The Possible Roles of the Dentate Granule Cell’s Leptin and Other Ciliary Receptors in Alzheimer’s Neuropathology
Abstract
:1. Introduction
2. The Contribution of the Dentate Gyrus to Memory Formation
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.
3. Granule Cells
4. Involvement of the Granule Cell’s Primary Cilium in Adult Neurogenesis
5. Looking into the Dentate Granule Cell’s Cilium
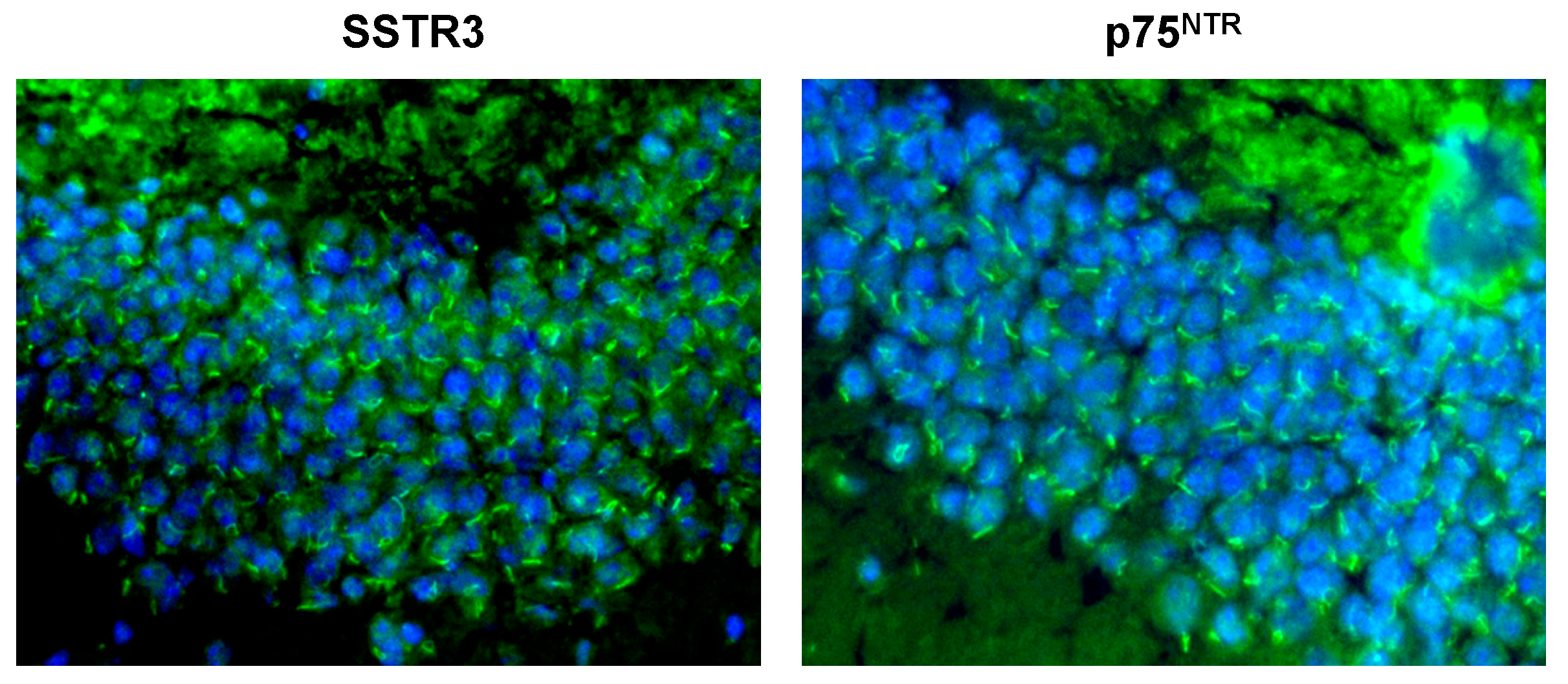
6. The Ciliary Leptin Receptor in Adult Neurogenesis and AD
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.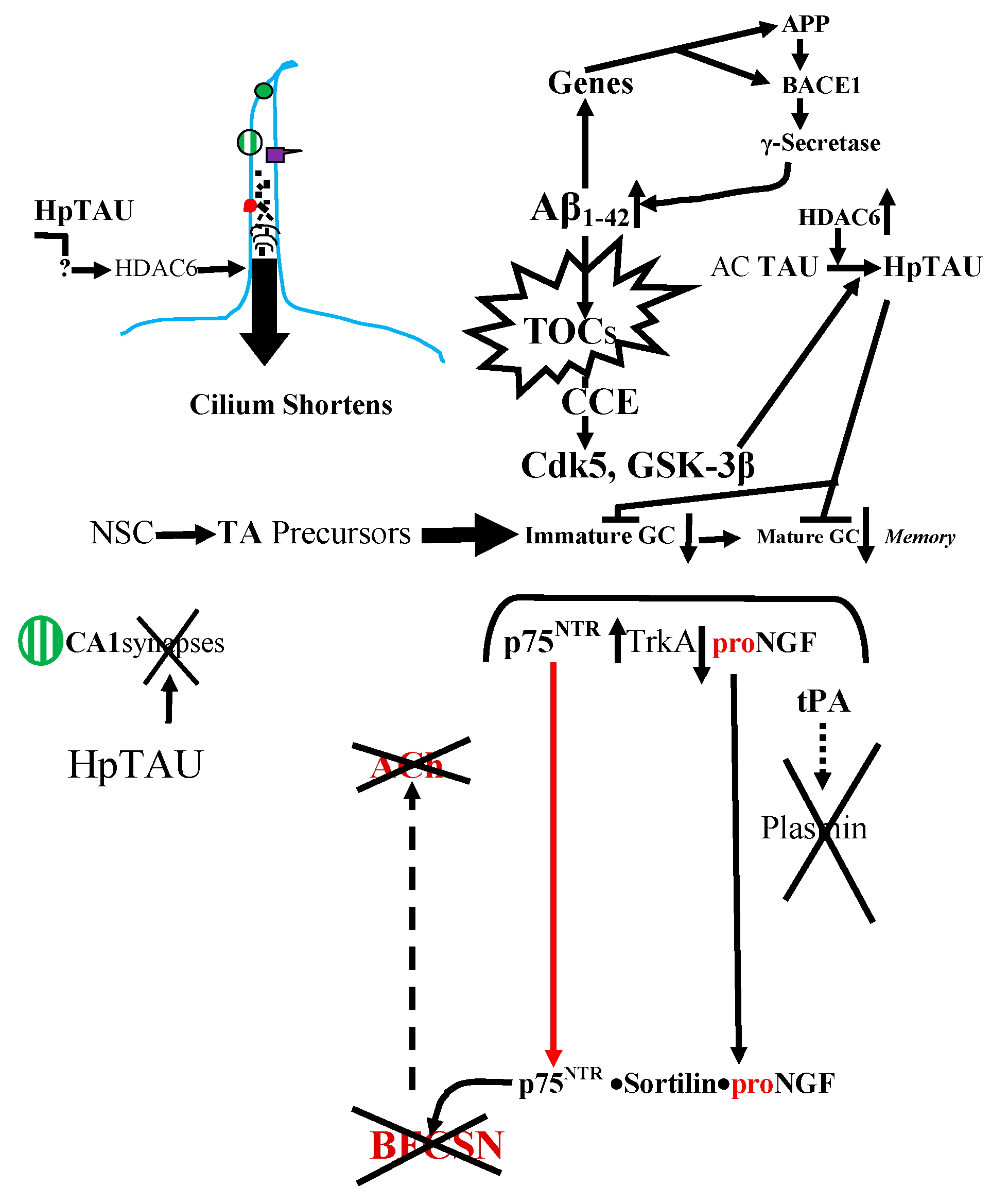
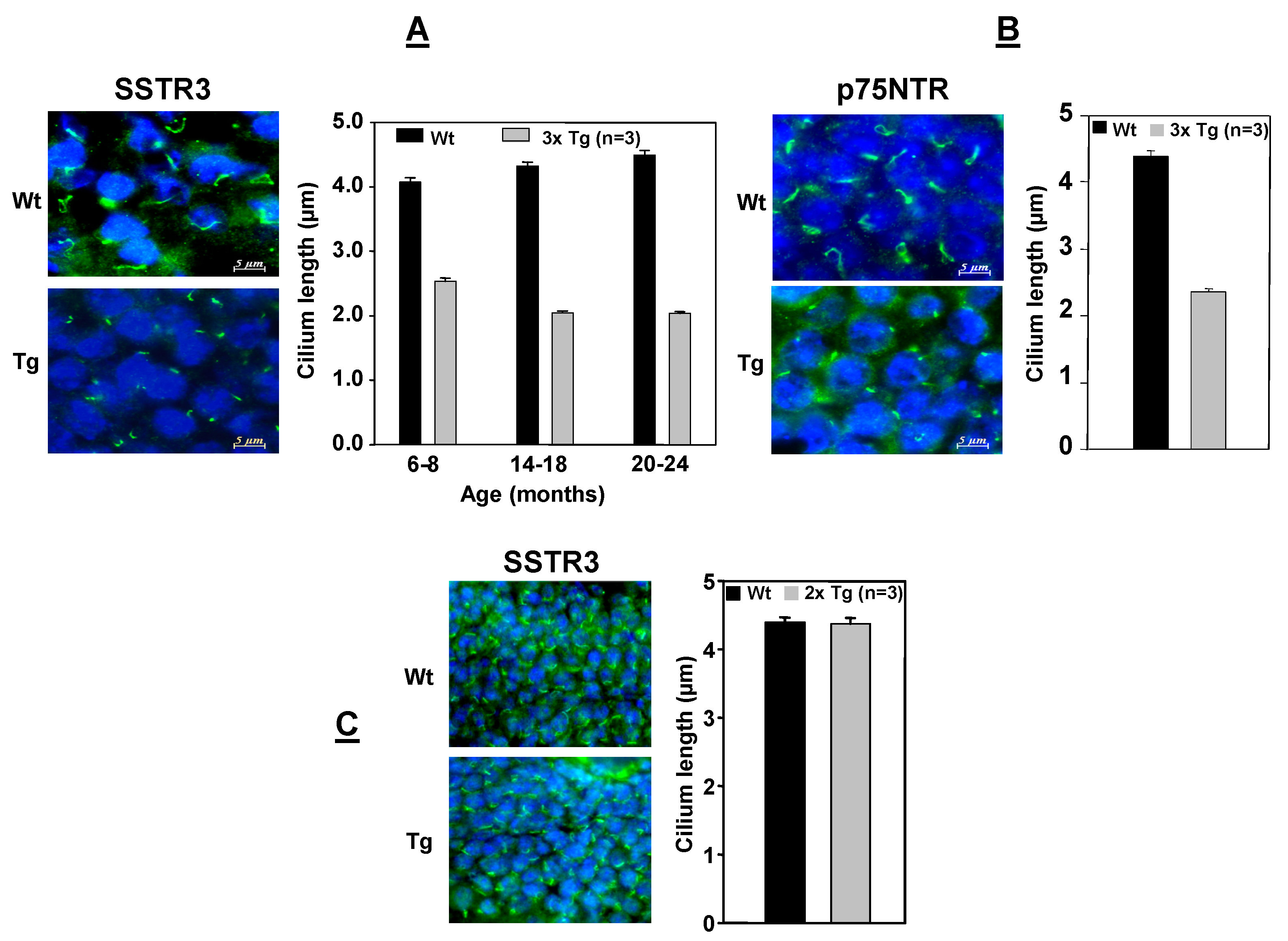
7. Leptin Reduces Aβs Production
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.
