Inhibition of Tumor Microenvironment-Driven JAK-STAT Signaling Enhances Response to Arginine Deprivation Therapy in Triple-Negative Breast Cancer
Highlights
- Arginine depletion suppresses TNBC cell growth in vitro but not in vivo, due to a TME-mediated arginine supply and JAK-STAT activation.
- ASS1 expression in human TNBC tumors correlates with JAK-STAT gene expression.
- Combining JAK inhibition with arginine depletion significantly suppresses tumor growth.
- ASS1 expression may help identify breast tumors with active cytokine/JAK-STAT signaling and refine patients’ stratification for targeted therapies.
- Inducing metabolic vulnerability through arginine depletion uncovers a targetable TME-driven survival mechanism, suggesting a new potential immunotherapeutic approach for TNBC.
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Animal Studies
2.2. Flow Cytometry of 4T1 Tumors
2.3. NMF and BMDM Production
2.4. Cell Lines and Lentiviral Infection
2.5. Arginine-Manipulated Plasmax Medium
2.6. Three-Dimensional (3D) Spheroid Culture and Co-Culture Systems
2.7. Flow Cytometry of In Vitro Samples and Apoptosis Assays
2.8. LC-MS Analysis of Amino Acids
2.9. XTT Survival Assay
2.10. Western Blotting
2.11. RNA Extraction and qPCR
2.12. Spheroid Area Quantification
2.13. Single-Cell RNA-Seq (scArg-Screen)
2.14. scRNA-Seq Pre-Processing, Clustering, and Differential Expression Analysis
2.15. Pseudo-Bulking for Robust DGE
2.16. IFNγ-JAK-STAT DEG Visualization
2.17. TCGA-TNBC Analysis
2.18. TCGA-BRCA: ASS1-High vs. ASS1-Low
2.19. Statistical Analysis
3. Results
