Hinokiflavone as a Potential Antitumor Agent: From Pharmacology to Pharmaceutics
Abstract
1. Introduction
2. Methodology
3. Pharmacological Mechanisms of HF’s Anticancer Effects
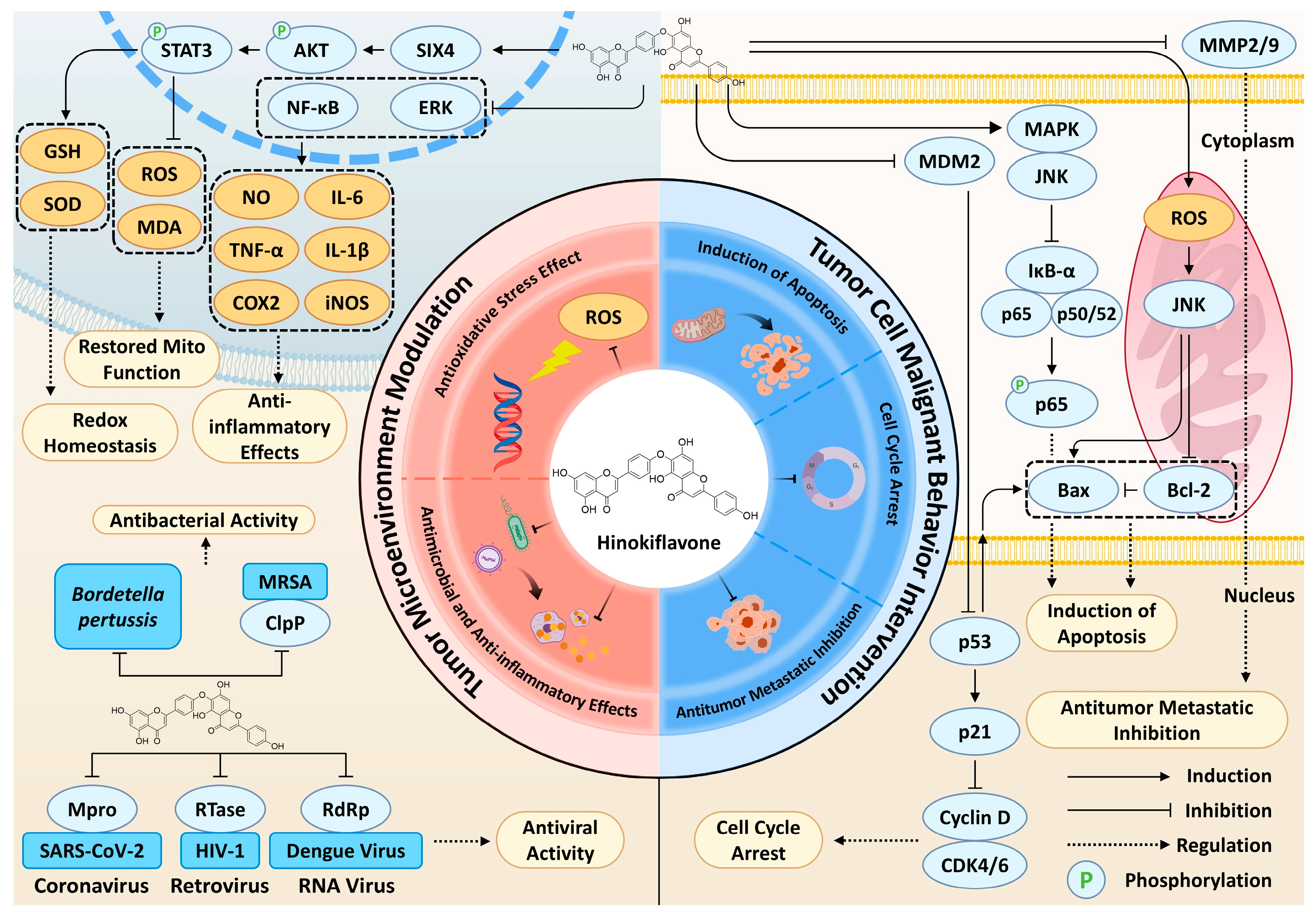
3.1. Induction of Apoptosis
3.2. Cell Cycle Arrest
3.3. Inhibition of Tumor Metastasis
3.4. Antioxidant Effects
3.5. Antimicrobial and Anti-Inflammatory Effects
3.6. Safety, Toxicity, and Pharmacological Limitations
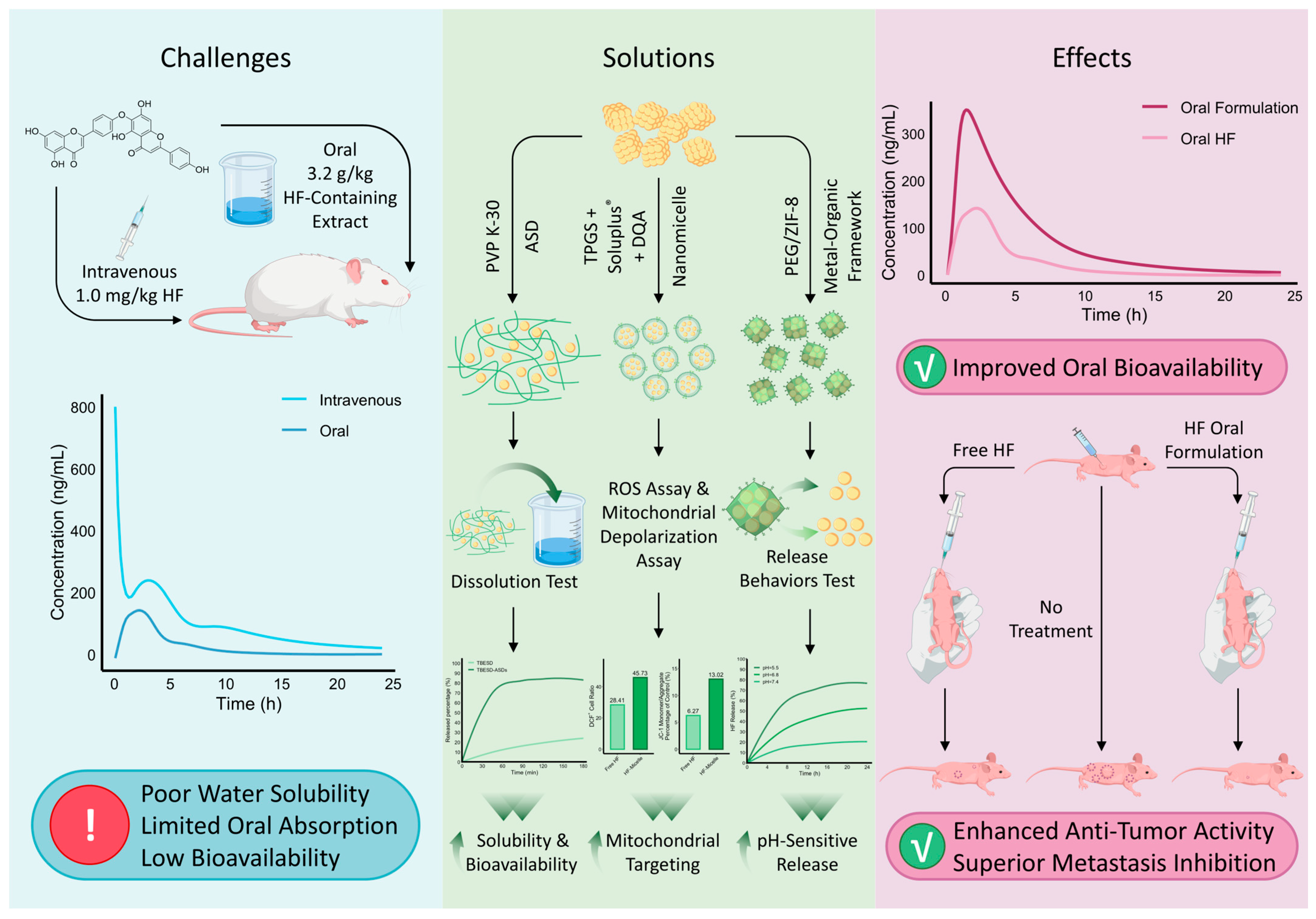
4. Pharmacokinetic Properties and Formulation Development of HF
5. Discussion and Perspectives
5.1. Unified Hierarchical Model of HF’s Anticancer Mechanisms and Context Dependence
5.2. Comparative Analysis of HF with Structurally Related Amentoflavone and Clinical MDM2 Inhibitors
5.3. Critical Evaluation of Inconsistent Findings and Limitations in HF Research
5.4. Clinical Translation Strategies and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Eslami, M.; Memarsadeghi, O.; Davarpanah, A.; Arti, A.; Nayernia, K.; Behnam, B. Overcoming chemotherapy resistance in metastatic cancer: A comprehensive review. Biomedicines 2024, 12, 183. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Tsalikis, J.; Abdel-Nour, M.; Farahvash, A.; Sorbara, M.T.; Poon, S.; Philpott, D.J.; Girardin, S.E. Isoginkgetin, a natural biflavonoid proteasome inhibitor, sensitizes cancer cells to apoptosis via disruption of lysosomal homeostasis and impaired protein clearance. Mol. Cell. Biol. 2019, 39, e00489-18. [Google Scholar] [CrossRef]
- Rye, I.H.; Trinh, A.; Sætersdal, A.B.; Nebdal, D.; Lingjærde, O.C.; Almendro, V.; Polyak, K.; Børresen-Dale, A.L.; Helland, Å.; Markowetz, F. Intratumor heterogeneity defines treatment-resistant HER 2+ breast tumors. Mol. Oncol. 2018, 12, 1838–1855. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Wei, J.; Li, W.; Zhang, P.; Guo, F.; Liu, M. Current trends in sensitizing immune checkpoint inhibitors for cancer treatment. Mol. Cancer 2024, 23, 279. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Navarrete, T.G.; Mejía-Guerra, K.; Mukundi, E.; Eubank, T.D.; Grotewold, E.; Arango, D.; Doseff, A.I. Transcriptome reprogramming through alternative splicing triggered by apigenin drives cell death in triple-negative breast cancer. Cell Death Dis. 2023, 14, 824. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Zhang, P.-M.; Jiang, M.; Yu, S.-W.; Wang, L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement. Med. Ther. 2020, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Z.; Li, Z.; Peng, H.; Luo, Y.; Deng, M.; Li, R.; Dai, C.; Xu, Y.; Liu, S. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget 2015, 6, 10460. [Google Scholar] [CrossRef] [PubMed]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J. Phase I/Ib study of tenalisib (RP6530), a dual PI3K δ/γ inhibitor in patients with relapsed/refractory T-cell lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef]
- Xu, K.; Ren, X.; Wang, J.; Zhang, Q.; Fu, X.; Zhang, P.-C. Clinical development and informatics analysis of natural and semi-synthetic flavonoid drugs: A critical review. J. Adv. Res. 2024, 63, 269–284. [Google Scholar] [CrossRef]
- Jurcevic Sangut, I.; Sarkanj, B.; Karalija, E.; Samec, D. A Comparative Analysis of Radical Scavenging, Antifungal and Enzyme Inhibition Activity of 3′-8″-Biflavones and Their Monomeric Subunits. Antioxidants 2023, 12, 1854. [Google Scholar] [CrossRef]
- Lotfi, M.-S.; Jafari-Sabet, M. Comparative in Silico study of apigenin and its dimeric forms on PIM1 kinase in glioblastoma multiform. Comput. Biol. Chem. 2024, 113, 108253. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X.A. Proceedings of chemistry, pharmacology, pharmacokinetics and synthesis of biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Goossens, J.-F.; Goossens, L.; Bailly, C. Hinokiflavone and related C–O–C-type biflavonoids as anti-cancer compounds: Properties and mechanism of action. Nat. Prod. Bioprospect. 2021, 11, 365–377. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.-P.; Zhou, X.; Liu, X.-G.; Gao, W.; Dong, X.; Li, H.-J.; Li, P.; Yang, H. Screening of minor bioactive compounds from herbal medicines by in silico docking and the trace peak exposure methods. J. Chromatogr. A 2016, 1436, 91–99. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Wang, M.; Ma, X.; Peng, X. Studies on the inhibition of α-glucosidase by biflavonoids and their interaction mechanisms. Food Chem. 2023, 420, 136113. [Google Scholar] [CrossRef]
- Coulerie, P.; Eydoux, C.; Hnawia, E.; Stuhl, L.; Maciuk, A.; Lebouvier, N.; Canard, B.; Figadère, B.; Guillemot, J.-C.; Nour, M. Biflavonoids of Dacrydium balansae with potent inhibitory activity on dengue 2 NS5 polymerase. Planta Med. 2012, 78, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ouyang, X.; Cai, R.; Chen, D. 3′, 8″-Dimerization enhances the antioxidant capacity of flavonoids: Evidence from acacetin and isoginkgetin. Molecules 2019, 24, 2039. [Google Scholar] [CrossRef]