 LepRb;
LepRb;  p75NTR
p75NTR  SSTR3.
SSTR3.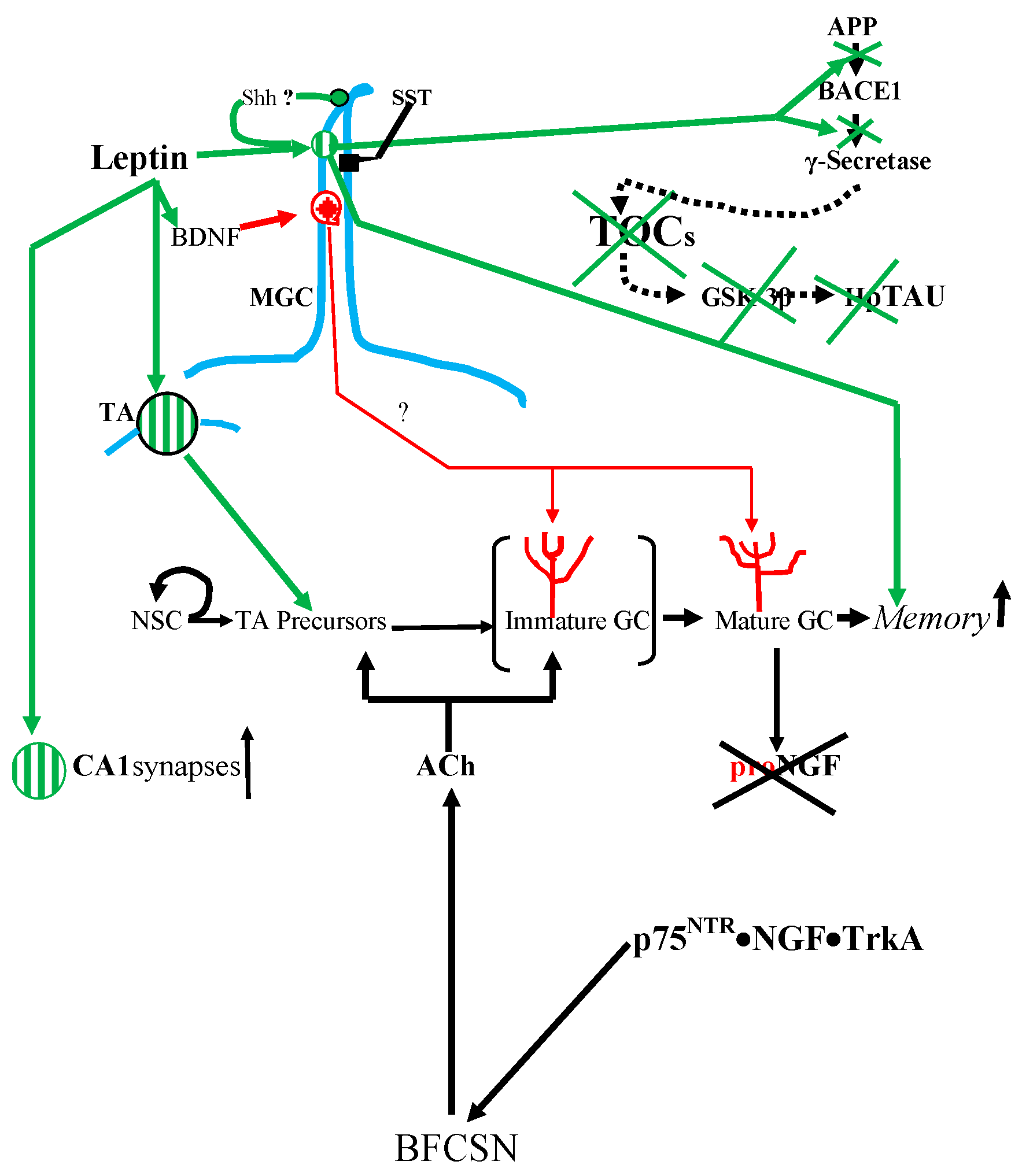
8. Where Do Dentate Granule Cells Put their LepRb?
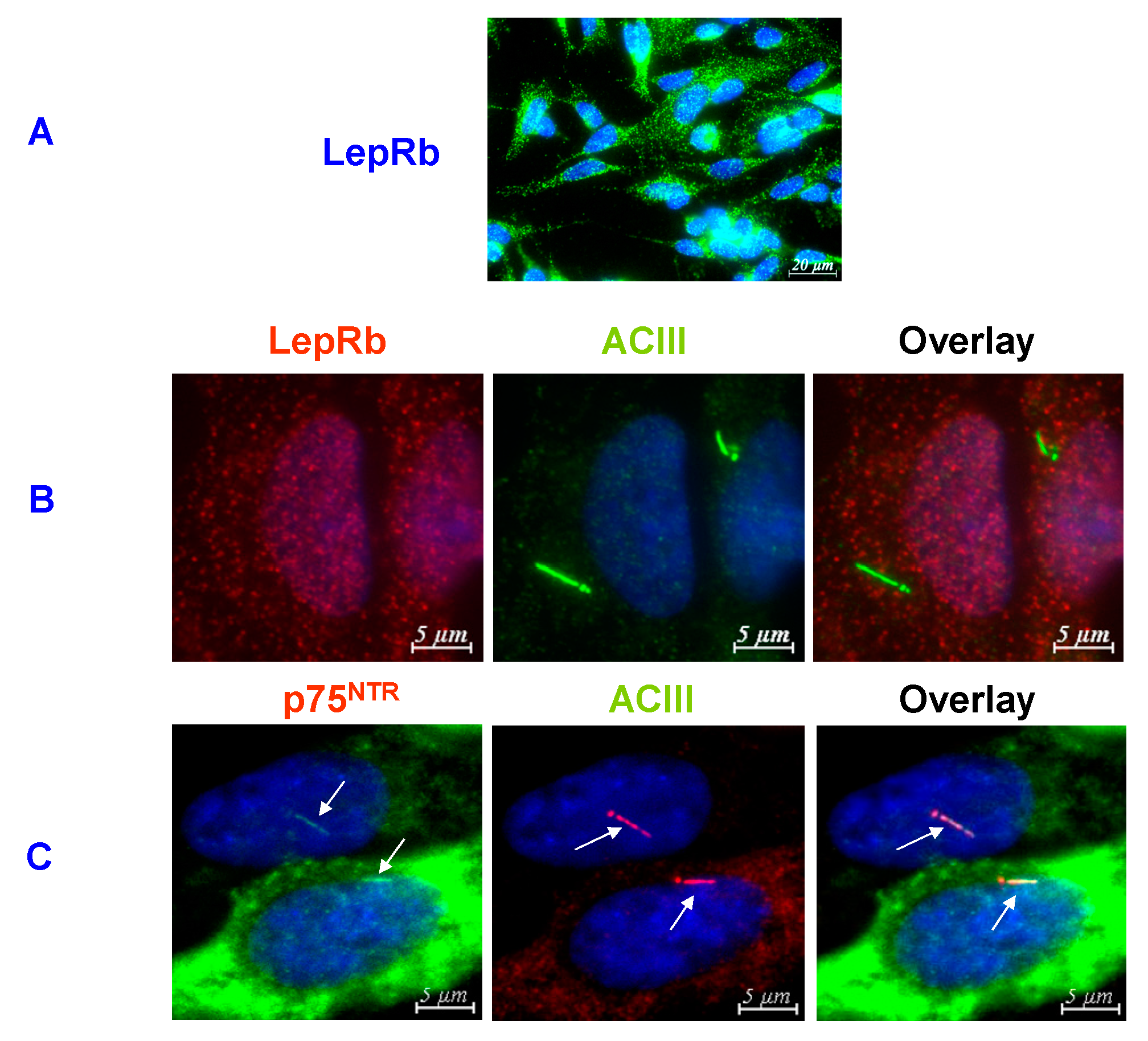
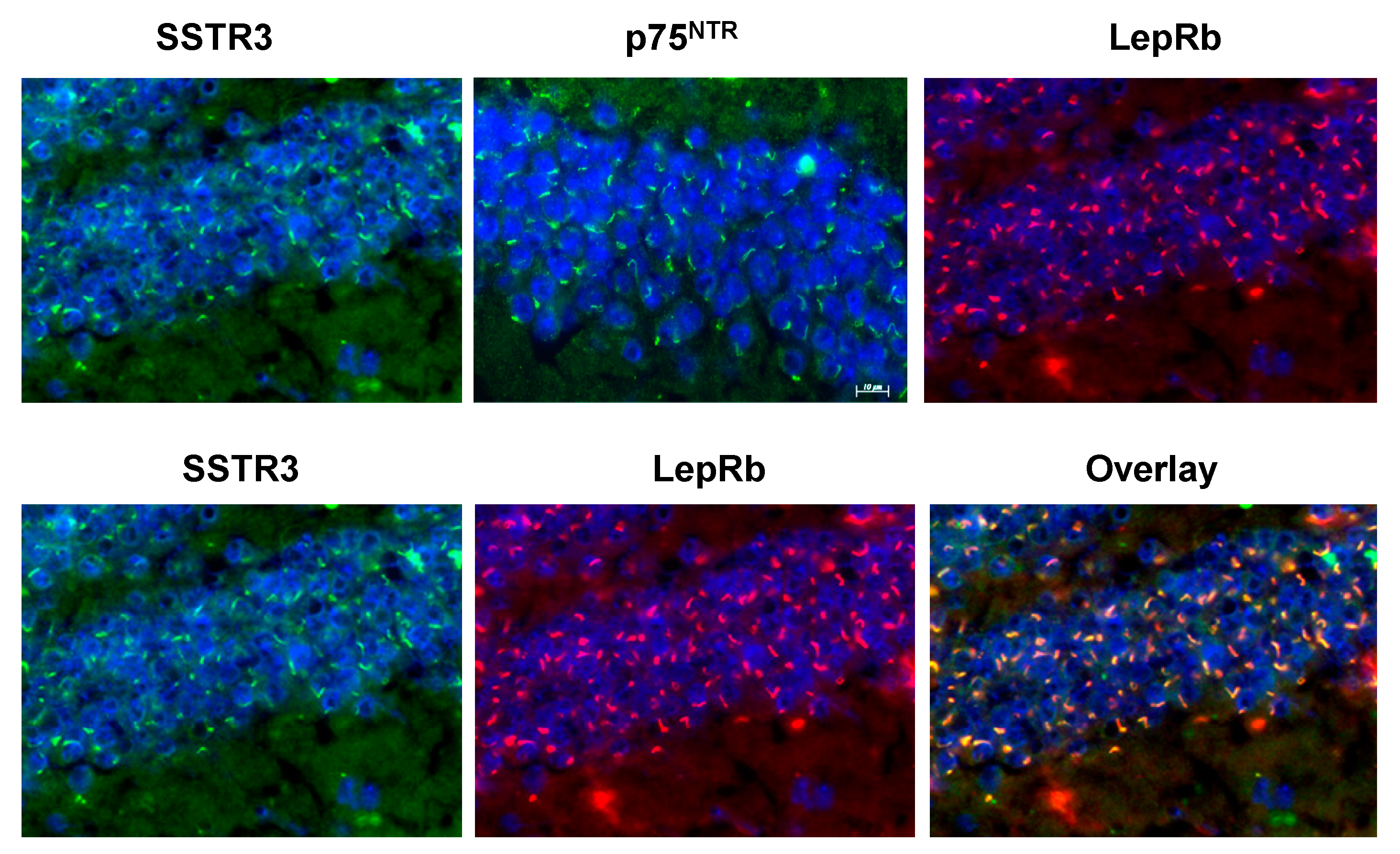
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Duvernoy, H.; Cattin, F.; Risold, P.Y. The Human Hippocampus, 4th ed.; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013. [Google Scholar]
- Ladyman, S.R.; Grattan, D.R. JAK-STAT and feeding. JAKSTAT 2013, 2, e23675. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Magnan, C.; Dunn-Meynell, A.; Le Foll, C. Metabolic sensing in the brain: Who, what, where and how? Endocrinology 2011, 152, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-D. Leptin and beyond: An odyssey to the central control of body weight. Yale J. Biol. Med. 2011, 84, 1–7. [Google Scholar] [PubMed]
- Tezapsidis, N.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Casadesus, G.; Robakis, N.K.; Wo-lozin, B.; Perry, G.; Zhu, X.; Greco, S.J.; et al. Leptin: A novel therapeutic strategy for Alz-heimer’s disease. J. Alzheimers Dis. 2009, 16, 731–740. [Google Scholar] [PubMed]
- Irving, A.J.; Harvey, J. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 369, 20130155. [Google Scholar] [CrossRef] [PubMed]
- Tezapsidis, N. Leptin as an anti-amyloidogenic biologic and methods for delaying the onset and reducing Alzheimer’s disease-like pathology. US Patent 8227408 B2, 24 July 2012. [Google Scholar]
- Dehaene, S. Consciousness and the Brain: Deciphering How the Brain Decodes Our Thoughts. The Viking Press: New York, NY, USA, 2014. [Google Scholar]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.J.; Fusi, S.; Hen, R. Adult neurogenesis in the mammalian hippocampus: Why the dentate gyrus? Learn. Mem. 2013, 20, 710–729. [Google Scholar] [CrossRef] [PubMed]
- Hunsaker, M.R.; Kesner, R.P. The operation of pattern separation and pattern completion processes associated with attributes or domains of memory. Neurosci. Behav. Rev. 2013, 37, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Seib, D.R.H.; Martin-Villalba, A. Neurogenesis in the normal ageing hippocampus: A mini review. Gerontology 2014. [Google Scholar] [CrossRef] [PubMed]
- Witter, M.P. Intrinsic and extrinsic wiring of CA3: Indications for connectional heterogeneity. Learn. Mem. 2007, 14, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.X.; Marchetto, M.C.; Gage, F.H. How to make a hippocampal dentate gyrus granule neuron. Development 2014, 141, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Adult neurogenesis in the dentate gyrus. Neurology 2013, 8, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Moscovitch, M.; Rosenbaum, R.S.; Gilboa, A.; Addis, D.R.; Westmacott, R.; Grady, C.; McAndrews, M.P.; Levine, B.; Black, S.M.; Winocur, G.; et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account. J. Anat. 2005, 207, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Rugg, M.D.; Johnson, J.D.; Park, H.; Uncapher, M.R. Encoding-retrieval overlap in human episodic memory: A functional neuroimaging perspective. Prog. Brain Res. 2008, 169, 339–352. [Google Scholar] [PubMed]
- Kempermann, G. Adult Neurogenesis 2; Oxford University Press: Oxford, UK; New York, NY, USA, 2011. [Google Scholar]
- Treves, A.; Tashiro, A.; Witter, M.E.; Moser, E.I. What is the dentate gyrus good for? Neuroscience 2008, 154, 1155–1172. [Google Scholar] [CrossRef] [PubMed]