3.1. ASS1 Expression in Breast Tumors Correlates with Altered TME Composition and Signaling
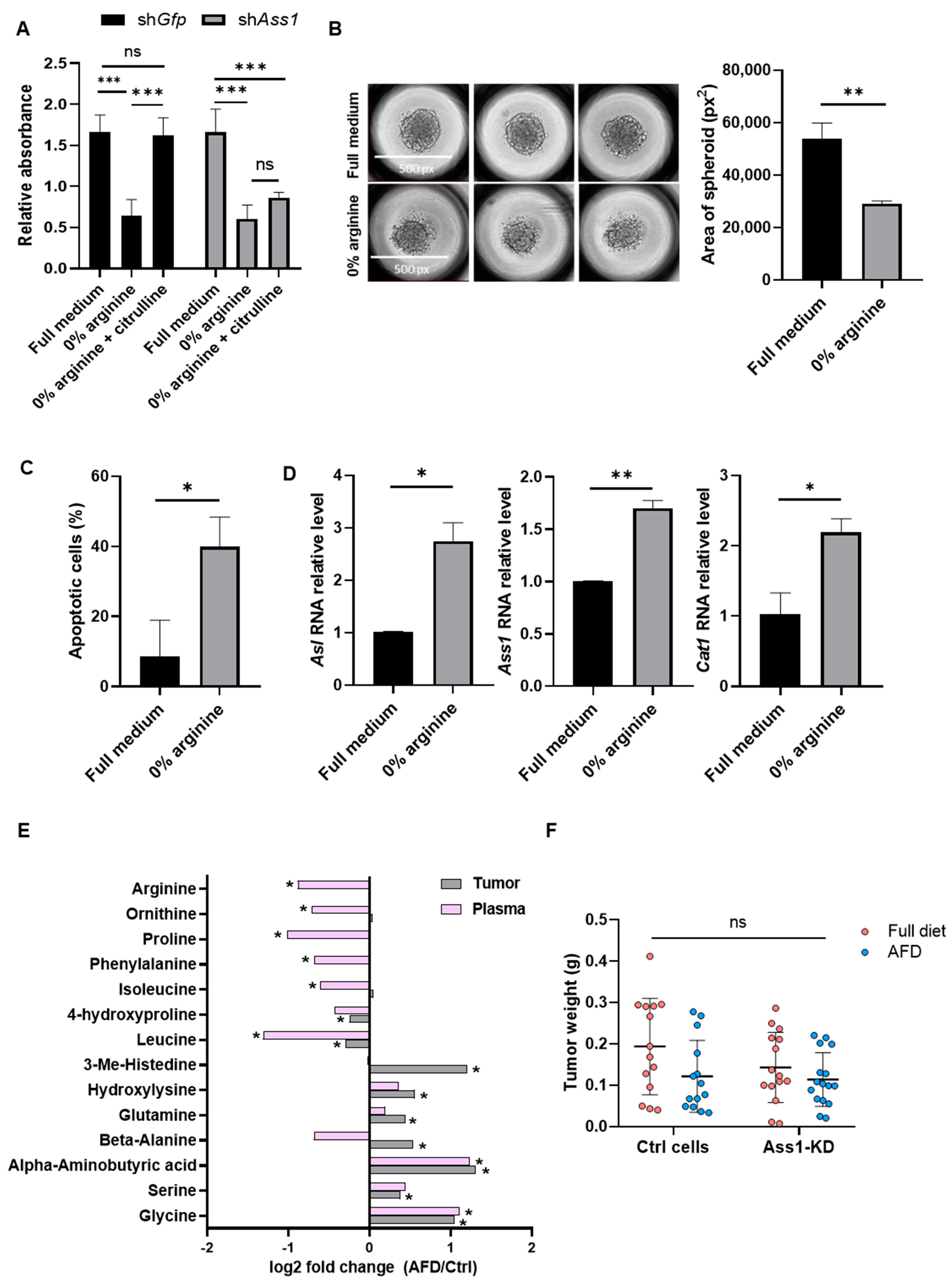
3.2. Arginine Deprivation Strongly Impairs the Survival of 4T1 TNBC Cells In Vitro
3.3. 4T1 TNBC Tumors Resist Arginine Depletion In Vivo
3.4. Arginine Deprivation Triggers Stress Responses and Broad Transcriptional Changes in Cancer Cells In Vivo
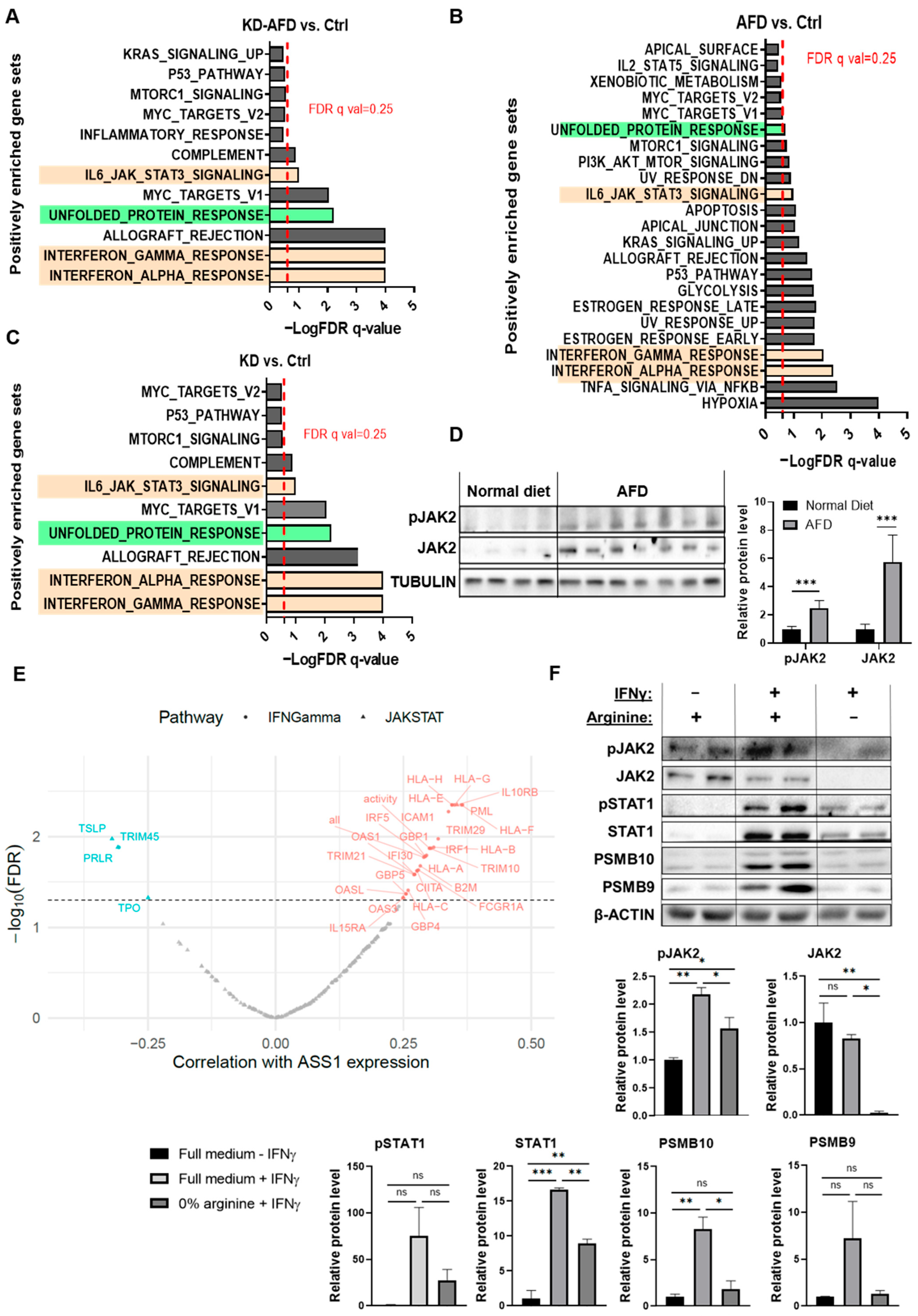
3.5. Arginine Starvation Upregulates IFNγ-JAK-STAT Signaling in 4T1 Tumors
3.6. 4T1 Cancer Cells Show Suppressed JAK-STAT Signaling Under Arginine Depletion
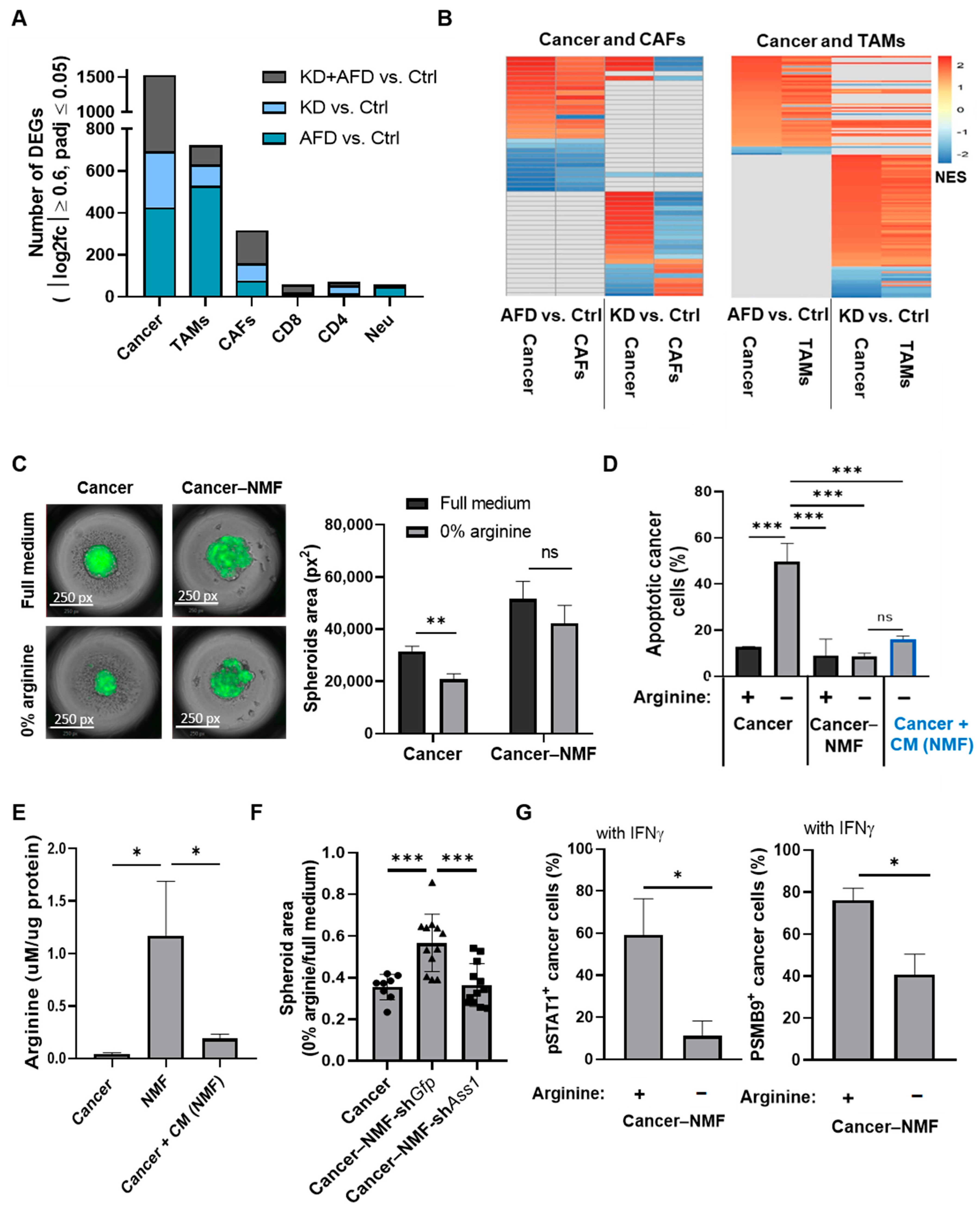
3.7. Stromal and Immune Cells Exhibit Distinct Transcriptional Responses to Arginine Depletion
3.8. Fibroblast-Derived Arginine Supports Cancer Cell Survival but Does Not Trigger Elevated IFNγ-JAK-STAT Signaling Following Arginine Deprivation
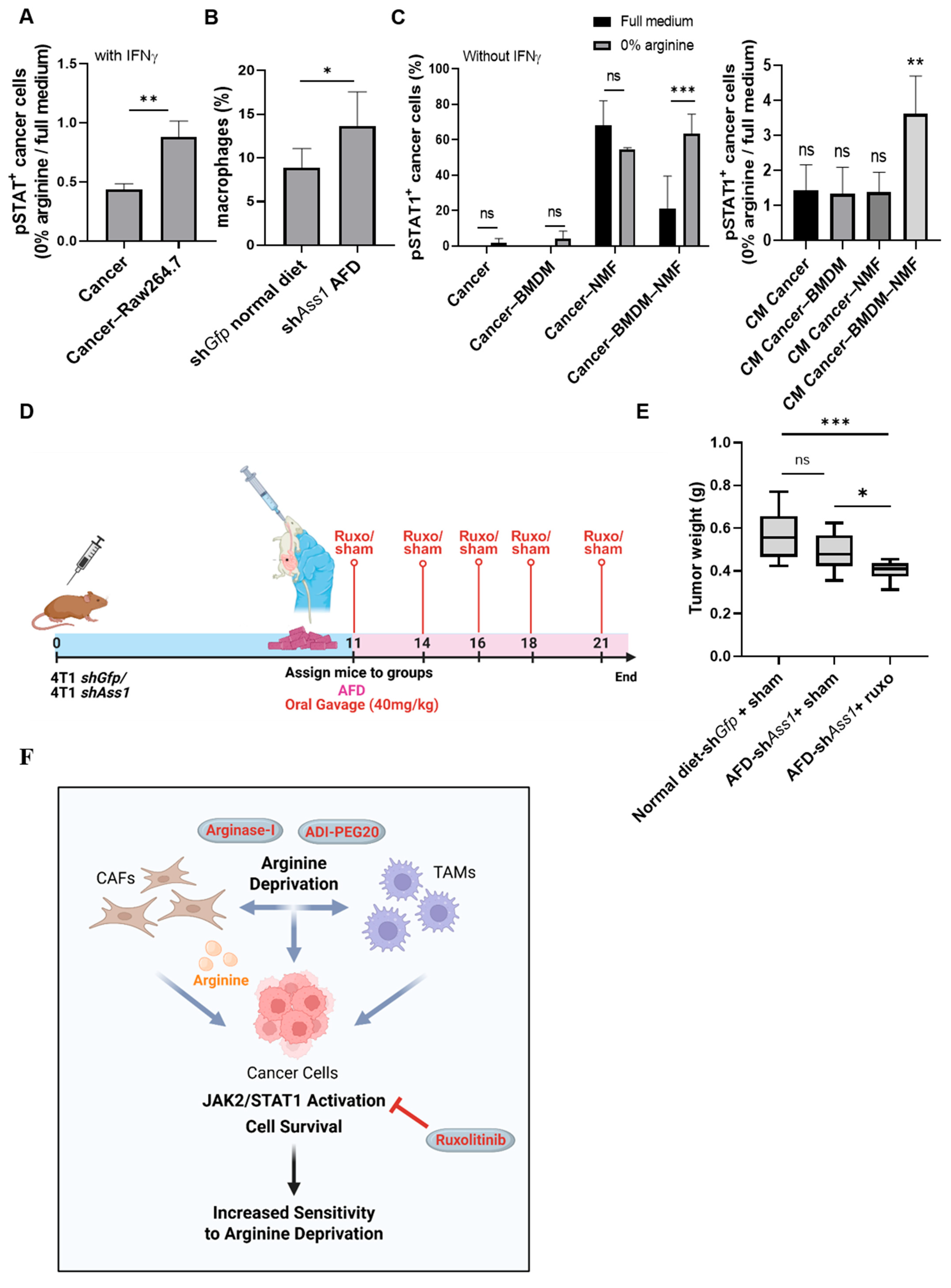
3.9. Cooperative Cross-Talk Between Cancer Cells, Fibroblasts, and Macrophages Mediates JAK-STAT Activation Under Arginine Deprivation
3.10. Combined Arginine Deprivation and JAK Inhibition Reveals a Therapeutically Targetable Vulnerability in TNBC
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronte, V.; Zanovello, P. Regulation of Immune Responses by L-Arginine Metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2020, 39, 28–37. [Google Scholar] [CrossRef]
- Bai, D.; Zhou, Y.; Jing, L.; Guo, C.; Yang, Q. Arginine Metabolism in Cancer Biology and Immunotherapy. Immune Netw. 2025, 25, e30. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Creelan, B.C.; Sarkodie, T.; Nolan, L.; Taylor, P.; Olevsky, O.; Grosso, F.; Cortinovis, D.; Chitnis, M.; Roy, A.; et al. Pegargiminase Plus First-Line Chemotherapy in Patients with Nonepithelioid Pleural Mesothelioma: The ATOMIC-Meso Randomized Clinical Trial. JAMA Oncol. 2024, 10, 475–483. [Google Scholar] [CrossRef]