- Thapa, A.; Woo, E.-R.; Chi, E.Y.; Sharoar, M.G.; Jin, H.-G.; Shin, S.Y.; Park, I.-S. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-β toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry 2011, 50, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Sugita, P.; Agusta, D.D.; Dianhar, H.; Suparto, I.H.; Kurniawanti, K.; Rahayu, D.U.C.; Irfana, L. The cytotoxicity and SAR analysis of biflavonoids isolated from Araucaria hunsteinii K. Schum. leaves against MCF-7 and HeLa cancer cells. J. Appl. Pharm. Sci. 2023, 13, 199–209. [Google Scholar] [CrossRef]
- Agusta, D.D.; Dianhar, H.; Rahayu, D.U.C.; Suparto, I.H.; Sugita, P. Anticancer and antivirus activities of two biflavonoids from Indonesian Araucaria hunsteinii K Schum leaves. J. Hunan Univ. Nat. Sci. 2022, 49, 168–177. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Xia, Z.-M.; Tian, Y.; Zhang, D.; Rui, W.-J.; Dong, J.-X.; Xiao, F.-J. Anticancer effects of five biflavonoids from ginkgo biloba l. Male flowers in vitro. Molecules 2019, 24, 1496. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Anderson, H.; Flavin, M.T.; Pai, Y.-H.S.; Mata-Greenwood, E.; Pengsuparp, T.; Pezzuto, J.M.; Schinazi, R.F.; Hughes, S.H.; Chen, F.-C. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997, 60, 884–888. [Google Scholar] [CrossRef]
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.-C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B. Structure-activity relationship study of biflavonoids on the Dengue virus polymerase DENV-NS5 RdRp. Planta Med. 2013, 79, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-H.; Hsieh, C.-L.; Liu, T.-T.; Huang, C.-S.; Chen, Y.-C.; Chuang, Y.-C.; Lin, S.-S.; Hsu, F.-T. Amentoflavone induces apoptosis and inhibits NF-ĸB-modulated anti-apoptotic signaling in glioblastoma cells. In Vivo 2018, 32, 279–285. [Google Scholar]
- Wu, L.; Qian, C.; Zhang, W.; Shi, M.; Chen, X.; Wang, Y.; Lin, F. Ginkgetin suppresses ovarian cancer growth through inhibition of JAK2/STAT3 and MAPKs signaling pathways. Phytomedicine 2023, 116, 154846. [Google Scholar] [CrossRef]
- Jian, H.Y.; Zhang, J.T.; Liu, Z.; Zhang, Z.; Zeng, P.H. Amentoflavone reverses epithelial-mesenchymal transition in hepatocellular carcinoma cells by targeting p53 signalling pathway axis. J. Cell. Mol. Med. 2024, 28, e18442. [Google Scholar] [CrossRef]
- Yao, J.; Tang, S.; Shi, C.; Lin, Y.; Ge, L.; Chen, Q.; Ou, B.; Liu, D.; Miao, Y.; Xie, Q. Isoginkgetin, a potential CDK6 inhibitor, suppresses SLC2A1/GLUT1 enhancer activity to induce AMPK-ULK1-mediated cytotoxic autophagy in hepatocellular carcinoma. Autophagy 2023, 19, 1221–1238. [Google Scholar] [CrossRef]
- Li, P.; Yue, G.G.-L.; Kwok, H.-F.; Long, C.-l.; Lau, C.B.-S.; Kennelly, E.J. Using ultra-performance liquid chromatography quadrupole time of flight mass spectrometry-based chemometrics for the identification of anti-angiogenic biflavonoids from edible Garcinia species. J. Agric. Food Chem. 2017, 65, 8348–8355. [Google Scholar] [CrossRef]
- Shao, M.; Lou, D.; Yang, J.; Lin, M.; Deng, X.; Fan, Q. Curcumin and wikstroflavone B, a new biflavonoid isolated from Wikstroemia indica, synergistically suppress the proliferation and metastasis of nasopharyngeal carcinoma cells via blocking FAK/STAT3 signaling pathway. Phytomedicine 2020, 79, 153341. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, X.; Li, J.; Yao, X.-C.; Liu, W.-L.; Kang, F.-H.; Zou, Z.-X.; Xu, K.-P.; Xu, P.-S.; Tan, G.-S. Identification of a new natural biflavonoids against breast cancer cells induced ferroptosis via the mitochondrial pathway. Bioorg. Chem. 2021, 109, 104744. [Google Scholar] [CrossRef]
- Lima, C.A.D.; Maquedano, L.K.; Jaalouk, L.S.; Santos, D.C.D.; Longato, G.B. Biflavonoids: Preliminary reports on their role in prostate and breast cancer therapy. Pharmaceuticals 2024, 17, 874. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.C.; Lima, I.S.; Santos, C.C.D.; Nonaka, C.K.V.; Souza, B.S.D.F.; David, J.M.; Ulrich, H.; Nascimento, R.P.D.; Costa, M.D.F.D.; Dos Santos, B.L. Agathisflavone Inhibits Viability and Modulates the Expression of miR-125b, miR-155, IL-6, and Arginase in Glioblastoma Cells and Microglia/Macrophage Activation. Molecules 2025, 30, 158. [Google Scholar] [CrossRef]
- Patel, D.K. Biological Potential and Therapeutic Effectiveness of Hinokiflavone in Medicine: The Effective Components of Herbal Medicines for Treatment of Cancers and Associated Complications. Curr. Nutr. Food Sci. 2024, 20, 439–449. [Google Scholar] [CrossRef]
- Sawada, T. Studies on flavanoids in the leaves of Coniferae and allied plants. V. Relation between the disribution of bisflavanoids and taxonomical position of the plants. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1958, 78, 1023–1027. [Google Scholar] [CrossRef]
- Fukui, Y.; Kawano, N. The structure of hinokiflavone, a new type bisflavonoid. J. Am. Chem. Soc. 1959, 81, 6331. [Google Scholar] [CrossRef]
- Nakazawa, K. Syntheses of Ring-substituted Flavonoids and Allied Compounds. XI. Synthesis of Hinokiflavone. Chem. Pharm. Bull. 1968, 16, 2503–2511. [Google Scholar] [CrossRef]