- Nikashiba, T.; Cushman, J.D.; Pelkey, K.A.; Renauddineau, S.; Buhl, D.L.; McHugh, T.J.; Barrera, V.; Chittajallu, R.; Iwamoto, K.S.; McBain, C.J. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 2012, 149, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Mongiat, L.A.; Schinder, A.F. Neuroscience: A price to pay for adult neurogenesis. Science 2014, 344, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Basak, O.; Taylor, V. Stem cells of the adult mammalian brain and their niche. Cell Mol. Life Sci. 2009, 66, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Chen, Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat. Neurosci. 2011, 14, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Morrens, J.; van den Broeck, W.; Kempermann, G. Glial cells in adult neurogenesis. Glia 2012, 60, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Deng, W.; Gage, F.H. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009, 25, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Cameron, H.A. Regulation of neuronal birth, migration, and death in the rat dentate gyrus. Dev. Neurosci. 1996, 18, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Nacher, J.; Rosell, D.R.; Alonso-Liosa, G.; McEwen, B.S. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur. J. Neurosci. 2001, 13, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Antequara, D.; Portero, A.; Bolos, M.; Orive, G.; Hernández, R.M.; Pedraz, J.L.K.; Carro, E. Encapsulated VEGF-secreting cells enhance proliferation of neuronal progenitors in the hippocampus of AβPP/Ps1 mice. J. Alzheimers Dis. 2012, 29, 187–200. [Google Scholar]
- Attardo, A.; Fabel, K.; Krebs, J.; Haubensak, W.; Huttner, W.B.; Kempermann, G. Tis21 expression marks not only populations of neurogenic precursor cells but also new post-mitotic neurons in adult hippocampal neurogenesis. Cereb. Cortex 2010, 20, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Gervacio, S.; Murray, K.; Lledoi, P.-M. NKCCl controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev. 2011. [Google Scholar] [CrossRef]
- Bruel-Jungerman, E.; Lucassen, P.L.; Francis, F. Cholinergic influences on cortical development and adult neurogenesis. Behav. Brain Res. 2011, 221, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Van der Borght, K.; Mulder, J.; Keijser, J.; Eggen, B.J.; Luiten, P.G.; van der Zee, E.A. Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res. Bull. 2005, 67, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Krzisch, M.; Temprana, S.G.; Mongiat, L.A.; Armida, J.; Schmutz, V.; Virtanen, M.A.; Kocher-Braissant, J.; Kraftsik, R.; Vutskits, L.; Conzelmann, K.K. Pre-existing astrocytes form functional processes on neurons generated in the adult hippocampus. Brain Struct. Funct. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dahl, H.A. Fine structure of cilia in the rat cerebral cortex. Z. Zellforsch. Mikrosk. Anat. 1963, 60, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Berbari, N.F.; Malarkey, E.B.; Zaki, S.M.; Yazdi, S.M.; McNair, A.D.; Kippe, J.M.; Croyle, M.J.; Kraft, T.W.; Yoder, B.K. Hippocampal and cortical primary cilia are required for aversive memory in mice. PLoS ONE 2014, 9, e106576. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, B.; Gaudet, C.; Ménard, M.; Brown, L.; Atkinson, T.; Laferla, F.M.; Ito, S.; Armato, U.; dal Prà, I.; Whitfield, J. Reduction of the immunostainable length of the hppocampal dentate granule cells’ primary cilia in 3 x AD-transgenic mice producing human Aβ(1–42) and tau. Biochem. Biophys. Res. Commun. 2012, 427, 218–222. [Google Scholar] [CrossRef]
- Chakravarthy, B.; Gaudet, C.; Ménard, M.; Atkinson, T.; Chiarini, A.; dal Prà, I.; Whit-Field, J. The p75 neurotrophin receptor is localized to primary cilia in adult mouse hippocampal dentate gyrus granule cells. Biochem. Biophys. Res. Commun. 2010, 401, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Händel, M.; Schultz, S.; Stenarius, A.; Schreff, M.; Erdtmann-Vouriliotis, M.; Schmidt, H.; Wolf, G.; Höllt, V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 1999, 89, 909–926. [Google Scholar] [CrossRef]
