Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects
Abstract
1. Introduction
2. Microfragmented Adipose Tissue in OA
| Time | Country | Patient Number | Position (KL) | Age (Mean, Years) | Follow-Up | Results | Complications | Reference |
|---|---|---|---|---|---|---|---|---|
| 2017 | Croatia | 17 (12M/5F) | Knee OA (III-IV) | 69 ± 12 | 12 months | ↑ GAG ↓ VAS, CRP | None | [39] |
| 2019 | USA | 35 (12M/23F) | Knee OA(I-IV) | 63 ± 11 | 1.09 ± 0.49 year | ↓ VAS ↑ KOOS, EQOL score | None | [40] |
| 2019 | Croatia | 10 | Knee OA(III-IV) | 69 ± 12 | 24 months | ↑ GAG | None | [26] |
| 2020 | UK | 110 (60M/50F) | Knee OA(I-IV) | 42–94 | 12 months | ↓ VAS ↑ OKS, EQ-5D | None | [27] |
| 2020 | USA | 25 | GHJ OA (II-IV) | >40 | 12 months | ↓ VAS, DASH ↑ GHJ space | None | [36] |
| 2021 | United Arab Emirates, USA, Italy | 75 (34.7%M/65.3%F) | Knee OA (II-IV) | 69.6 | 24 months | ↓ VAS, ↑ KOOS—ADL, KOOS-Pain | Adipose tissue harvest site pain (49% of patients) and swelling/bruising (28% of patients); knee swelling (13% of patients) | [29] |
| 2021 | Belgium | 64 (48.4%M/51.6%F) | Knee OA (I-IV) | 54.2 ± 9.1 | 12 months | ↓ VAS ↑ KOOS—ADL, QOL; EQ-5D | Knee pain, swelling, and stiffness (79% of patients); knee instability (2 patients) and calf muscle soreness (1 patient) | [28] |
| 2021 | UK | 220 (125M/95F) | Knee OA (III-IV) | - | 24 months | ↑ EQ-5D, OKS | Adipose tissue harvest site pain and bleeding (6%, 4% of patients); Knee pain and swelling (14% of patients); severe reactions to injections (1 patient) | [41] |
| 2022 | Italy | 202 (97M/105F) | Knee OA (I-IV) | 54.0 ± 9.0 | 24.5 ± 9.6 months | ↓ VAS ↑ KOOS | None | [42] |
| 2022 | Italy | 53 (28M/25F) | Knee OA (I-IV) | 54.5 ± 12.1 | 24 months | ↓ VAS ↑ IKDC Subjective scoring, KOOS-Pain | Mild or moderate knee pain, joint swelling and/or effusion (10 patients), pain and oedema in the treated leg (1 patient) | [43] |
| 2022 | Italy | 55 (22M/33F) | Hip OA (I-IV) | 52.5 ± 10.9 | 35 ± 6 months | ↑ OHS | Adipose tissue harvest site bruising (1 patient) | [33] |
| 2023 | UK | 46 (28M/18F) 13 (4M/9F) | Knee OA (I-IV) GHJ OA (III-IV) |
66.9 ± 1.0
64.2 ± 2.4 | 52 weeks | ↓ VAS, DASH ↑ OKS, Lysholm score | None | [44] |
| 2023 | China | 20 (8M/12F) | Knee OA (I-IV) | 54.63 ± 3.90 | 18 months | ↓ VAS, WOMAC score (%) ↑ HSS, KSS, knee oedema | None | [45] |
| 2024 | USA | 26 (8M/18F) | Knee OA (I-IV) | 56.7 ± 7.8 | 12 months | ↓ VAS ↑ KOOS, Tegner score | None | [46] |
| 2025 | USA | 23 (15M/8F) | Knee OA (I-IV) | 62.6 | 12 months | ↓ VAS, WOMAC score (%) ↑ KOOS | Adipose tissue harvest site morbidity of mild pain and ecchymosis (minimal patients) | [31] |
3. Adipose Tissue Stromal Vascular Rich Fraction in OA
4. Dedifferentiated Adipocytes in OA
5. Adipose Tissue-Derived Stem Cells in OA
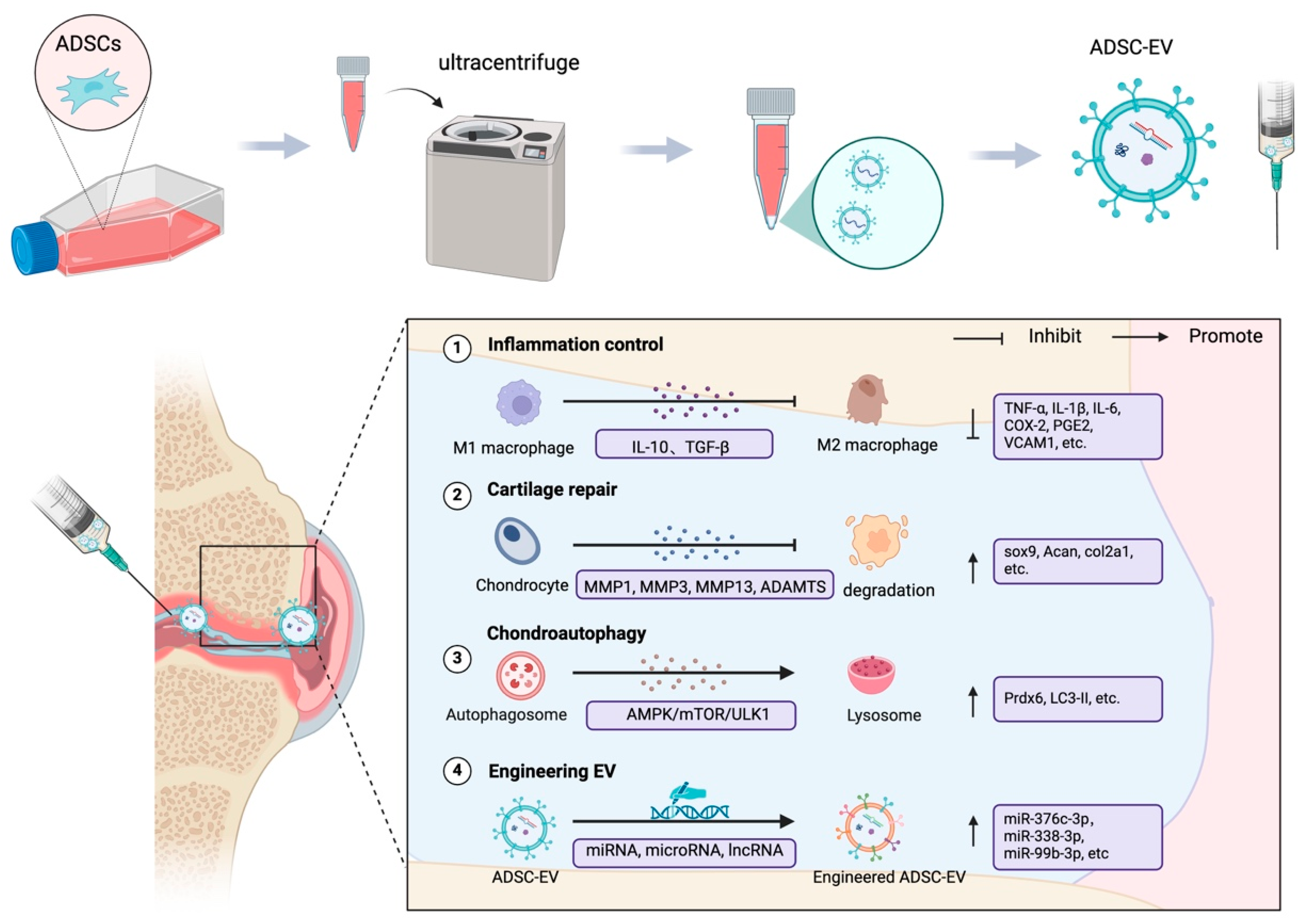
6. Adipose-Derived Stem Cell Extracellular Vesicles in OA
6.1. ADSC-EVs and the Regulation of Inflammation in OA
6.2. ADSC-EVs Promote Cartilage Repair
6.3. Promotion of Chondrocyte Autophagy
6.4. Engineering ADSC-EVs
| Time | Model | Moulding Method | Mechanism | Results | Reference |
|---|---|---|---|---|---|
| 2017 | OA osteoblasts | IL-1β | ↓ HNE-modified proteins ↑ mitochondrial membrane potential | ↓ ageing-related β-galactosidase activity and γ H2AX, IL-6, PGE 2 | [161] |
| 2018 | OA chondrocytes | IL-1β | ↓ NF-κB and activator protein-1 ↑ membrane-bound protein A1 | ↓ PGE 2, MMP-13 ↑ IL-10, collagen II | [147] |
| 2020 | OA chondrocytes; rats | IL-1β, MIA, DMM | - | ↑ chondrocyte proliferation and migration, collagen II ↓ MMP-1, MMP-3, MMP-13, and ADAMTS-5, M1 Macrophage infiltration | [144] |
| 2020 | OA chondrocytes, synovial fibroblasts, periosteal cells | H2O2 | ↑ miR-145, miR-221 Wnt/β-catenin pathway | ↓ IL-6, NF-κB, TNF-α ↑ IL-10, Collagen II and β-catenin | [146] |