- Fenwick, N.; Weston, R.; Wheatley, K.; Hodgson, J.; Marshall, L.; Elliott, M.; Makin, G.; Ng, A.; Brennan, B.; Lowis, S.; et al. PARC: A Phase I/II Study Evaluating the Safety and Activity of Pegylated Recombinant Human Arginase BCT-100 in Relapsed/Refractory Cancers of Children and Young Adults. Front. Oncol. 2024, 14, 1296576. [Google Scholar] [CrossRef]
- Assi, G.; Faour, W.H. Arginine Deprivation as a Treatment Approach Targeting Cancer Cell Metabolism and Survival: A Review of the Literature. Eur. J. Pharmacol. 2023, 953, 175830. [Google Scholar] [CrossRef]
- Phillips, M.M.; Sheaff, M.T.; Szlosarek, P.W. Targeting Arginine-Dependent Cancers with Arginine-Degrading Enzymes: Opportunities and Challenges. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2013, 45, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Li, R.; Wang, Q.; Yu, L.; Zi, F. A Pan-Cancer Analysis of the Role of Argininosuccinate Synthase 1 in Human Tumors. Front. Oncol. 2023, 13, 1049147. [Google Scholar] [CrossRef]
- Keshet, R.; Lee, J.S.; Adler, L.; Iraqi, M.; Ariav, Y.; Lim, L.Q.J.; Lerner, S.; Rabinovich, S.; Oren, R.; Katzir, R.; et al. Targeting Purine Synthesis in ASS1-Expressing Tumors Enhances the Response to Immune Checkpoint Inhibitors. Nat. Cancer 2020, 1, 894–908. [Google Scholar] [CrossRef] [PubMed]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine Deprivation