- Pelter, A.; Warren, R.; Usmani, J.; Ilyas, M.; Rahman, W. The use of solvent induced methoxy shifts as a guide to hinokiflavone structure. Tetrahedron Lett. 1969, 10, 4259–4263. [Google Scholar] [CrossRef]
- Miki, K.; Nagai, T.; Nakamura, T.; Tuji, M.; Koyama, K.; Kinoshita, K.; Furuhata, K.; Yamada, H.; Takahashi, K. Synthesis and evaluation of influenza virus sialidase inhibitory activity of hinokiflavone-slailc acid conjugates. Heterocycles 2008, 75, 879–886. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary; National Center for Biotechnology Information: Bethesda, MD, USA, 2021.
- Aruwa, C.E.; Sabiu, S. Staphylococcus aureus AgrA Modulators From South African Antimicrobial Plants. Chem. Biodivers. 2025, 22, e202403220. [Google Scholar] [CrossRef]
- Darwish, R.S.; Hammoda, H.M.; Ghareeb, D.A.; Abdelhamid, A.S.; Harraz, F.M.; Shawky, E. Seasonal dynamics of the phenolic constituents of the cones and leaves of oriental Thuja (Platycladus orientalis L.) reveal their anti-inflammatory biomarkers. RSC Adv. 2021, 11, 24624–24635. [Google Scholar] [CrossRef]
- Sakar, M.K.; Friedrich, H. Flavonoide in Blättern von Juniperus drupacea. Planta Med. 1984, 50, 108–109. [Google Scholar] [CrossRef]
- Lee, S.; Park, N.-J.; Bong, S.-K.; Jegal, J.; Park, S.-a.; Kim, S.-N.; Yang, M.H. Ameliorative effects of Juniperus rigida fruit on oxazolone-and 2, 4-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2018, 214, 160–167. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, D.; Xu, L.; Yang, S. Chemical constitutents of Podocarpus imbricatus BI.(II). China J. Chin. Mater. Medica 1997, 22, 169–170, 192. [Google Scholar]
- Yuan, Y.; Wang, B.; Chen, L.; Luo, H.; Fisher, D.; Sutherland, I.A.; Wei, Y. How to realize the linear scale-up process for rapid purification using high-performance counter-current chromatography. J. Chromatogr. A 2008, 1194, 192–198. [Google Scholar] [CrossRef]
- Dai, Z.; Ma, S.-C.; Wang, G.-L.; Wang, F.; Lin, R.-C. A new glucoside from Selaginella sinensis. J. Asian Nat. Prod. Res. 2006, 8, 529–533. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Chen, F.-C.; Lee, K.-H. Hinokiflavone, a Cytotoxic Principle from Rhus succedanea and the Cytotoxicity of the Related Biflavonoids1. Planta Med. 1989, 55, 166–168. [Google Scholar] [CrossRef] [PubMed]
- De, L.; Xing, N.; Du, Q.; Guo, S.; Wang, S. Investigating the anti-lung cancer properties of Zhuang medicine Cycas revoluta Thunb. leaves targeting ion channels and transporters through a comprehensive strategy. Comput. Biol. Chem. 2024, 112, 108156. [Google Scholar] [CrossRef]
- Heieren, B.T.; Dyrdal, A.S.; Herfindal, L.; Holmelid, B.; Brede, C.; Andersen, H.L.; Fossen, T. Cytotoxic Natural Products from Cryptomeria japonica (Thunb. ex L.) D.Don. Int. J. Mol. Sci. 2024, 25, 13735. [Google Scholar] [CrossRef]
- Nakazawa, K. Synthesis of hinokiflavone. Tetrahedron Lett. 1967, 8, 5223–5225. [Google Scholar] [CrossRef]
- Rahman, M.; Riaz, M.; Desai, U.R. Synthesis of biologically relevant biflavanoids–A review. Chem. Biodivers. 2007, 4, 2495–2527. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Lee, S.-g.; Lee, M. Biflavonoids isolated from Selaginella tamariscina and their anti-inflammatory activities via ERK 1/2 signaling. Molecules 2018, 23, 926. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yao, S.; Zhang, X.-X.; Song, H. Rapid screening and structural characterization of antioxidants from the extract of selaginella doederleinii hieron with DPPH-UPLC-Q-TOF/MS method. Int. J. Anal. Chem. 2015, 2015, 849769. [Google Scholar] [CrossRef]
- Ilic, V.K.; Egorova, O.; Tsang, E.; Gatto, M.; Wen, Y.; Zhao, Y.; Sheng, Y. Hinokiflavone inhibits MDM2 activity by targeting the MDM2-MDMX RING domain. Biomolecules 2022, 12, 643. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.; Zhang, X.; Liu, Y.; Lv, Q.; Liu, G.; Zhang, J.; Li, X. Hinokiflavone induces apoptosis via activating mitochondrial ROS/JNK/caspase pathway and inhibiting NF-κB activity in hepatocellular carcinoma. J. Cell. Mol. Med. 2020, 24, 8151–8165. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Luo, Y.; Xu, B.; Yao, Y.; Deng, Y.; Yang, F.; Ye, T.; Wang, G.; Cheng, Z. Hinokiflavone induces apoptosis in melanoma cells through the ROS-mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed. Pharmacother. 2018, 103, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.-P.; Teng, T.; Yan, L.; Tang, Q.-Z. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: Oxidative stress and cell death. Int. J. Biol. Sci. 2022, 18, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Sun, Y.; Zhao, G.; Wang, J.; Liu, L.; Liu, F.; Wang, P.; Yang, J.; Xu, X. Hinokiflavone, as a MDM2 inhibitor, activates p53 signaling pathway to induce apoptosis in human colon cancer HCT116 cells. Biochem. Biophys. Res. Commun. 2022, 594, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, X.; Guo, L.; Liu, J.; Yang, Y.; Zeng, Y.; Li, C.; Liu, W.; Ma, W. Hinokiflavone induces apoptosis, cell cycle arrest and autophagy in chronic myeloid leukemia cells through MAPK/NF-κB signaling pathway. BMC Complement. Med. Ther. 2022, 22, 100. [Google Scholar] [CrossRef]