| 2021 | OA chondrocytes, synovial cells | IL-1β | ↓ NF-κB pathway | ↓ IL-6, IL-8, MCP-1, MMP-1, MMP-10 and ADAMTS5 | [162] |
| 2021 | OA chondrocytes | IL-1β | Peroxidase 6 | ↓ IL-6, MMP-13 ↑ autophagy protein LC3B | [152] |
| 2022 | OA chondrocytes, synovial cell | IL-1β | ↓ NF-κB pathway | ↓ IL-6, IL-8, MCP-1, COX-2 and VEGF, MMP-1, MMP-13 and ADAMTS-4, TNF-α | [163] |
| 2022 | OA chondrocytes, synovial cell, mice | IL-1β, DMM | ↓ endoplasmic reticulum stress (miR-486-5p) | ↓M1 macrophage, IL-6, TNF-α, MMP13 ↓ CHOP, Caspase-3 and GRP78 ↑ collagen II | [164] |
| 2022 | OA chondrocytes, synovial cells, rats | IL-1β, lipopolysaccharide, MIA | Targets the WNT-β-catenin pathway (MicroRNA-376c-3p) | ↑ collagen II, β-catenin, Aggrecan ↓ TNF-α and IL-6, IL-1β, IFN-γ, α-SMA and collagen III, MMP13, ADAMTS5 | [154] |
| 2023 | Rats | IL-1β | Targets ADAMTS9 to activate the PI3K/AKT/mTOR pathway (miR-93-5p) | ↓ IL-6, IL-1β, TNF-α, and iNOS | [145] |
| 2023 |
Primary articular chondrocytes,
rats | IL-1β, ACLT | Protoelastin induces miR-451-5p | ↑ collagen II and SOX 9; cartilage ECM ↑ OARSI and Mankin scores | [165] |
| 2023 |
Primary articular chondrocytes,
rats | IL-1β, MIA | miR-429 targets FEZ2 | ↑ chondrocyte autophagy | [153] |
| 2023 |
Primary articular chondrocytes,
rats | IL-1β, ACLT | Hypoxia inhibits SASP secretion | ↓ ADAMTS5, MMP13, IL-6, and TNF-α ↑ proteoglycans and collagen II | [142] |
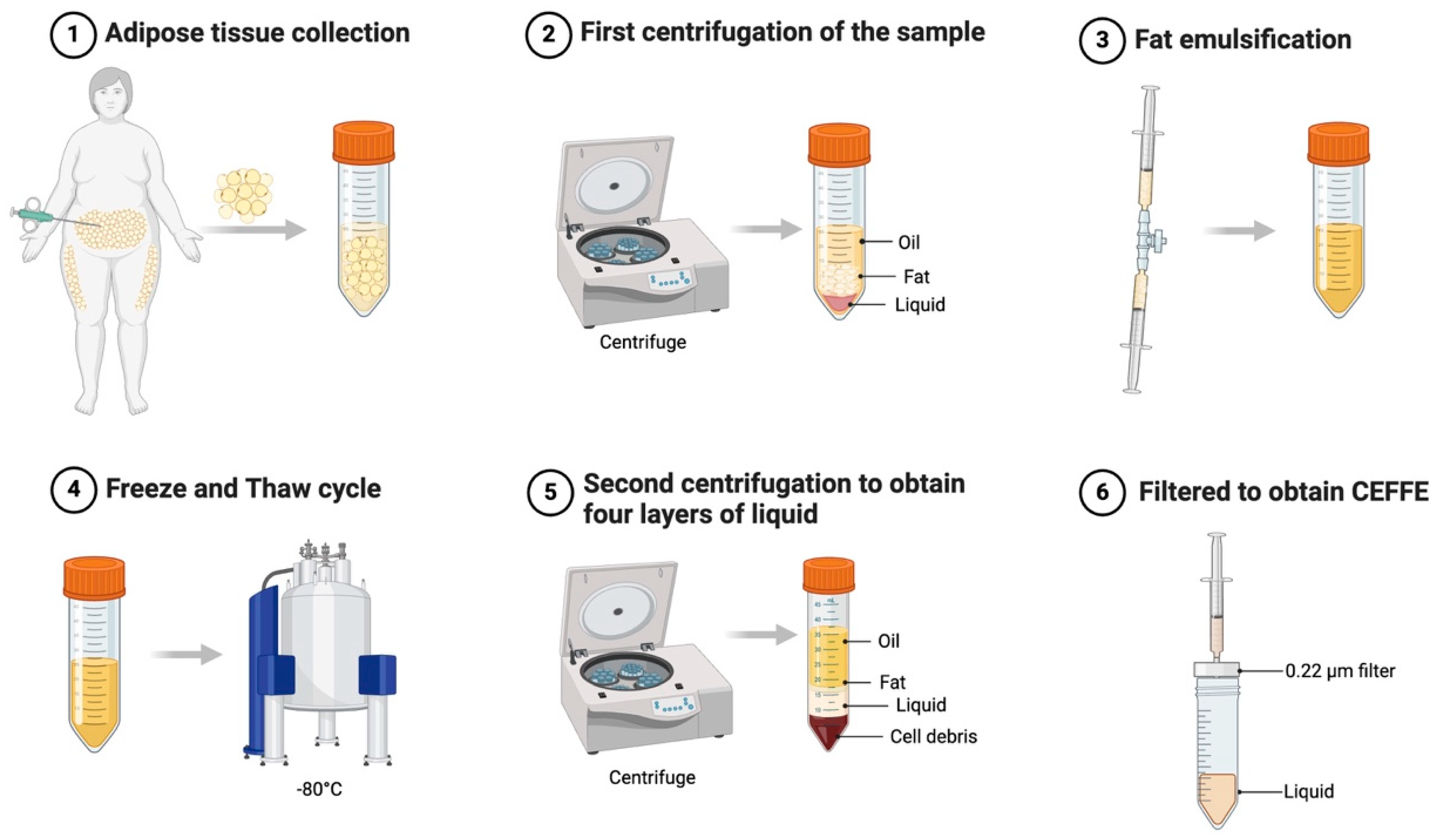
7. Cell-Free Fat Extracts for OA
8. Controversies and Challenges
9. Regulations and Limitations
10. Current Status and Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, B.; Liu, X.; Zeng, H.; Chen, B.; Wang, Z.; Yang, Q.; Peng, J.; Hao, L. Temporal trends in the burden of musculoskeletal diseases in China from 1990 to 2021 and predictions for 2021 to 2030. Bone 2024, 191, 117332. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, E.; Manjoo, A.; Martins, D.; Bhandari, M.; Sethi, P.; Nicholls, M. Intra-articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties. CARTILAGE 2023, 14, 424–432. [Google Scholar] [CrossRef]
- Jüni, P.; Hari, R.; Rutjes, A.W.; Fischer, R.; Silletta, M.G.; Reichenbach, S.; da Costa, B.R. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. 2015, 2015, CD005328. [Google Scholar] [CrossRef]
- Orfanos, G.; McCarthy, H.S.; Williams, M.; Dugard, N.; Gallacher, P.D.; Glover, A.W.; Roberts, S.; Wright, K.T.; Kuiper, J.H. A Randomized Controlled Trial Comparing “Early” Versus “Late” Periosteal Patch Attachment to Knee Chondral Defects in Autologous Chondrocyte Implantation. CARTILAGE 2024. [Google Scholar] [CrossRef]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Xu, H.; Wei, J.; Chen, D.; Li, Y.; Shen, Q. Assessing causality between osteoarthritis and gastrointestinal disorders: A Mendelian randomization study. Sci. Rep. 2023, 13, 1–8. [Google Scholar] [CrossRef]
- Burn, E.; Murray, D.; Hawker, G.; Pinedo-Villanueva, R.; Prieto-Alhambra, D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: A real-world cohort study. Osteoarthr. Cartil. 2019, 27, 1627–1635. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. STEM CELLS Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Placenta Extracellular Vesicles: Messengers Connecting Maternal and Fetal Systems. Biomolecules 2024, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Onorato, F.; Rucci, M.; Alessio-Mazzola, M.; Bistolfi, A.; Castagnoli, C.; Formica, M.; Ferracini, R. Autologous microfragmented adipose tissue treatment of knee osteoarthritis demonstrates effectiveness in 68% of patients at 4-year follow-up. Arch. Orthop. Trauma Surg. 2024, 144, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Jeyaraman, N.; Jayakumar, T.; Ramasubramanian, S.; Ranjan, R.; Jha, S.K.; Gupta, A. Efficacy of stromal vascular fraction for knee osteoarthritis: A prospective, single-centre, non-randomized study with 2 years follow-up. World, J. Orthop. 