and Argininosuccinate Synthetase Expression in the Treatment of Cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- Almazrouei, K.M.; Mishra, V.; Pandya, H.; Sambhav, K.; Bhavsar, S.N. Tumor Microenvironment and Its Role in Cancer Progression: An Integrative Review. Cureus 2025, 17, e92707. [Google Scholar] [CrossRef]
- Vande Voorde, J.; Ackermann, T.; Pfetzer, N.; Sumpton, D.; Mackay, G.; Kalna, G.; Nixon, C.; Blyth, K.; Gottlieb, E.; Tardito, S. Improving the Metabolic Fidelity of Cancer Models with a Physiological Cell Culture Medium. Sci. Adv. 2019, 5, eaau7314. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Patkar, S.; Lee, J.S.; Gertz, E.M.; Robinson, W.; Schischlik, F.; Crawford, D.R.; Schäffer, A.A.; Ruppin, E. Deconvolving Clinically Relevant Cellular Immune Cross-Talk from Bulk Gene Expression Using CODEFACS and LIRICS Stratifies Patients with Melanoma to Anti-PD-1 Therapy. Cancer Discov. 2022, 12, 1088–1105. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Rickard, J.A.; McDonald, W.J.; Thomas, L.N.; Too, C.K.L. CAT-1-Mediated Arginine Uptake and Regulation of Nitric Oxide Synthases for the Survival of Human Breast Cancer Cell Lines. J. Cell. Biochem. 2011, 112, 1084–1092. [Google Scholar] [CrossRef]
- Lurie, R.H.; Platanias, L.C. Mechanisms of Type-I- and Type-II-Interferon-Mediated Signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Megger, D.A.; Philipp, J.; Le-Trilling, V.T.K.; Sitek, B.; Trilling, M. Deciphering of the Human Interferon-Regulated Proteome by Mass Spectrometry-Based Quantitative Analysis Reveals Extent and Dynamics of Protein Induction and Repression. Front. Immunol. 