- Pawellek, A.; Ryder, U.; Tammsalu, T.; King, L.J.; Kreinin, H.; Ly, T.; Hay, R.T.; Hartley, R.C.; Lamond, A.I. Characterisation of the biflavonoid hinokiflavone as a pre-mRNA splicing modulator that inhibits SENP. eLife 2017, 6, e27402. [Google Scholar] [CrossRef]
- Cui, C.-P.; Wong, C.C.-L.; Kai, A.K.-L.; Ho, D.W.-H.; Lau, E.Y.-T.; Tsui, Y.-M.; Chan, L.-K.; Cheung, T.-T.; Chok, K.S.-H.; Chan, A.C. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut 2017, 66, 2149–2159. [Google Scholar] [CrossRef]
- Cheng, J.; Kang, X.; Zhang, S.; Yeh, E.T. SUMO-specific protease 1 is essential for stabilization of HIF1α during hypoxia. Cell 2007, 131, 584–595. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Kuo, H.-P.; Chen, M.-C.; Lin, Y.-C.; Lin, B.-J.; Hsu, K.-C.; Chen, C.-H. Hinokiflavone is a novel CK2 inhibitor promoting apoptosis and synergizing with chemotherapeutic agents in cisplatin resistant bladder cancer cells. Sci. Rep. 2025, 15, 20922. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Goldblatt, Z.E.; Cirka, H.A.; Billiar, K.L. Mechanical regulation of apoptosis in the cardiovascular system. Ann. Biomed. Eng. 2021, 49, 75–97. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Carlsen, L.; Hernandez Borrero, L.; Seyhan, A.A.; Tian, X.; El-Deiry, W.S. Advanced strategies for therapeutic targeting of wild-type and mutant p53 in cancer. Biomolecules 2022, 12, 548. [Google Scholar] [CrossRef]
- Andrysik, Z.; Espinosa, J.M. Harnessing p53 for targeted cancer therapy: New advances and future directions. Transcription 2025, 16, 3–46. [Google Scholar] [CrossRef]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef]
- Nag, S.; Qin, J.; Srivenugopal, K.S.; Wang, M.; Zhang, R. The MDM2-p53 pathway revisited. J. Biomed. Res. 2013, 27, 254. [Google Scholar] [CrossRef] [PubMed]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating p53-based therapies for cancer into the clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef]
- Huang, W.; Liu, C.; Liu, F.; Liu, Z.; Lai, G.; Yi, J. Hinokiflavone induces apoptosis and inhibits migration of breast cancer cells via EMT signalling pathway. Cell Biochem. Funct. 2020, 38, 249–256. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, R.; Ye, T.; Yang, S.; Li, Y.; Yang, F.; Wang, G.; Xie, Y.; Li, Q. Antitumor activity in colorectal cancer induced by hinokiflavone. J. Gastroenterol. Hepatol. 2019, 34, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Abdelhamid, A.M.; Saber, S.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Yahya, G.; Salama, M.M. Cell cycle machinery in oncology: A comprehensive review of therapeutic targets. FASEB J. 2024, 38, e23734. [Google Scholar] [CrossRef] [PubMed]
- Massacci, G.; Perfetto, L.; Sacco, F. The Cyclin-dependent kinase 1: More than a cell cycle regulator. Br. J. Cancer 2023, 129, 1707–1716. [Google Scholar] [CrossRef]
- Provenzano, L.; Dieci, M.; Curigliano, G.; Giuliano, M.; Botticelli, A.; Lambertini, M.; Rizzo, G.; Pedersini, R.; Sirico, M.; La Verde, N. Real-world effectiveness comparison of first-line palbociclib, ribociclib or abemaciclib plus endocrine therapy in advanced HR+/HER2-BC patients: Results from the multicenter PALMARES-2 study. Ann. Oncol. 2025, 36, 762–774. [Google Scholar] [CrossRef]
- Youssef, K.K.; Narwade, N.; Arcas, A.; Marquez-Galera, A.; Jiménez-Castaño, R.; Lopez-Blau, C.; Fazilaty, H.; García-Gutierrez, D.; Cano, A.; Galcerán, J.; et al. Two distinct epithelial-to-mesenchymal transition programs control invasion and inflammation in segregated tumor cell populations. Nat. Cancer 2024, 5, 1660–1680. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K. NADPH oxidases: Current aspects and tools. Redox Biol. 2020, 34, 101512. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Ashraf, R.; Kumar, S. Mfn2-mediated mitochondrial fusion promotes autophagy and suppresses ovarian cancer progression by reducing ROS through AMPK/mTOR/ERK signaling. Cell. Mol. Life Sci. 2022, 79, 573. [Google Scholar] [CrossRef]
- Sun, B.; Ding, P.; Song, Y.; Zhou, J.; Chen, X.; Peng, C.; Liu, S. FDX1 downregulation activates mitophagy and the PI3K/AKT signaling pathway to promote hepatocellular carcinoma progression by inducing ROS production. Redox Biol. 2024, 75, 103302. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: A review of the molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids regulate inflammation and oxidative stress in cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, S.; Wang, Y.; Huang, K. Target-guided isolation and purification of antioxidants from Selaginella sinensis by offline coupling of DPPH-HPLC and HSCCC experiments. J. Chromatogr. B 2011, 879, 191–196. [Google Scholar] [CrossRef]