- Stanić, D.; Malmgren, H.; He, H.; Scott, L.; Aperia, A.; Hökfelt, T. Developmental changes in frequency of the somatostatin receptor 3 protein. Brain Res. 2009, 1249, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, N.; Gu, Y.; Wang, J.; Jamoschka, S.; Takemaru, K.-L.; Levine, J.; Ge, S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci. 2012, 15, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Amador-Arjona, A.; Eelliott, J.; Miller, A.; Ginbey, A.; Pazour, G.J.; Enikolopov, G.; Roberts, A.J.; Terskikh, A.V. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: Implications for learning and memory. J. Neurosci. 2011, 31, 9933–9944. [Google Scholar] [CrossRef] [PubMed]
- Malicki, J.; Avidor-Reiss, T. From cytoplasm into the cilium: Bon voyage. Organogenesis 2014, 10, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Verteb-rate smoothened functions at the primary cilium. Nature 2005, 437, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog signaling and primary cilia are required for formation of adult neural stem cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Ocbina, P.J.R.; Anderson, K.V. The primary cilium as a hedgehog signal transduction machine. Methods Cell Biol. 2009, 94, 199–222. [Google Scholar] [PubMed]
- Breunig, J.J.; Sarkisian, M.R.; Arellano, J.I.; Morozov, Y.M.; Ayoub, A.E.; So-jitra, S.; Wang, B.; Flavell, R.A.; Rakic, P.; Town, T. Primary cilia regulate hippo-campal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 13127–13132. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; dal Prà, I.; Whitfield, J.F.; Armato, U. The killing of neurons by beta-amyloid peptides, prions, and proinflammatory cytokines. Ital. J. Anat. Embryol. 2006, 111, 221–246. [Google Scholar] [PubMed]
- Sotthibundhu, A.; Li, Q.X.; Thangnipon, W.; Coulson, E.J. Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol. Aging 2009, 30, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Makuch, R.; Baratta, J.; Karaelias, L.D.; Lauterborn, J.C.; Gall, C.M.; Yu, J.; Robertson, R.T. Arrival of afferents and the differentiation of target neurons: Studies of developing cholinergic projections to the dentate gyrus. Neuroscience 2001, 104, 81–91. [Google Scholar] [CrossRef]
- Chakravarthy, B.; Ménard, M.; Ito, S.; Gaudet, C.; dal Prà, I.; Armato, U.; Whit-Field, J.F. Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 1–10. [Google Scholar]
- Bernabeu, R.O.; Longo, F.M. The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Catts, V.S.; Al-Menhali, N.; Burne, T.H.; Colditz, M.J.; Coulson, E.J. The p75 neuro-trophin receptor regulates hippocampal neurogenesis and related behaviours. Eur. J. Neurosci. 2010, 28, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Colditz, M.J.; Catts, V.S.; Al-Menhali, N.; Osborne, G.W.; Bartlett, P.F.; Coulson, E.J. p75 neurotrophin receptor regulates basal and fluoxetine-stimulated hippocampal neurogenesis. Exp. Brain Res. 2010, 200, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Brigadski, T.; Erdmann, K.S.; Holtmann, B.; Sendtner, M.; Narz, F.; Lessmann, V. Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. J. Cell Sci. 2004, 117, 5803–5814. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Conway, D.; Parada, L.F.; Barbacid, M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell 1990, 61, 647–656. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Lo, D.C. Truncated and full-length trkB receptors regulate distinct modes of dendritic growth. Nat. Neurosci. 2000, 3, 342–349. [Google Scholar] [PubMed]
- He, Q.; Wang, G.; Wakade, S.; Dasgupta, S.; Dinkins, M.; Kong, J.N.; Spassieva, S.D.; Bieberich, E. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol. Biol. Cell 2014, 25, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Krishnamurthy, K.; Bieberich, E. Regulation of primary cilia formation by ceramide. J. Lipid Res. 2009, 50, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Brann, A.B.; Scott, R.; Neuberger, Y.; Abulafia, D.; Boldin, S.; Fainzilber, M.; Futerman, A.H. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J. Neurosci. 1999, 19, 8199–8206. [Google Scholar] [PubMed]