2017, 8, 291020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. Molecular Mechanisms of IFN-Gamma to up-Regulate MHC Class I Antigen Processing and Presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT Pathway: Extracellular Signals, Diseases, Immunity, and Therapeutic Regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef]
- Plaster, M.; Singh, S.; Tavana, H. Fibroblasts Promote Proliferation and Matrix Invasion of Breast Cancer Cells in Co-Culture Models. Adv. Ther. 2019, 2, 1900121. [Google Scholar] [CrossRef]
- Sharon, Y.; Raz, Y.; Cohen, N.; Ben-Shmuel, A.; Schwartz, H.; Geiger, T.; Erez, N. Tumor-Derived Osteopontin Reprograms Normal Mammary Fibroblasts to Promote Inflammation and Tumor Growth in Breast Cancer. Cancer Res. 2015, 75, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Madera, L.; Greenshields, A.; Coombs, M.R.P.; Hoskin, D.W. 4T1 Murine Mammary Carcinoma Cells Enhance Macrophage-Mediated Innate Inflammatory Responses. PLoS ONE 2015, 10, 0133385. [Google Scholar] [CrossRef]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-Associated Macrophage Derived IL-6 Enriches Cancer Stem Cell Population and Promotes Breast Tumor Progression via Stat-3 Pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef]
- Ostojic, A.; Vrhovac, R.; Verstovsek, S. Ruxolitinib: A New JAK1/2 Inhibitor That Offers Promising Options for Treatment of Myelofibrosis. Future Oncol. 2011, 7, 1035. [Google Scholar] [CrossRef]