- Gao, P.C.; Tan, X.H.; Xia, M.C.; Li, K.F.; Zhao, F.Z.; Ying, H.G.; Zhou, Z.; Yuan, Y.M.; Nan, T.G.; Guan, R.L. Hinokiflavone alleviates high-fat diet-induced erectile dysfunction via the EGFR/PI3K/Akt/eNOS signaling pathway. Sex. Med. 2025, 13, qfaf059. [Google Scholar] [CrossRef]
- Alqasoumi, S.I.; Farraj, A.I.; Abdel-Kader, M.S. Study of the hepatoprotective effect of Juniperus phoenicea constituents. Pak. J. Pharm. Sci. 2013, 26, 999–1009. [Google Scholar] [PubMed]
- Abdel-Kader, M.S.; Abulhamd, A.T.; Hamad, A.M.; Alanazi, A.H.; Ali, R.; Alqasoumi, S.I. Evaluation of the hepatoprotective effect of combination between hinokiflavone and Glycyrrhizin against CCl4 induced toxicity in rats. Saudi Pharm. J. 2018, 26, 496–503. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Zhang, Y.; Shan, G.-Y.; Cheng, J.-Y.; Wan, H.; Zhang, Y.-X.; Li, H.-J. Hinokiflavone exerts dual regulation on apoptosis and pyroptosis via the SIX4/Stat3/Akt pathway to alleviate APAP-induced liver injury. Life Sci. 2024, 354, 122968. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Bedhiafi, T.; Mestiri, S.; Taib, N.; Uddin, S.; Merhi, M.; Dermime, S. Chronic inflammation and cancer; the two sides of a coin. Life Sci. 2024, 338, 122390. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Q.; Cheng, X.-X.; He, S.; Xia, T.; Li, Y.-Q.; Peng, W.; Zhou, Y.-Q.; Xu, Z.-H.; He, M.-S.; Liu, Y. Super-enhancer–driven EFNA1 fuels tumor progression in cervical cancer via the FOSL2-Src/AKT/STAT3 axis. J. Clin. Investig. 2025, 135, e177599. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Ameya, G.; Birri, D.J. The molecular mechanisms of virus-induced human cancers. Microb. Pathog. 2023, 183, 106292. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, G.; Shao, S.; Yao, Y. Helicobacter pylori triggers inflammation and oncogenic transformation by perturbing the immune microenvironment. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189139. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T. Studies on Inhibitors of Skin Tumor Promotion-5-Inhibitory Effects of Flavonoids on Epstein--Barr Virus Activation-2. Jpn. J. Pharmacogn. 1989, 43, 135–141. [Google Scholar]
- Lin, Y.-M.; Flavin, M.T.; Schure, R.; Chen, F.-C.; Sidwell, R.; Barnard, D.I.; Huffmann, J.H.; Kern, E.R. Antiviral activities of biflavonoids. Planta Med. 1999, 65, 120–125. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Ma, S.-C.; Wei, F.; Lin, R.-C.; But, P.P.-H.; Lee, S.H.-S.; Lee, S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003, 51, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Zembower, D.E.; Lin, Y.-M.; Flavin, M.T.; Chen, F.-C.; Korba, B.E. Robustaflavone, a potential non-nucleoside anti-hepatitis B agent. Antivir. Res. 1998, 39, 81–88. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, A.; Mallick, T.; Begum, N.A. Exploring the efficacy of naturally occurring biflavone based antioxidants towards the inhibition of the SARS-CoV-2 spike glycoprotein mediated membrane fusion. Virology 2021, 556, 133–139. [Google Scholar] [CrossRef]
- Sawant, S.; Patil, R.; Khawate, M.; Zambre, V.; Shilimkar, V.; Jagtap, S. Computational assessment of select antiviral phytochemicals as potential SARS-CoV-2 main protease inhibitors: Molecular dynamics guided ensemble docking and extended molecular dynamics. In Silico Pharmacol. 2021, 9, 44. [Google Scholar] [CrossRef]
- Belhassan, A.; Zaki, H.; Chtita, S.; Alaqarbeh, M.; Alsakhen, N.; Benlyas, M.; Lakhlifi, T.; Bouachrine, M. Camphor, artemisinin and sumac phytochemicals as inhibitors against COVID-19: Computational approach. Comput. Biol. Med. 2021, 136, 104758. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the antibacterial activity and efflux pump inhibitory effect of Cycas thouarsii R. Br. extract against Klebsiella pneumoniae clinical isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, B.; Chen, X.; Wang, L.; Wang, X.; Hou, J.; Wei, L.; Sui, L.; Zhang, C.; Guan, J. Hinokiflavone attenuates the virulence of methicillin-resistant Staphylococcus aureus by targeting caseinolytic protease P. Antimicrob. Agents Chemother. 2022, 66, e00240-22. [Google Scholar] [CrossRef]
- Lale, A.; Herbert, J.; Augereau, J.; Billon, M.; Leconte, M.; Gleye, J. Ability of different flavonoids to inhibit the procoagulant activity of adherent human monocytes. J. Nat. Prod. 1996, 59, 273–276. [Google Scholar] [CrossRef] [PubMed]
- El-Banna, A.A.; Shawky, E.; Celik, I.; Ghareeb, D.A.; Abdulmalek, S.A.; Darwish, R.S. Deciphering the putative bioactive metabolites and the underlying mechanism of Juniperus horizontalis Moench (Creeping juniper) in the treatment of inflammation using network pharmacology and molecular docking. J. Pharm. Pharmacol. 2024, 76, 514–533. [Google Scholar] [CrossRef]
- Yin, R.; Xiong, K.; Wen, S.; Wang, Y.; Xu, F. Development and validation of an LC–MS/MS method for the determination of hinokiflavone in rat plasma and its application to a pharmacokinetic study. Biomed. Chromatogr. 2017, 31, e3821. [Google Scholar] [CrossRef]