2024, 15, 457–468. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Colombini, A.; Viganò, M.; de Girolamo, L. Secreted Factors and EV-miRNAs Orches-trate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1582. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, B.; Cai, Y.; Chen, C.; Yu, Z.; Li, W.; Zhang, W. Cell-free fat extract attenuates osteoarthritis via chondrocytes regeneration and macrophages immunomodulation. Stem Cell Res. Ther. 2022, 13, 133. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Prantl, L.; Eigenberger, A.; Reinhard, R.; Siegmund, A.; Heumann, K.; Felthaus, O. Cell-Enriched Lipotransfer (CELT) Improves Tissue Regeneration and Rejuvenation without Substantial Manipulation of the Adipose Tissue Graft. Cells 2022, 11, 3159. [Google Scholar] [CrossRef]
- Carvalho, P.P.; Gimble, J.M.; Dias, I.R.; Gomes, M.E.; Reis, R.L. Xenofree Enzymatic Products for the Isolation of Human Adipose-Derived Stromal/Stem Cells. Tissue Eng. Part C: Methods 2013, 19, 473–478. [Google Scholar] [CrossRef]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.S.; Wu, X.; Dietrich, M.; Rood, J.; Gimble, J.M. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy 2013, 15, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Borrone, A.; Follenzi, A.; Messaggio, F.; Tremolada, C.; Cannas, M. Human Lipoaspirate as Autologous Injectable Active Scaffold for One-Step Repair of Cartilage Defects. Cell Transplant. 2016, 25, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, X.; Yang, Q.; Liu, X.; Fang, J.; Dai, X. Autologous Micro-Fragmented Adipose Tissue as Stem Cell-Based Natural Scaffold for Cartilage Defect Repair. Cell Transplant. 2019, 28, 1709–1720. [Google Scholar] [CrossRef]
- Shi, Z.; He, J.; He, J.; Xu, Y. Micro-fragmented adipose tissue regulated the biological functions of osteoarthritis synoviocytes by upregulating MiR-92a-3p expression. Tissue Cell. 2022, 74, 101716. [Google Scholar] [CrossRef]
- Borić, I.; Hudetz, D.; Rod, E.; Jeleč, Ž.; Vrdoljak, T.; Skelin, A.; Polašek, O.; Plečko, M.; Trbojević-Akmačić, I.; Lauc, G.; et al. A 24-Month Follow-Up Study of the Effect of Intra-Articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2019, 10, 1051. [Google Scholar] [CrossRef]
- Heidari, N.; Noorani, A.; Slevin, M.; Zerbi, A.; Wilson, A. Patient-Centered Outcomes of Microfragmented Adipose Tissue Treatments of Knee Osteoarthritis: An Observational, Intention-to-Treat Study at Twelve Months. Stem Cells Int. 2020, 2020, 8881405. [Google Scholar] [CrossRef]
- Van Genechten, W.; Vuylsteke, K.; Martinez, P.R.; Swinnen, L.; Sas, K.; Verdonk, P. Autologous Micro-Fragmented Adipose Tissue (MFAT) to Treat Symptomatic Knee Osteoarthritis: Early Outcomes of a Consecutive Case Series. J. Clin. Med. 2021, 10, 2231. [Google Scholar] [CrossRef]
- Gobbi, A.; Dallo, I.; Rogers, C.; Striano, R.D.; Mautner, K.; Bowers, R.; Rozak, M.; Bilbool, N.; Murrell, W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: A multi-centric, international study. Int. Orthop. 2021, 45, 1179–1188. [Google Scholar] [CrossRef]
- Russo, A.; Cortina, G.; Condello, V.; Collarile, M.; Orlandi, R.; Gianoli, R.; Giuliani, E.; Madonna, V. Autologous micro-fragmented adipose tissue injection provides significant and prolonged clinical improvement in patients with knee osteoarthritis: A case-series study. J. Exp. Orthop. 2023, 10, 1–15. [Google Scholar] [CrossRef]