- Schwarz, A.; Futerman, A.H. Inhibition of sphingolipid synthesis, but not degradation, alters the rate of dendritic growth in cultured hippocampal neurons. Brain Res. Dev. Brain Res. 1998, 108, 125–130. [Google Scholar] [CrossRef]
- Dobrowski, R.T.; Carter, B.D. Coupling of the p75 neurotrophin receptor to sphingo-lipid signaling. Ann. N. Y. Acad. Sci. 1998, 845, 32–45. [Google Scholar] [CrossRef]
- Berbari, N.F.; Johnson, A.D.; Lewis, J.S.; Askwith, C.C.; Mykytyn, K. Identification ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 2008, 19, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Einstein, E.B.; Patterson, C.A.; Hon, B.J.; Regan, K.A.; Reddi, J.; Melnikoff, D.E.; Mateer, M.J.; Schultz, S.; Johnson, B.N.; Tallent, M.K. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J. Neurosci. 2010, 30, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Gu, C.; Mykytyn, K. Heteromerization of ciliary G protein-coupled receptors in the mouse brain. PLOS ONE 2012, 7, e46304. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Liu, L.; Provenzano, F.A.; Berman, D.E.; Profaci, C.P.; Sloan, R.; Mayeux, R.; Duff, K.E.; Small, S.A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 2014, 17, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Penzes, P.; Vanleeuwen, J.E. Impaired regulation of synaptic actin cytoskeleton in Alzheimer’s disease. Brain Res. 2011, 67, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hubin, E.; van Nuland, N.A.; Broersen, K.; Pauwels, K. Transient dynamics of Aβ contribute to toxicity in Alzheimer’s disease. Cell. Mol. Life Sci. 2014, 71, 3507–3521. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L. Synaptotoxic amyloid-β oligomers: A molecular basis for the cause, diag-nosis and treatment of Alzheimer’s disease? J. Alzheimers Dis. 2013, 33 (Suppl. S1), S49–S63. [Google Scholar] [PubMed]
- Selkoe, D.J.; Mandelkow, E.; Holtzman, D.M. (Eds.) The Biology of Alzheimer Disease; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012.
- Cheng, X.; Wu, J.; Geng, M.; Xiong, J. The role of synaptic activity in the regulation of amyloid beta levels in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Choy, R.W.; Cheng, Z.; Schekman, R. Amyloid precursor protein (APP) traffic from the cell surface via endosomes for amyloid β production in the trans-Golgi network. Proc. Natl. Acad. Sci. USA 2012, 109, E2077–E2082. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, S.; Wirths, O.; Bayer, T.A. Pyroglutamate amyloid-β (Aβ): A hatchet man in Alzheimer disease. J. Biol. Chem. 2011, 286, 38825–38832. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ramos, E.; Hervás-Aguilar, A.; Aguado-Liera, D.; Puebla-Jiménez, L.; Hernández-Pinto, A.M.; Barrios, V.; Arilla-Ferreiro, E. Somatostatin and Alzheimer’s disease. Mol. Cell. Endocrinol. 2008, 286, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Baglietto-Vargas, D.; del Rio, J.C.; Moreno-Gonzalez, I.; Santa-Maria, C.; Jimenez, S.; Caballero, C.; Lopez-Tellez, J.F.; Khan, Z.U.; Ruano, D.; et al. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PSI × APP transgenic model of Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ménard, M.; Atkinson, T.; Gaudet, C.; Brown, L.; Whitfield, J.; Chakravarthy, B. Involvement of insulin-like growth factor 1 receptor signaling in the amyloid-beta peptide oligomers-induced p75 neurotrophin receptor protein expression in mouse hippocampus. J. Alzheimers Dis. 2012, 31, 493–506. [Google Scholar] [PubMed]