- Shan, C.-X.; Guo, S.-C.; Yu, S.; Shan, M.-Q.; Li, S.F.Y.; Chai, C.; Cui, X.-B.; Zhang, L.; Ding, A.-W.; Wu, Q.-N. Simultaneous determination of quercitrin, afzelin, amentoflavone, hinokiflavone in rat plasma by UFLC–MS-MS and its application to the pharmacokinetics of platycladus orientalis leaves extract. J. Chromatogr. Sci. 2018, 56, 895–902. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Li, L.; Zhang, X.; Song, K.; Diao, X.; Sun, Y.; Zhang, L. UHPLC-Q-TOF-MS/MS method based on four-step strategy for metabolites of hinokiflavone in vivo and in vitro. J. Pharm. Biomed. Anal. 2019, 169, 19–29. [Google Scholar] [CrossRef]
- Zhang, H.; Ban, Y.M.; Li, D.M.; Wang, G.; Gu, J.; Zhu, L. Amentoflavone for treating cardiocerebrovascular diseases and neurological disorders. Front. Pharmacol. 2024, 15, 1406510. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zhang, Y.; Huang, K.; Liu, H.; Xu, D.; Li, S.; Liu, Q.; Huang, J.; Yao, H. Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion. Drug Deliv. 2020, 27, 309–322. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Li, L.; Song, K.; Zhang, L. Preparation and antitumor evaluation of hinokiflavone hybrid micelles with mitochondria targeted for lung adenocarcinoma treatment. Drug Deliv. 2020, 27, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qiu, J.; Liu, L.; Li, X.; Liu, X.; Zhang, Y. Preparation of PEG/ZIF-8@ HF drug delivery system for melanoma treatment via oral administration. Drug Deliv. 2022, 29, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Guembe-Michel, N.; Nguewa, P.; González-Gaitano, G. Soluplus®-Based Pharmaceutical Formulations: Recent Advances in Drug Delivery and Biomedical Applications. Int. J. Mol. Sci. 2025, 26, 1499. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Li, L.; Zhang, Y.; Zhang, L.; Zhang, Z. Preparation, evaluation and metabolites study in rats of novel amentoflavone-loaded TPGS/soluplus mixed nanomicelles. Drug Deliv. 2020, 27, 137–150. [Google Scholar] [CrossRef]
- Markham, K.R.; Sheppard, C.; Geiger, H. 13C NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry 1987, 26, 3335–3337. [Google Scholar] [CrossRef]
- Faiyaz, S.S.M.; Ahmad, A.; Fatima, D.; Shahid, S.M.A.; Kaushik, G.; Verma, C.; Tiwari, R.K.; Kumar, V. Amentoflavone Impedes NF-kappaB Activation and Mediates Apoptosis Induction in Lung Cancer Cells: An in vitro and in silico exploration. J. Inflamm. Res. 2025, 18, 8657–8673. [Google Scholar] [CrossRef]
- Frota, L.S.; Pessoa, C.D.O.; Costa, D.N.; Maranhao, S.S.; Ishiki, H.M.; Morais, S.M. Cytotoxic effects of amentoflavone from Ouratea fieldingiana on HCT-116, SNB-19, and pC-3 cells with structural insights from molecular docking. Nat. Prod. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, K.H.; Ku, H.J.; Lee, K.C.; Lin, S.S.; Hsu, F.T. Amentoflavone induces caspase-dependent/-independent apoptosis and dysregulates cyclin-dependent kinase-mediated cell cycle in colorectal cancer in vitro and in vivo. Environ. Toxicol. 2023, 38, 1078–1089. [Google Scholar] [CrossRef]
- Lee, K.C.; Chen, W.T.; Liu, Y.C.; Lin, S.S.; Hsu, F.T. Amentoflavone Inhibits Hepatocellular Carcinoma Progression Through Blockage of ERK/NF-kB Activation. In Vivo 2018, 32, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, P.; Zhao, Z.; Choe, H.; Ali, T.; Ding, K.; Wu, X.; Ma, J.; Zhang, L. Network pharmacology and experimental study on the inhibition of glycolysis by amentoflavone in pancreatic cancer. Discov. Oncol. 2025, 16, 1875. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef]
- Bao, C.; Chen, J.; Kim, J.T.; Qiu, S.; Cho, J.S.; Lee, H.J. Amentoflavone inhibits tumorsphere formation by regulating the Hedgehog/Gli1 signaling pathway in SUM159 breast cancer stem cells. J. Funct. Foods 2019, 61, 103501. [Google Scholar] [CrossRef]
- Sun, Q.; Zhen, P.; Li, D.; Liu, X.; Ding, X.; Liu, H. Amentoflavone promotes ferroptosis by regulating reactive oxygen species (ROS)/5’AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) to inhibit the malignant progression of endometrial carcinoma cells. Bioengineered 2022, 13, 13269–13279. [Google Scholar] [CrossRef]
- Chen, B.; Luo, H.; Chen, W.; Huang, Q.; Zheng, K.; Xu, D.; Li, S.; Liu, A.; Huang, L.; Zheng, Y.; et al. Pharmacokinetics, Tissue Distribution, and Human Serum Albumin Binding Properties of Delicaflavone, a Novel Anti-Tumor Candidate. Front. Pharmacol. 2021, 12, 761884. [Google Scholar] [CrossRef]
- Pi, L.; Rooprai, J.; Allan, D.S.; Atkins, H.; Bredeson, C.; Fulcher, A.J.; Ito, C.; Ramsay, T.; Shorr; Stanford, W.L.; et al. Evaluating dose-limiting toxicities of MDM2 inhibitors in patients with solid organ and hematologic malignancies: A systematic review of the literature. Leuk. Res. 2019, 86, 106222. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Song, J.-S.; Lee, B.-H.; Son, Y.K.; Kim, Y. Qualitative and quantitative analysis of the major bioactive components of JuniPerus chinensis L. Using LC-QTOF-MS and LC-MSMS and investigation of antibacterial activity against pathogenic bacteria. Molecules 2023, 28, 3937. [Google Scholar] [CrossRef] [PubMed]