- Richter, D.L.; Harrison, J.L.; Faber, L.; Schrader, S.; Zhu, Y.; Pierce, C.; Watson, L.; Shetty, A.K.; Schenck, R.C. Microfragmented Adipose Tissue Injection Reduced Pain Compared With a Saline Control Among Patients With Symptomatic Osteoarthritis of the Knee During 1-Year Follow-Up: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 41, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Natali, S.; Screpis, D.; Farinelli, L.; Iacono, V.; Vacca, V.; Gigante, A.; Zorzi, C. The use of intra-articular injection of autologous micro-fragmented adipose tissue as pain treatment for ankle osteoarthritis: A prospective not randomized clinical study. Int. Orthop. 2021, 45, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Natali, S.; Screpis, D.; Romeo, M.; Magnanelli, S.; Rovere, G.; Andrea, A.; Camarda, L.; Zorzi, C. Is intra-articular injection of autologous micro-fragmented adipose tissue effective in hip osteoarthritis? A three year follow-up. Int. Orthop. 2022, 47, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Slevin, M.; Zeinolabediny, Y.; Meloni, D.; Olgiati, S.; Wilson, A.; Noorani, A.; Azamfirei, L. Comparison of the Effect of MFAT and MFAT + PRP on Treatment of Hip Osteoarthritis: An Observational, Intention-to-Treat Study at One Year. J. Clin. Med. 2022, 11, 1056. [Google Scholar] [CrossRef]
- Striano, R.D.; Malanga, G.A.; Bilbool, N.; Azatullah, K. Refractory shoulder pain with osteoarthritis, and rotator cuff tear, treated with micro-fragmented adipose tissue. Orthop. Spine Sports Med. 2018, 2, 1–5. [Google Scholar]
- Vinet-Jones, H.; K, F.D. Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis. Regen Med. 2020, 15, 2153–2161. [Google Scholar] [CrossRef]
- Baria, M.; George, R.; Barker, T.; Flanigan, D.; Kaeding, C.; Magnussen, R.A. Relationship of Body Mass Index on Patient-Reported Outcomes After Platelet-Rich Plasma Versus Microfragmented Adipose Tissue for Knee Osteoarthritis: A Secondary Analysis of a Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2024, 103, 1006–1011. [Google Scholar] [CrossRef]
- Miller, D.; Grant, A.; Durgam, S.; El-Hayek, K.; Flanigan, D.C.; Malanga, G.; Vasileff, W.K.; Baria, M.R. Adipose-Derived Stem Cells, Obesity, and In-flammation: A Systematic Review and Implications for Osteoarthritis Treatment. Am. J. Phys. Med. Rehabil. 2022, 101, 879–887. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević-Akmačić, I.; Plečko, M.; et al. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes 2017, 8, 270. [Google Scholar] [CrossRef]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. STEM CELLS Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Heidari, N.; Borg, T.-M.; Olgiati, S.; Slevin, M.; Danovi, A.; Fish, B.; Wilson, A.; Noorani, A. Microfragmented Adipose Tissue Injection (MFAT) May Be a Solution to the Rationing of Total Knee Replacement: A Prospective, Gender-Bias Mitigated, Reproducible Analysis at Two Years. Stem Cells Int. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Andriolo, L.; Boffa, A.; Poggi, A.; Cenacchi, A.; Busacca, M.; Kon, E.; Filardo, G.; Di Martino, A. Microfragmented Adipose Tissue Versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up. Am. J. Sports Med. 2022, 50, 2881–2892. [Google Scholar] [CrossRef]
- Fan, F.A.; Grant, R.; Whitehead, J.P.; Yewlett, A.; Lee, P.Y.F. An Observational Study Evaluating the Efficacy of Microfragmented Adipose Tissue in the Treatment of Osteoarthritis. Regen. Med. 2022, 18, 113–121. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, Q.; Li, S.; Liu, M.; Sun, H.; Li, L.; Han, K.; Liu, P. Intra-Articular Injection of Autologous Micro-Fragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: A Prospective Interventional Study. J. Pers. Med. 2023, 13, 504. [Google Scholar] [CrossRef]
- Baria, M.; Barker, T.; Durgam, S.; Pedroza, A.; Flanigan, D.; Jia, L.; Kaeding, C.; Magnussen, R. Microfragmented Adipose Tissue Is Equivalent to Platelet-Rich Plasma for Knee Osteoarthritis at 12 Months Posttreatment: A Randomized Controlled Trial. Orthop. J. Sports Med. 2024, 12, 23259671241233916. [Google Scholar] [CrossRef]