- Bruno, A.M.; Leon, W.; Fragosos, G.; Mushynski, W.; Almazan, G.; Cuello, A.C. Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 2009, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Shruster, A.; Melamed, E.; Offen, D. Neurogenesis in the aged and neurodegenerative brain. Apoptosis 2010, 15, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Waldau, R.; Shetty, A.K. Behavior of neural stem cells in the Alzheimer brain. Cell. Mol. Life Sci. 2008, 65, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Zhang, Y.; Luo, H.; Zhu, S.; Yang, Y.; Zhao, T.; Wu, J.; Huang, Y.; Kong, J.; et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus 2009, 19, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Barucker, C.; Harmeier, A.; Weiske, J.; Fauler, B.; Albring, K.F.; Prokop, S.; Hildebrand, P.; Lurz, R.; Heppner, F.L.; Huber, O.; et al. Nuclear trans-location uncovers the amyloid peptide Aβ42 as a regulator of gene transcription. J. Biol. Chem. 2014, 289, 20182–20191. [Google Scholar] [CrossRef] [PubMed]
- Maloney, B.; Lahiri, D.K. The Alzheimer’s amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: Characterizing a new regulatory motif. Gene 2011, 488, 1–12. [Google Scholar]
- Dal Prà, I.; Chiarini, A.; Gui, L.; Chakravarthy, B.; Pacchiana, R.; Gardenal, E.; Whitfield, J.F.; Armato, U. Do astrocytes collaborate with neurons in spreading the “infectious” Aβ and Tau drivers of Alzheimer’s disease? Neuroscientist 2015, 21, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease patho-genesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Dolan, P.J.; Johnson, G.V.W. Histone deacetylase 6 interacts with the micro-tubule-associated protein tau. J. Neurochem. 2008, 106, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhao, K.; Wu, J.; Xu, Z.; Jin, S.; Zhang, Y.Q. HDAC6 mutations rescue human tauinduced induced microtubule defects in Drosophila. Proc. Natl. Acad. Sci. USA 110, 4604–4609. [CrossRef] [PubMed]
- Seward, M.E.; Swanson, E.; Norambuena, A.; Reimann, A.; Cochran, J.N.; Li, R.; Robertson, E.D.; Bloom, G.S. Amyloid-β signals through tau to drive ectopic neuronal cell cycle reentry in Alzheimer’s disease. J. Cell Sci. 2013, 126, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.; Sherman, M.A.; Amar, F.; Nuvolone, M.; Schneider, J.A.; Bennett, D.A.; Aguzzi, A.; Lesnć, S.E. The complex PrP(c) Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. J. Neurosci. 2012, 32, 16857–16871. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Himmelstein, D.S.; Lancia, J.K.; Binder, L.I. Tau oligomers and tau toxicity in neurodegenerative disease. Biochem. Soc. Trans. 2012, 40, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, R.M.; Polanco, J.-C.; Ittner, L.M.; Götz, J. Tau aggregation and its interplay amyloid-β. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Absalon, S.; Kochanek, D.M.; Raghavan, V.; Krichevsky, A.M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry tau phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 2013, 33, 14645–14659. [Google Scholar] [CrossRef] [PubMed]
- Hussman, J.W.; Nochlin, D.; Vincent, J. Mitotic activation: A convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol. Aging 2000, 21, 815–828. [Google Scholar] [CrossRef]
- Greco, S.J.; Sarkar, S.; Casadesus, G.; Zhu, X.; Smith, M.A.; Ashford, J.W.; Johston, J.M.; Tezapsidis, N. Leptin inhibits glycogen synthase kinase-3β to prevent tau phosphorylation in neuronal cells. Neurosci. Lett. 2009, 455, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Ghribi, O. Leptin signaling and Alzheimer’s disease. Am. J. Neurodegener. Dis. 2012, 1, 245–265. [Google Scholar] [PubMed]
