muller, G.A.; Colasanti, G.; D’Amico, G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002, 62, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ji, X.J.; Zhou, Y.X.; Yao, X.Q.; Liu, Y.Q.; Zhang, F.; Yin, X.X. Quercetin inhibits the mtorc1/p70s6k signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol. Res. 2015, 99, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Han, M.; Luo, Y.; Li, C.; Pei, G.; Liao, W.; Bai, S.; Ge, S.; Liu, X.; Xu, G. Role of sema4c in tgf-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. Nephrol. Dialysis Transplant. 2011, 26, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Xiao, Y.; Shi, M.; Zhang, G.; Guo, B. Snon as a key regulator of the high glucose-induced epithelial-mesenchymal transition in cells of the proximal tubule. Kidney Blood Pressure Res. 2012, 35, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Lin, S.L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010, 176, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, I.; Wolf, G. Transforming growth factor-beta and the progression of renal disease. Nephrol. Dialysis Transplant. 2014, 29 (Suppl. 1), i37–i45. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nature Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, Y.S.; Dai, C.; Kiss, L.P.; Wen, X.; Liu, Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 2008, 172, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.C.; Kantharidis, P.; Thomas, M.C. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 2007, 185, 222–231. [Google Scholar] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Carew, R.M.; Wang, B.; Kantharidis, P. The role of emt in renal fibrosis. Cell. Tissue Res. 2012, 347, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001, 159, 1465–1475. [Google Scholar] [CrossRef]
- Phanish, M.K.; Wahab, N.A.; Colville-Nash, P.; Hendry, B.M.; Dockrell, M.E. The differential role of smad2 and smad3 in the regulation of pro-fibrotic tgfbeta1 responses in human proximal-tubule epithelial cells. Biochem. J. 2006, 393, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Herman-Edelstein, M.; Koh, P.; Burns, W.; Jandeleit-Dahm, K.; Watson, A.; Saleem, M.; Goodall, G.J.; Twigg, S.M.; Cooper, M.E.; et al. E-cadherin expression is regulated by mir-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 2010, 59, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Koh, P.; Winbanks, C.; Coughlan, M.T.; McClelland, A.; Watson, A.; Jandeleit-Dahm, K.; Burns, W.C.; Thomas, M.C.; Cooper, M.E.; et al. Mir-200a prevents renal fibrogenesis through repression of tgf-beta2 expression. Diabetes 2011, 60, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Iwano, M.; Suzuki, D.; Nakatani, K.; Kimura, K.; Harada, K.; Kubo, A.; Akai, Y.; Toyoda, M.; Kanauchi, M.; et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Amer J. Kidney Dis. 2009, 54, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lv, L.L.; Ni, J.; Ni, H.F.; Li, Q.; Ma, K.L.; Liu, B.C. Urinary podocyte-associated mrna profile in various stages of diabetic nephropathy. PloS ONE 2011, 6, e20431. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.Y.; Zheng, M.; Tang, R.N.; Ni, J.; Ma, K.L.; Li, Q.; Liu, B.C. Effects of angiotensin receptor blocker on phenotypic alterations of podocytes in early diabetic nephropathy. Amer. J. Med. Sci. 2011, 341, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Yao, J.; Caruana, G.; Ricardo, S.D.; Yamamoto, Y.; Yamamoto, H.; Bertram, J.F. Blockade of endothelial-mesenchymal transition by a smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 2612–2624. [Google Scholar] [CrossRef] [PubMed]
- Guerrot, D.; Dussaule, J.C.; Kavvadas, P.; Boffa, J.J.; Chadjichristos, C.E.; Chatziantoniou, C. Progression of renal fibrosis: The underestimated role of endothelial alterations. Fibrogenesis Tissue Repair 2012, 5, S15. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, Y.; Koya, D.; Kanasaki, K. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Li, Z.; Jimenez, S.A. Role of endothelial-mesenchymal transition (endomt) in the pathogenesis of fibrotic disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, M.H.; Dong, Z.; Yang, T. Prostaglandin e2 is a potent inhibitor of epithelial-to-mesenchymal transition: Interaction with hepatocyte growth factor. Amer. J. Physiol-Renal Physiol. 2006, 291, F1323–F1331. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, M.J.; Song, I.K.; Choi, S.Y.; Nam, J.O.; Kim, C.D.; Lee, B.H.; Park, R.W.; Park, K.M.; Kim, Y.J.; et al. Erythropoietin decreases renal fibrosis in mice with ureteral obstruction: Role of inhibiting TGF-beta-induced epithelial-to-mesenchymal transition. J. Amer. Soc. Nephrol. 2007, 18, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.C.; Twigg, S.M.; Forbes, J.M.; Pete, J.; Tikellis, C.; Thallas-Bonke, V.; Thomas, M.C.; Cooper, M.E.; Kantharidis, P. Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: Implications for diabetic renal disease. J. Amer. Soc. Nephrol. 2006, 17, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.H.; Kim, J.S.; Chang, J.W.; Kim, S.B.; Park, J.S.; Lee, S.K. Amp-activated protein kinase inhibits tgf-beta-, angiotensin ii-, aldosterone-, high glucose-, and albumin-induced epithelial-mesenchymal transition. Amer. J. Physiol-Renal Physiol. 2013, 304, F686–F697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, W.; Xu, J.; Shao, L.; Wang, Y. Effect of bmp7 on podocyte transdifferentiation and smad7 expression induced by hyperglycemia. Clin. Nephrol. 