- Hix, L.M.; Karavitis, J.; Khan, M.W.; Shi, Y.H.; Khazaie, K.; Zhang, M. Tumor STAT1 Transcription Factor Activity Enhances Breast Tumor Growth and Immune Suppression Mediated by Myeloid-Derived Suppressor Cells. J. Biol. Chem. 2013, 288, 11676–11688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, S.; Liu, X.; Qi, D.; He, X.; Chen, D. STAT1 Mediates the Transcription of CircIFI30 and Promotes the Progression of Triple-Negative Breast Cancer by Up-Regulating CDCA4. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 1–13. [Google Scholar] [CrossRef]
- Beziaud, L.; Young, C.M.; Alonso, A.M.; Norkin, M.; Minafra, A.R.; Huelsken, J. IFNγ-Induced Stem-like State of Cancer Cells as a Driver of Metastatic Progression Following Immunotherapy. Cell Stem Cell 2023, 30, 818–831.e6. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S.; Srivastava, R.K.; Nandi, A.; Thacker, G.; Murali, H.; Kim, S.; Baldeon, M.; Tobias, J.; Blanco, M.A.; et al. Loss of ELF5-FBXW7 Stabilizes IFNGR1 to Promote the Growth and Metastasis of Triple-Negative Breast Cancer through Interferon-γ Signalling. Nat. Cell Biol. 2020, 22, 591–602. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, R.; Wu, W.; Lei, L. Comprehensive Network Analysis of the Molecular Mechanisms Associated with Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Genet. 2020, 245, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sabaawy, H.E.; Ryan, B.M.; Khiabanian, H.; Pine, S.R. JAK/STAT of All Trades: Linking Inflammation with Cancer Development, Tumor Progression and Therapy Resistance. Carcinogenesis 2021, 42, 1411. [Google Scholar] [CrossRef]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ Signaling and Response: The Barricade to Tumor Immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science (1979) 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tishler, H.; Ziman, S.; Cheng, K.; Wang, K.; Sanghvi, N.; Adler, L.; Stelzer, G.; Maniriho, H.; Dassa, B.; Bab-Dinitz, E.; et al. Inhibition of Tumor Microenvironment-Driven JAK-STAT Signaling Enhances Response to Arginine Deprivation Therapy in Triple-Negative Breast Cancer. Cells 2026, 15, 25. https://doi.org/10.3390/cells15010025
Tishler H, Ziman S, Cheng K, Wang K, Sanghvi N, Adler L, Stelzer G, Maniriho H, Dassa B, Bab-Dinitz E, et al. Inhibition of Tumor Microenvironment-Driven JAK-STAT Signaling Enhances Response to Arginine Deprivation Therapy in Triple-Negative Breast Cancer. Cells. 2026; 15(1):25. https://doi.org/10.3390/cells15010025
Chicago/Turabian StyleTishler, Hila, Shahar Ziman, Kuoyuan Cheng, Kun Wang, Neel Sanghvi, Lital Adler, Gil Stelzer, Hillary Maniriho, Bareket Dassa, Elizabeta Bab-Dinitz, and et al. 2026. "Inhibition of Tumor Microenvironment-Driven JAK-STAT Signaling Enhances Response to Arginine Deprivation Therapy in Triple-Negative Breast Cancer" Cells 15, no. 1: 25. https://doi.org/10.3390/cells15010025
APA StyleTishler, H., Ziman, S., Cheng, K., Wang, K., Sanghvi, N., Adler, L., Stelzer, G., Maniriho, H., Dassa, B., Bab-Dinitz, E., Levi, M., Galai, S., Goldman, O., Ariav, Y., Darzi, N., Ezagouri, S., Nimni, N., Rosenfeld, N., Rotkopf, R., ... Erez, A. (2026). Inhibition of Tumor Microenvironment-Driven JAK-STAT Signaling Enhances Response to Arginine Deprivation Therapy in Triple-Negative Breast Cancer. Cells, 15(1), 25. https://doi.org/10.3390/cells15010025