- McGregor, G.; Malekizadeh, Y.; Harvey, J. Minireview: Food for thought: Regulation of synaptic function by metabolic hormones. Mol. Endocrinol. 2015, 29, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Raza, S.; Meiers, C.; Ghribi, O. Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochim. Biophys Acta 2014, 1842, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, R.; Alvira-Botero, M.X.; Robayo, O.; Antequera, D.; Garzón, M.; Martin-Moreno, A.M.; Brera, B.; de Ceballos, M.I.; Carro, E. Leptin gene therapy attenuates neural damages evoked by amyloid-β and reduces memory deficits in APP/PS1 mice. Gene Ther. 2014, 21, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.-Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.-Y. Leptin restores adult hippo-campal neurogenesis suppressed by chronic unpredictable stress and reverses glucocorticoid-induced inhibition of GSK3β/β-catenin signaling. Mol. Psychiatry 2012, 17, 790–808. [Google Scholar]
- Pérez-González, R.; Antequera, D.; Vargas, T.; Spuch, C.; Bolós, M.; Carro, E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kenney, A.M.; Rowitch, D.H. Sonic hedgehog promotes G1 cyclin expression expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 2000, 20, 9055–9067. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.J.; O’Malley, D.; Irving, A.J.; Ashford, M.L.; Harvey, J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI3-kinase-driven activation of BK channels. J. Physiol. 2002, 545, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Armato, U.; Chiarini, A.; Chakravarthy, B.; Chioffi, F.; Pacchiana, R.; Colarusso, E.; Whitfield, J.F.; dal Prà, I. Calcium-sensing receptor antagonist (calcilytic) (NPS2143 specifically blocks the the increased secretion of endogenous fibrillary or soluble Aβ25–35 in human cortical astrocytes and neurons—Therapeutic relevance to Alzheimer’s disease. Biochim. Biophys. Acta 2013, 1832, 1634–1652. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, B.; Ménard, M.; Brown, L.; Hewitt, M.; Atkinson, T.; Whitfield, J.F. A synthetic peptide corresponding to a region of the pericentriolar material 1 (PCM-1) protein binds β amyloid (Aβ1–42) oligomers. J. Neurochem. 2013, 126, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.J.; Sarkar, S.; Johnston, J.M.; Zhu, X.; Su, B.; Casadesus, G.; Ashford, J.W.; Smith, M.A.; Tezapsidis, N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem. Biophys. Res. Commun. 2008, 376, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, B.; Gaudet, C.; Ménard, M.; Atkinson, T.; Brown, L.; Laferla, F.M.; Armato, U.; Whitfield, J.F. Amyloid-beta peptides stuimulate the expression of the p75(NTR) neurotrophin receptor in SHSY5Y human neuroblastoma cells and AD transgenic mice. J. Alzheimers Dis. 2010, 19, 915–925. [Google Scholar] [PubMed]
- Whitfield, J.F.; Chakravarthy, B.; Chiarini, A.; Dal Prá, I.; Armato, U. The leptin receptor, a driver of adult neurogenesis that may treat Alzheimer’s disease, has been found in murine dentate gran-ule cells’ ciliary toolbox. In Horizons in Neuroscience Research; Costa, A., Villalba, E., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2015; Volume 21. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitfield, J.F.; Chiarini, A.; Dal Prà, I.; Armato, U.; Chakravarthy, B. The Possible Roles of the Dentate Granule Cell’s Leptin and Other Ciliary Receptors in Alzheimer’s Neuropathology. Cells 2015, 4, 253-274. https://doi.org/10.3390/cells4030253
Whitfield JF, Chiarini A, Dal Prà I, Armato U, Chakravarthy B. The Possible Roles of the Dentate Granule Cell’s Leptin and Other Ciliary Receptors in Alzheimer’s Neuropathology. Cells. 2015; 4(3):253-274. https://doi.org/10.3390/cells4030253
Chicago/Turabian StyleWhitfield, James F., Anna Chiarini, Ilaria Dal Prà, Ubaldo Armato, and Balu Chakravarthy. 2015. "The Possible Roles of the Dentate Granule Cell’s Leptin and Other Ciliary Receptors in Alzheimer’s Neuropathology" Cells 4, no. 3: 253-274. https://doi.org/10.3390/cells4030253
APA StyleWhitfield, J. F., Chiarini, A., Dal Prà, I., Armato, U., & Chakravarthy, B. (2015). The Possible Roles of the Dentate Granule Cell’s Leptin and Other Ciliary Receptors in Alzheimer’s Neuropathology. Cells, 4(3), 253-274. https://doi.org/10.3390/cells4030253