2015, 84, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ibrini, J.; Fadel, S.; Chana, R.S.; Brunskill, N.; Wagner, B.; Johnson, T.S.; El Nahas, A.M. Albumin-induced epithelial mesenchymal transformation. Nephron Exp. Nephrol. 2012, 120, e91–e102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.P.; Lyons, J.G.; Tan, T.K.; Wang, Y.P.; Hsu, T.T.; Min, D.Q.; Succar, L.; Rangan, G.K.; Hu, M.; Henderson, B.R.; et al. Disruption of e-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta 1 in renal tubular epithelial cells. Amer. J. Pathol. 2009, 175, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Li, Y.; Dai, C.; Kiss, L.P.; Wu, C.; Liu, Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010, 78, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Danoff, T.M.; Kalluri, R.; Neilson, E.G. Early role of fsp1 in epithelial-mesenchymal transformation. Amer. J. Physiol. 1997, 273, F563–F574. [Google Scholar] [PubMed]
- Yang, J.; Liu, Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J. Amer. Soc. Nephrol. 2002, 13, 96–107. [Google Scholar]
- Cufi, S.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Martin-Castillo, B.; Joven, J.; Menendez, J.A. Metformin against tgfbeta-induced epithelial-to-mesenchymal transition (emt): From cancer stem cells to aging-associated fibrosis. Cell. Cycle 2010, 9, 4461–4468. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Siamantouras, E.; Smith, S.W.; Cockwell, P.; Liu, K.K.; Squires, P.E. TGF beta modulates cell-to-cell communication in early epithelial-to-mesenchymal transition. Diabetologia 2012, 55, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Price, G.W.; Squires, P.E. Mind the gap: Connexins and cell-cell communication in the diabetic kidney. Diabetologia 2015, 58, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Kerr, M.I.; Wall, M.J.; Squires, P.E. Visfatin reduces gap junction mediated cell-to-cell communication in proximal tubule-derived epithelial cells. Cell. Physiol. Biochem. 2013, 32, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lv, L.L.; Cao, Y.H.; Zhang, J.D.; Wu, M.; Ma, K.L.; Phillips, A.O.; Liu, B.C. Urinary mrna markers of epithelial-mesenchymal transition correlate with progression of diabetic nephropathy. Clin. Endocrinol. 2012, 76, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Salvidio, G.; Lupia, E.; Ruotsalainen, V.; Verzola, D.; Deferrari, G.; Camussi, G. Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin ii. Diabetes 2003, 52, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Turk, T.; Leeuwis, J.W.; Gray, J.; Torti, S.V.; Lyons, K.M.; Nguyen, T.Q.; Goldschmeding, R. Bmp signaling and podocyte markers are decreased in human diabetic nephropathy in association with ctgf overexpression. J. Histochem. Cytochem. 2009, 57, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.T. The origin of renal fibroblasts and progression of kidney disease. Am. J. Pathol. 2010, 176, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Koesters, R.; Kaissling, B.; Lehir, M.; Picard, N.; Theilig, F.; Gebhardt, R.; Glick, A.B.; Hahnel, B.; Hosser, H.; Grone, H.J.; et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 2010, 177, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Szcykalski, L.M.; Springer, F.; Barnes, J.L. Origin of interstitial fibroblasts in an accelerated model of angiotensin ii-induced renal fibrosis. Am. J. Pathol. 2005, 167, 1193–1205. [Google Scholar] [CrossRef]
- Ru, Y.; Eyden, B.; Curry, A.; McWilliam, L.J.; Coyne, J.D. Actin filaments in human renal tubulointerstitial fibrosis: significance for the concept of epithelial-myofibroblast transformation. J. Submicrosc . Cytol. Pathol. 2003, 35, 221–233. [Google Scholar] [PubMed]
- Leroy, P.; Mostov, K.E. Slug is required for cell survival during partial epithelial-mesenchymal transition of hgf-induced tubulogenesis. Mol. Biol. Cell 2007, 18, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Duffield, J.S.; Humphreys, B.D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediat. Nephrol. 2012, 27, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Falke, L.L.; Gholizadeh, S.; Goldschmeding, R.; Kok, R.J.; Nguyen, T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015, 11, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Umezawa, A.; Takenaka, T.; Suzuki, H.; Okada, H. The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models. Kidney Int. 2015, 87, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Quaggin, S.E.; Kapus, A. Scar wars: Mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Harumi, T.; Watanabe, J.; Fujita, Y.; Honjo, J.; Shimizu, N.; Makino, Y.; Haneda, M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: A possible role of hif-1alpha expression and oxygen metabolism. Diabetes 2011, 60, 981–992. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeffler, I.; Wolf, G. Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells 2015, 4, 631-652. https://doi.org/10.3390/cells4040631
Loeffler I, Wolf G. Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells. 2015; 4(4):631-652. https://doi.org/10.3390/cells4040631
Chicago/Turabian StyleLoeffler, Ivonne, and Gunter Wolf. 2015. "Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction?" Cells 4, no. 4: 631-652. https://doi.org/10.3390/cells4040631
APA StyleLoeffler, I., & Wolf, G. (2015). Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells, 4(4), 631-652. https://doi.org/10.3390/cells4040631