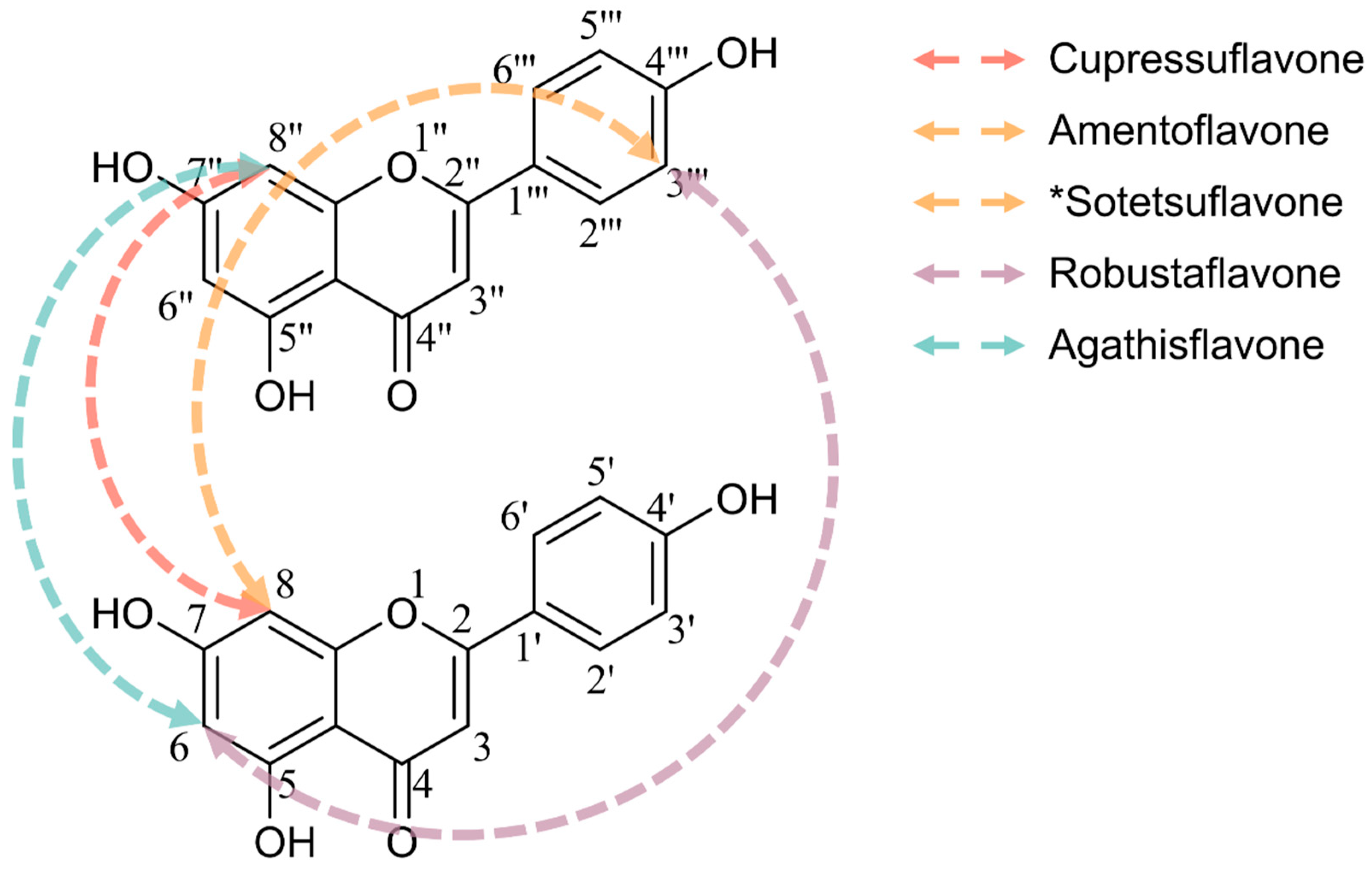
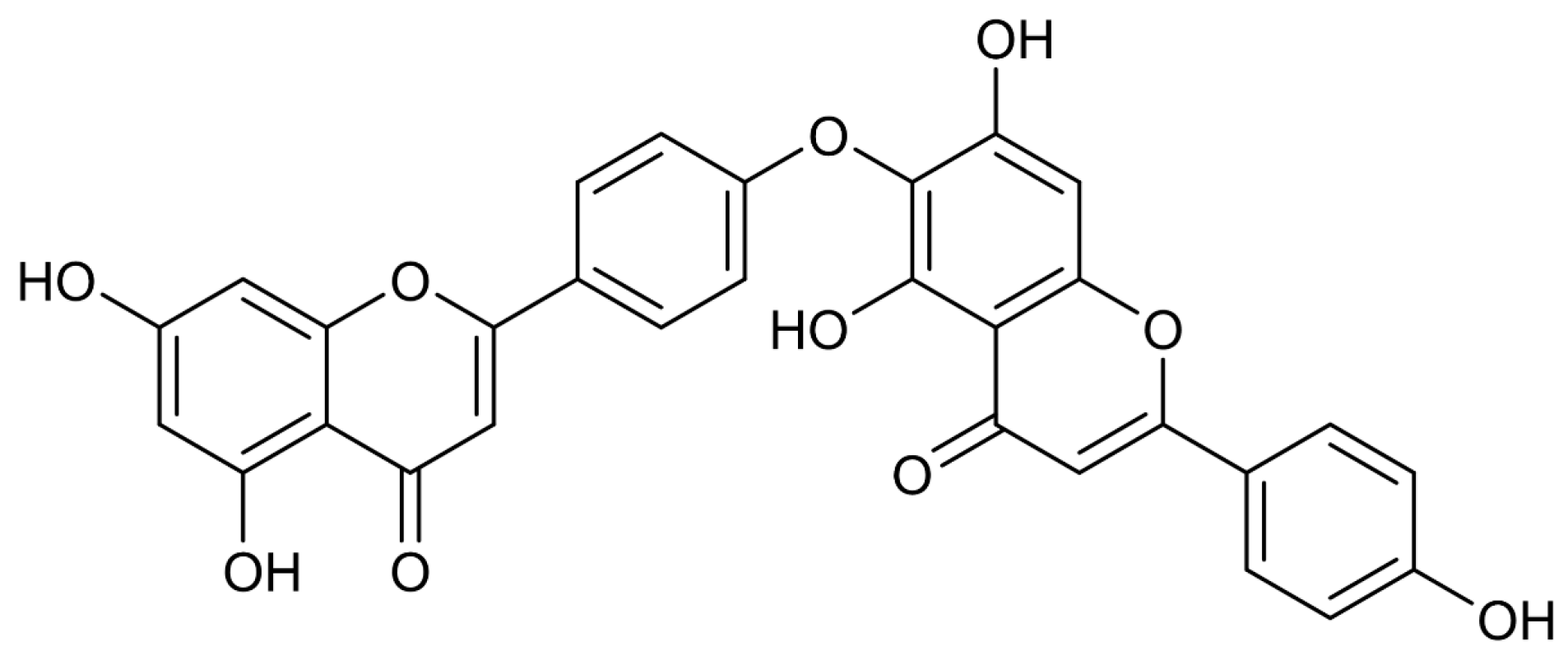
| Cell Type | Cell Line | Time Point (h) | IC50 (μM) | Assay Method | Refs |
|---|---|---|---|---|---|
| Leukemia | AML-2 | 24 | 4.93 ± 1.16 | Celltiter Glo | [63] |
| Leukemia | HL-60 | 24 | 10.95 ± 0.19 | Celltiter Glo | [63] |
| Chronic Myeloid Leukemia | K562 | 24 | 23.38 ± 1.78 | CCK-8 Assay | [68] |
| Chronic Myeloid Leukemia | K562 | 48 | 8.84 ± 1.62 | CCK-8 Assay | [68] |
| Colorectal Cancer | HCT116 p53-deficient | 24 | 32.66 ± 0.31 | Celltiter Glo | [63] |
| Colorectal Cancer | HCT116 | 24 | 14.19 ± 2.04 | Celltiter Glo | [63] |
| Colorectal Cancer | HCT116 | 48 | 13 | MTT Assay | [84] |
| Colorectal Cancer | CT26 | 48 | 12 | MTT Assay | [84] |
| Colorectal Cancer | HT29 | 48 | 13 | MTT Assay | [84] |
| Colorectal Cancer | SW48 | 48 | 14 | MTT Assay | [84] |
| Colorectal Cancer | SW480 | 48 | 17 | MTT Assay | [84] |
| Colorectal Cancer | DLD-1 | 48 | 17 | MTT Assay | [84] |
| Colorectal Cancer | SW620 | 48 | 18 | MTT Assay | [84] |
| Osteosarcoma | U2OS | 24 | 15.90 ± 2.07 | Celltiter Glo | [63] |
| Breast Cancer | MCF-7 | 24 | 17.33 ± 1.90 | Celltiter Glo | [63] |
| Melanoma | B16 | 24 | 20 | MTT Assay | [65] |
| Melanoma | B16 | 48 | 10 | MTT Assay | [65] |
| Melanoma | A375 | 24 | 23 | MTT Assay | [65] |
| Melanoma | A375 | 48 | 10 | MTT Assay | [65] |
| Melanoma | CHL-1 | 24 | 25 | MTT Assay | [65] |
| Melanoma | CHL-1 | 48 | 12 | MTT Assay | [65] |
| Breast Cancer | MDA-MB-231 | 48 | ≈20 | MTT Assay | [83] |
| Breast Cancer | 4T1 | 48 | >80 | MTT Assay | [83] |
| HCC | SMMC-7721 | 24 | 74.4 ± 8.1 | CCK-8 Assay | [64] |
| HCC | SMMC-7721 | 48 | 60.3 ± 2.9 | CCK-8 Assay | [64] |
| HCC | HepG 2 | 24 | 80.8 ± 2.6 | CCK-8 Assay | [64] |
| HCC | HepG 2 | 48 | 57.5 ± 5.3 | CCK-8 Assay | [64] |
| Normal Human Hepatocytes | L02 | 24 | 75 | MTT Assay | [65] |
| Normal Human Hepatocytes | L02 | 24 | 159.1 ± 5.6 | CCK-8 Assay | [64] |
| Normal Human Hepatocytes | L02 | 48 | 104.7 ± 4.5 | CCK-8 Assay | [64] |
| Normal Monkey Kidney Cells | Vero | 24 | 45 | MTT Assay | [65] |
| Normal Monkey Kidney Cells | Vero | 48 | 29 | MTT Assay | [65] |
| Normal Human Fibroblast Cell Line | BJ-FB | 24 | >50 | Celltiter Glo | [63] |
| Pharmacological Effect | Cell Line(s) | Cancer Type/Model | In Vivo Validation | Main Targets/Pathways | Mechanism Description | Refs |
|---|---|---|---|---|---|---|
| Induction of Apoptosis | AML-2 | Leukemia | No in vivo confirmation | MDM2-p53 | HF targets the MDM2-MDMX RING domain, inhibits MDM2’s E3 ubiquitin ligase activity, reducing p53 ubiquitination and degradation | [63] |
| HL-60 | Leukemia | |||||
| U2OS | Osteosarcoma | |||||
| MCF-7 | Breast Cancer | |||||
| HCT116 | Colorectal Cancer | No in vivo confirmation | HF time- and dose-dependently suppresses MDM2 mRNA synthesis, relieving MDM2-mediated p53 inhibition | [67] | ||
| MDA-MB-231 | Breast Cancer | Mouse xenograft model (MDA-MB-231): IHC confirmation | Bax/Bcl-2 | HF downregulates Bcl-2 and dose-dependently upregulates Bax, inducing caspase-dependent apoptosis | [83] | |
| A375 | Melanoma | [65] | ||||
| CT26, HCT116 | Colorectal Cancer | No in vivo confirmation | [84] | |||
| SMMC-7721, HepG2 | HCC | Mouse xenograft model (SMCC-7721): Western blot and IHC confirmation | JNK, p38 | HF dose-dependently activates JNK and p38, lowering Bcl-2/Bax ratio and triggering intrinsic apoptosis | [64] | |
| K562 | Leukemia | No in vivo confirmation | In addition to apoptosis, HF induces autophagy in K562 cells | [68] | ||
| SMMC-7721, HepG2 | HCC | Mouse xenograft model (SMCC-7721): Western blot and IHC confirmation | NF-κB | HF significantly reduces NF-κB activity by inhibiting IKBα phosphorylation and p65 nuclear translocation | [64] | |
| K562 | Leukemia | No in vivo confirmation | In leukemia cell lines, it has been confirmed that HF inhibits NF-κB activity by activating the JNK/p38 signaling pathway | [68] | ||
| Cell Cycle Arrest | SMMC-7721, HepG2 | HCC | No in vivo confirmation | CDK4, CDK6, p21 | Downregulates cyclin D1, CDK4, CDK6, upregulates p53, induces G0/G1 arrest | [64] |
| K562 | Leukemia | No in vivo confirmation | Cdc2, p21 | Upregulates p21, downregulates Cdc2, induces G2/M arrest | [68] | |
| HCT116 | Colorectal Cancer | No in vivo confirmation | p21, 14-3-3σ | Promotes transcription of p21 and 14-3-3σ, inducing G2/M arrest | [67] | |
| A357, B16 | Melanoma | No in vivo confirmation | - | Induces S phase arrest | [65] | |
| Inhibition of Tumor Metastasis | CT26, HCT116 | Colorectal Cancer | Mouse syngeneic model (CT-26): IHC confirmation | MMP2, MMP9, TIMP2 | Inhibits MMP2 and MMP9 expression, upregulates TIMP2 expression, reducing tumor cell migration | [84] |
| A375 | Melanoma | No in vivo confirmation | MMP2, MMP9 | HF decreases MMP2 and MMP9 levels, inhibiting tumor cell invasion and migration | [65] | |