- Yang, W.-T.; Ke, C.-Y.; Yeh, K.-T.; Huang, S.-G.; Lin, Z.-Y.; Wu, W.-T.; Lee, R.-P. Stromal-vascular fraction and adipose-derived stem cell therapies improve cartilage regeneration in osteoarthritis-induced rats. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Anjiki, K.; Matsumoto, T.; Kuroda, Y.; Fujita, M.; Hayashi, S.; Nakano, N.; Tsubosaka, M.; Kamenaga, T.; Takashima, Y.; Kikuchi, K.; et al. Heterogeneous Cells as well as Adipose-Derived Stromal Cells in Stromal Vascular Fraction Contribute to Enhance Anabolic and Inhibit Catabolic Factors in Osteoarthritis. Stem Cell Rev. Rep. 2023, 19, 2407–2419. [Google Scholar] [CrossRef]
- Fujita, M.; Matsumoto, T.; Hayashi, S.; Hashimoto, S.; Nakano, N.; Maeda, T.; Kuroda, Y.; Takashima, Y.; Kikuchi, K.; Anjiki, K.; et al. Paracrine effect of the stromal vascular frac-tion containing M2 macrophages on human chondrocytes through the Smad2/3 signaling pathway. J. Cell Physiol. 2022, 237, 3627–3639. [Google Scholar] [CrossRef]
- Onoi, Y.; Matsumoto, T.; Anjiki, K.; Hayashi, S.; Nakano, N.; Kuroda, Y.; Tsubosaka, M.; Kamenaga, T.; Ikuta, K.; Tachibana, S.; et al. Human uncultured adipose-derived stromal vas-cular fraction shows therapeutic potential against osteoarthritis in immunodeficient rats via direct effects of transplanted M2 macrophages. Stem Cell Res Ther. 2024, 15, 325. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int. Orthop. 2018, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, S.; Vaish, A.; Chavada, V.; Murrell, W.D.; Vaishya, R. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis—A systematic review. Int. Orthop. 2021, 45, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis. Cell Transplant. 2021, 30, 9636897211067454. [Google Scholar] [CrossRef]
- Çimen, O.; Irgıt, K.S.; Bekmezci, T.; Büyüktopçu, Ö.; Şahbat, Y.; Korucu, A. Midterm results of intra-articular stromal vascular fraction injection for the treatment of knee osteoarthritis. Knee Surgery, Sports Traumatol. Arthrosc. 2023, 31, 5012–5017. [Google Scholar] [CrossRef]
- Rogers, C.J.; Harman, R.; Sheinkop, M.B.; Hanson, P.; Ambach, M.A.; David, T.; Desai, R.; Sampson, S.; Aufierro, D.; Bowen, J.; et al. Clinical Evaluation of Safety and Efficacy of a Central Current Good Manufacturing Practices Laboratory Produced Autologous Adipose-Derived Stromal Vascular Fraction Cell Therapy Product for the Treatment of Knee Osteoarthritis. Stem Cells Dev. 2024, 33, 168–176. [Google Scholar] [CrossRef]
- Labarre, K.W.; Zimmermann, G. Infiltration of the Hoffa’s fat pad with stromal vascular fraction in patients with osteoar-thritis of the knee -Results after one year of follow-up. Bone Rep. 2022, 16, 101168. [Google Scholar] [CrossRef]
- Labarre, K.W.; Zimmermann, G. Long-term effects of infrapatellar fat pad SVF infiltration in knee osteoarthritis management: A prospective cohort study. Bone Rep. 2025, 24, 101827. [Google Scholar] [CrossRef]
- Han, J.H.; Jung, M.; Chung, K.; Moon, H.-S.; Jung, S.-H.; Byun, J.; Kim, S.-H. Intra-articular Stromal Vascular Fraction and Mesenchymal Stem Cell Injections Show Variable Efficacy and Higher Potential Complications Compared to Corticosteroid and Hyaluronic Acid in Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2025. [Google Scholar] [CrossRef]
- Onoi, Y.; Matsumoto, T.; Sobajima, S.; Tsubosaka, M.; Hayashi, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Clinical use of autologous adipose-derived stromal vascular fraction cell injections for hip osteoarthritis. Regen. Ther. 2023, 24, 94–102. [Google Scholar] [CrossRef]
- Yokota, N.; Yamakawa, M.; Shirata, T.; Kimura, T.; Kaneshima, H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen. Ther. 2017, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated adipose tissue infusion for the treatment of knee osteoarthritis: Clinical and histological observations. Int. Orthop. 2018, 43, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med, J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Simunec, D.; Salari, H.; Meyer, J. Treatment of Grade 3 and 4 Osteoarthritis with Intraoperatively Separated Adipose Tissue-Derived Stromal Vascular Fraction: A Comparative Case Series. Cells 2020, 9, 2096. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, Q.; Luo, J.; Tong, Y.; Yu, T.; Zhang, Q. The Effect of Autologous Adipose-Derived Stromal Vascular Fractions on Cartilage Regeneration Was Quantitatively Evaluated Based on the 3D-FS-SPGR Sequence: A Clinical Trial Study. BioMed Res. Int. 2022, 2022, 2777568. [Google Scholar] [CrossRef]
- Yokota, N.; Lyman, S.; Hanai, H.; Shimomura, K.; Ando, W.; Nakamura, N. Clinical Safety and Effectiveness of Adipose-Derived Stromal Cell vs Stromal Vascular Fraction Injection for Treatment of Knee Osteoarthritis: 2-Year Results of Parallel Single-Arm Trials. Am. J. Sports Med. 2022, 50, 2659–2668. [Google Scholar] [CrossRef]
- Shevela, E.Y.; Glebova, T.R.; Kotova, M.A.; Nitsa, N.A.; Kozhevnikov, Y.A.; Meledina, I.V.; Ostanin, A.A.; Chernykh, E.R. Comparative Efficacy of the Stromal-Vascular Fraction Cells of Lipoaspirate and Hyaluronic Acid in the Treatment of Gonarthrosis: Results of an Interim Analysis. Bull. Exp. Biol. Med. 2022, 174, 131–136. [Google Scholar] [CrossRef]
- Tantuway, V.; Thomas, W.; Parikh, M.B.; Sharma, R.; Jeyaraman, N.; Jeyaraman, M. Clinical Outcome of Minimally Manipulat-ed, Mechanically Isolated Autologous Adipose Tissue-Derived Stromal Vascular Fraction (Sahaj Therapy®) in Knee Osteoarthritis-Randomized Controlled Trial. Indian J. Orthop. 2023, 57, 1646–1658. [Google Scholar] [CrossRef]
- Fujita, M.; Matsumoto, T.; Sobajima, S.; Tsubosaka, M.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Clinical and Radiological Comparison of Single and Double Intra-articular Injection of Adipose-Derived Stromal Vascular Fraction for Knee Osteoarthritis. Cell Transplant. 2023, 32. [Google Scholar] [CrossRef]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Yun, K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation 1986, 31, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Jumabay, M.; Matsumoto, T.; Yokoyama, S.-I.; Kano, K.; Kusumi, Y.; Masuko, T.; Mitsumata, M.; Saito, S.; Hirayama, A.; Mugishima, H.; et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J. Mol. Cell. Cardiol. 2009, 47, 565–575. [Google Scholar] [CrossRef]
- Xue, M.; Liao, Y.; Jiang, W. Insights into the molecular changes of adipocyte dedifferentiation and its future research oppor-tunities. J. Lipid Res. 2024, 65, 100644. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Pan, Y.; Tian, L.; Choi, M.H.; Wang, L.; Kim, J.Y.; Zhang, J.; Cheng, S.H.; Zhang, L. Hypertonicity induces mitochondrial extracellular vesicles (MEVs) that activate TNF-α and β-catenin signaling to promote adipocyte dedifferentiation. Stem Cell Res. Ther. 2023, 14, 333. [Google Scholar] [CrossRef]
- Côté, J.A.; Lessard, J.; Pelletier, M.; Marceau, S.; Lescelleur, O.; Fradette, J.; Tchernof, A. Role of the TGF-β pathway in dedifferentiation of human mature adipocytes. FEBS Open Bio 2017, 7, 1092–1101. [Google Scholar] [CrossRef]
- Shimizu, M.; Matsumoto, T.; Kikuta, S.; Ohtaki, M.; Kano, K.; Taniguchi, H.; Saito, S.; Nagaoka, M.; Tokuhashi, Y. Transplantation of dedifferentiated fat cell-derived micromass pellets contributed to cartilage repair in the rat osteochondral defect model. J. Orthop. Sci. 2018, 23, 688–696. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Mattiucci, D.; Busilacchi, E.; Mancini, S.; Discepoli, G.; Amici, A.; Falconi, M.; Cinti, S.; Leoni, P. Biosafety evidence for human dedifferentiat-ed adipocytes. J. Cell. Physiol. 2015, 230, 1525–1533. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Z.; Zhang, X.; Zhai, Z.; Wu, Y.; Qu, F.; Xu, C. Conditioned Extracellular Vesicles Derived from Dedifferentiated Fat Cells Promote Bone Regeneration by Altering MicroRNAs. Pharmaceutics 2024, 16, 1430. [Google Scholar] [CrossRef]