| MDA-MB-231, 4T1 | Breast Cancer | Mouse xenograft model (MDA-MB-231): IHC confirmation | E-cadherin, N-cadherin | Dose-dependently upregulates E-cadherin and downregulates N-cadherin, reversing or inhibiting EMT to suppress invasion | [83] | |
| Antioxidant and Hepatoprotective Effects | - | CCl4-induced liver injury rats | Male Wistar rats: Histopathology confirmation | - | At biochemical level, HF’s hepatoprotective activity comparable to positive control silymarin, mechanism not detailed | [100] |
| - | CCl4-induced liver injury in rats | Male Wistar albino rats: Histopathology, electron microscopy and enzyme activity assay | - | Combination of HF and glycyrrhizin provides less hepatoprotection versus silymarin, mechanism unclear | [101] | |
| - | APAP-induced drug-induced liver injury | Female C57BL/6 mice: Histopathology, Western blot and enzyme activity assay | SIX4, Akt, Stat3 | HF activates SIX4-mediated Akt/Stat3 pathway, inhibiting inflammasome activation and pyroptosis induced by APAP | [102] | |
| Anti-inflammation Effects | RAW 264.7, HT-29 | - | No in vivo confirmation | ERK1/2, iNOS, COX-2 | HF and mHF inhibit ERK1/2, iNOS, COX-2 expression in LPS-stimulated cells concentration-dependently, reducing NO, IL-6, IL-8, TNF-α | [61] |
| Human leukocytes | - | Ex vivo human white blood cells: MTT assay and RT-qPCR | TNF-α, IL-6, IL-1β | HF inhibits expression of inflammatory cytokines TNF-α, IL-6, IL-1β; TNF-α inhibition comparable to positive control piroxicam | [120] |
| Parameter | Unit * | Oral Platycladus orientalis Leaf Extract (Ref. [122]) | Intravenous HF (Ref. [121]) |
|---|---|---|---|
| t1/2 | h | 2.11 ± 0.29 | 6.10 ± 1.86 |
| AUC0−t | ng·h/mL | 667.08 ± 94.31 | 2394.42 ± 466.86 |
| AUC0−∞ | ng·h/mL | 667.48 ± 94.59 | 2541.93 ± 529.85 |
| CL | L/h/kg | 393.6 ± 61.8 (CL/F) ** | 0.41 ± 0.08 (CL) |
| Tmax | h | 1.92 ± 0.20 | - |
| Cmax | ng/mL | 138.45 ± 12.33 | - |
| C2min | ng/mL | - | 803.42 ± 92.75 |
| MRT0−t | h | - | 6.01 ± 0.68 |
| MRT0−∞ | h | - | 7.55 ± 1.37 |
| Vd | L/kg | - | 3.54 ± 1.54 |
| Formulation Type | HF Hybrid Nanomicelles [127] | PEGylated ZIF-8@HF Drug Delivery System [128] |
|---|---|---|
| In vitro cytotoxicity | In A549 cells, HF-loaded TPGS/Soluplus + DQA micelles demonstrated a 2.48-fold increase in cytotoxic potency compared with free HF (IC50 = 7.81 μg/mL vs. 19.34 μg/mL). | In B16F10 melanoma cells, PEG/ZIF-8@HF exhibited an approximately 1.8-fold enhancement in cytotoxicity relative to free HF (IC50 ≈ 4 μM vs. 7.5 μM). |
| In vivo tumor inhibition | In A549 subcutaneous xenograft models, HF-micelles achieved a 1.41-fold improvement in tumor inhibition compared with free HF (tumor inhibition ratio: 64.76% vs. 45.92%). | In B16F10 melanoma–bearing nude mice, PEG/ZIF-8@HF produced an approximately 1.53-fold increase in antitumor efficacy (tumor inhibition ratio: 50.46% vs. 33.03%). |
| Pro-apoptotic effects | HF-micelles induced mitochondrial depolarization and apoptosis 1.57 times more effectively than free HF in vitro (47.23% vs. 30.11%). | PEG/ZIF-8@HF enhanced apoptosis induction by approximately 1.82-fold in vivo, as evidenced by a higher proportion of TUNEL-positive tumor regions (40.83% vs. 22.43%). |
| diPharmacological Effect | Supporting Evidence | Opposing Evidence |
|---|---|---|
| Antioxidant Activity | DPPH-UPLC-Q-TOF/MS evaluation showed HF had the strongest antioxidant capacity among biflavonoids from Selaginella doederleinii extracts [62] | HPLC-DPPH evaluation of Selaginella sinensis extracts showed HF only displayed DPPH scavenging activity at high concentrations, which was much weaker than quercetin and positive control rutin [98] |
| Hepatoprotective Effect | HF exhibited hepatoprotective effects comparable to the standard drug silymarin in CCl4-induced liver injury models [100] | HF alone or combined with glycyrrhizin showed better protection than either alone but did not surpass silymarin in CCl4-induced liver injury models [101] |
| Antimicrobial Activity | Cycas thouarsii extracts demonstrated antibacterial activity against clinical Klebsiella pneumoniae isolates, with HF being the most active purified component [117] | Juniperus chinensis L. ethanol extracts containing HF showed only weak inhibitory activity against Klebsiella pneumoniae [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Liu, F.; Li, R.; Zhou, X.; Li, X. Hinokiflavone as a Potential Antitumor Agent: From Pharmacology to Pharmaceutics. Cells 2026, 15, 17. https://doi.org/10.3390/cells15010017
Liu F, Li R, Zhou X, Li X. Hinokiflavone as a Potential Antitumor Agent: From Pharmacology to Pharmaceutics. Cells. 2026; 15(1):17. https://doi.org/10.3390/cells15010017
Chicago/Turabian StyleLiu, Fengrui, Ranyi Li, Xiaolei Zhou, and Xiaoyu Li. 2026. "Hinokiflavone as a Potential Antitumor Agent: From Pharmacology to Pharmaceutics" Cells 15, no. 1: 17. https://doi.org/10.3390/cells15010017
APA StyleLiu, F., Li, R., Zhou, X., & Li, X. (2026). Hinokiflavone as a Potential Antitumor Agent: From Pharmacology to Pharmaceutics. Cells, 15(1), 17. https://doi.org/10.3390/cells15010017