- Oki, Y.; Watanabe, S.; Endo, T.; Kano, K. Mature Adipocyte-Derived Dedifferentiated Fat Cells Can Trans-Differentiate into Osteoblasts In Vitro and In Vivo only by All-Trans Retinoic Acid. Cell Struct. Funct. 2008, 33, 211–222. [Google Scholar] [CrossRef]
- Jumabay, M.; Abdmaulen, R.; Urs, S.; Heydarkhan-Hagvall, S.; Chazenbalk, G.D.; Jordan, M.C.; Roos, K.P.; Yao, Y.; Boström, K.I. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J. Mol. Cell. Cardiol. 2012, 53, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Matsumoto, T.; Kazama, T.; Kano, K.; Shimizu, M.; Ryu, K.; Tokuhashi, Y.; Nakanishi, K. Therapeutic potential of dedifferentiated fat cells in a rat model of osteoarthritis of the knee. Regen. Ther. 2024, 26, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Shen, P.-H.; Wang, C.-C.; Liu, H.-Y.; Lu, C.-H.; Su, S.-C.; Liu, J.-S.; Li, P.-F.; Huang, C.-L.; Ho, L.-J.; et al. DFATs derived from infrapatellar fat pad hold advantage on chondrogenesis and adipogenesis to evade age mediated influence. J. Orthop. Transl. 2023, 42, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Nur, R.; Fukuda, N.; Matsumoto, T.; Medet, J.; Kano, K.; Yamamoto, C.; Maruyama, T.; Endo, M.; Matsumoto, K. Implantation of Dedifferentiated Fat Cells Ameliorates Habu Snake Venom-Induced Chronic Renal Dysfunction in Tenascin-C-Deficient Mice. Nephron Exp. Nephrol. 2008, 110, e91–e98. [Google Scholar] [CrossRef]
- Ohta, Y.; Takenaga, M.; Tokura, Y.; Hamaguchi, A.; Matsumoto, T.; Kano, K.; Mugishima, H.; Okano, H.; Igarashi, R. Mature Adipocyte-Derived Cells, Dedifferentiated Fat Cells (DFAT), Promoted Functional Recovery from Spinal Cord Injury-Induced Motor Dysfunction in Rats. Cell Transplant. 2008, 17, 877–886. [Google Scholar] [CrossRef]
- Sugawara, A.; Sato, S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Hum. Cell 2013, 27, 12–21. [Google Scholar] [CrossRef]
- Yamada, H.; Ito, D.; Oki, Y.; Kitagawa, M.; Matsumoto, T.; Watari, T.; Kano, K. Transplantation of mature adipocyte-derived dedifferentiated fat cells promotes locomotor functional recovery by remyelination and glial scar reduction after spinal cord injury in mice. Biochem. Biophys. Res. Commun. 2014, 454, 341–346. [Google Scholar] [CrossRef]
- Soejima, K.; Kashimura, T.; Asami, T.; Kazama, T.; Matsumoto, T.; Nakazawa, H. Effects of mature adipocyte-derived dedifferentiated fat (DFAT) cells on generation and vascularisation of dermis-like tissue after artificial dermis grafting. J. Plast. Surg. Hand Surg. 2014, 49, 25–31. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.-A.; Guo, S.-L.; Wang, S.; Wu, D.; Wang, Y.; Luo, D.; Zhou, B.-R. Antiphotoaging Effect of Conditioned Medium of Dedifferentiated Adipocytes on Skin In Vivo and In Vitro: A Mechanistic Study. Stem Cells Dev. 2015, 24, 1096–1111. [Google Scholar] [CrossRef]
- Maruyama, T.; Fukuda, N.; Matsumoto, T.; Kano, K.; Endo, M.; Kazama, M.; Kazama, T.; Ikeda, J.; Matsuda, H.; Ueno, T.; et al. Systematic implantation of dedifferentiated fat cells ameliorated monoclonal antibody 1-22-3-induced glomerulonephritis by immunosuppression with increases in TNF-stimulated gene 6. Stem Cell Res. Ther. 2015, 6, 80. [Google Scholar] [CrossRef]
- Ikado, Y.; Obinata, D.; Matsumoto, T.; Murata, Y.; Kano, K.; Fukuda, N.; Yamaguchi, K.; Takahashi, S. Transplantation of mature adipocyte-derived dedifferentiated fat cells for the treatment of vesicoureteral reflux in a rat model. Int. Urol. Nephrol. 2016, 48, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Mikrogeorgiou, A.; Sato, Y.; Kondo, T.; Hattori, T.; Sugiyama, Y.; Ito, M.; Saito, A.; Nakanishi, K.; Tsuji, M.; Kazama, T.; et al. Dedifferentiated Fat Cells as a Novel Source for Cell Therapy to Target Neonatal Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2017, 39, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, T.; Kishimoto, N.; Matsuyama, T.; Momota, Y. Functional recovery by application of human dedifferentiated fat cells on cerebral infarction mice model. Cytotechnology 2018, 70, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, H.; Matsumine, H.; Osaki, H.; Ueta, Y.; Kamei, W.; Shimizu, M.; Hashimoto, K.; Fujii, K.; Kazama, T.; Matsumoto, T.; et al. Dedifferentiated fat cells in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Regen. Ther. 2019, 11, 240–248. [Google Scholar] [CrossRef]
- Tateno, A.; Asano, M.; Akita, D.; Toriumi, T.; Tsurumachi-Iwasaki, N.; Kazama, T.; Arai, Y.; Matsumoto, T.; Kano, K.; Honda, M. Transplantation of dedifferentiated fat cells combined with a biodegradable type I collagen-recombinant peptide scaffold for critical-size bone defects in rats. J. Oral Sci. 2019, 61, 534–538. [Google Scholar] [CrossRef]
- Ishioka, S.; Hosokawa, T.; Ikeda, T.; Konuma, N.; Kaneda, H.; Ohashi, K.; Furuya, T.; Masuko, T.; Taniguchi, H.; Kano, K.; et al. Therapeutic potential of mature adipocyte-derived dedifferentiated fat cells for inflammatory bowel disease. Pediatr. Surg. Int. 2020, 36, 799–807. [Google Scholar] [CrossRef]
- Fujisaki, S.; Kajiya, H.; Yanagi, T.; Maeshiba, M.; Kakura, K.; Kido, H.; Ohno, J. Enhancement of jaw bone regeneration via ERK1/2 activation using dedifferentiated fat cells. Cytotherapy 2021, 23, 608–616. [Google Scholar] [CrossRef]
- Huang, G.; Xia, B.; Dai, Z.; Yang, R.; Chen, R.; Yang, H. Comparative study of dedifferentiated fat cell and adipose-derived stromal cell sheets for periodontal tissue regeneration: In vivo and in vitro evidence. J. Clin. Periodontol. 2022, 49, 1289–1303. [Google Scholar] [CrossRef]
- Utsunomiya, K.; Maruyama, T.; Shimizu, S.; Matsumoto, T.; Endo, M.; Kobayashi, H.; Kano, K.; Abe, M.; Fukuda, N. Implantation of dedifferentiated fat cells ameliorated antineutrophil cytoplasmic antibody glomerulonephritis by immunosuppression and increases in tumor necrosis factor-stimulated gene-6. Stem Cell Res. Ther. 2022, 13, 319. [Google Scholar] [CrossRef]
- Akita, D.; Kazama, T.; Tsukimura, N.; Taniguchi, Y.; Takahashi, R.; Arai, Y.; Tsurumachi-Iwasaki, N.; Yasuda, H.; Okubo, T.; Kano, K.; et al. Transplantation of Mature Adipocyte-Derived Dedifferentiated Fat Cells Facilitates Periodontal Tissue Regeneration of Class II Furcation Defects in Miniature Pigs. Materials 2022, 15, 1311. [Google Scholar] [CrossRef]
- Murata, Y.; Obinata, D.; Matsumoto, T.; Ikado, Y.; Kano, K.; Fukuda, N.; Yamaguchi, K.; Takahashi, S. Urethral injection of dedifferentiated fat cells ameliorates sphincter damage and voiding dysfunction in a rat model of persistence stress urinary incontinence. Int. Urol. Nephrol. 2022, 54, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Mimatsu, H.; Onoda, A.; Kazama, T.; Nishijima, K.; Shimoyama, Y.; Go, S.; Ueda, K.; Takahashi, Y.; Matsumoto, T.; Hayakawa, M.; et al. Dedifferentiated fat cells administration amelio-rates abnormal expressions of fatty acids metabolism-related protein expressions and intestinal tissue damage in experimental necrotizing enterocolitis. Sci. Rep. 2023, 13, 8266. [Google Scholar] [CrossRef] [PubMed]
- Kamidaki, Y.; Hosokawa, T.; Abe, N.; Fujita, E.; Yamaoka, B.; Ono, K.; Goto, S.; Kazama, T.; Matsumoto, T.; Uehara, S. Muscle regeneration therapy using dedifferentiated fat cell (DFAT) for anal sphincter dysfunction. Pediatr. Surg. Int. 2024, 40, 238. [Google Scholar] [CrossRef] [PubMed]
- Keerthi, N.; Chimutengwende-Gordon, M.; Sanghani, A.; Khan, W. The Potential of Stem Cell Therapy for Osteoarthritis and Rheumatoid Arthritis. Curr. Stem Cell Res. Ther. 2013, 8, 444–450. [Google Scholar] [CrossRef]
- Kong, L.; Zheng, L.-Z.; Qin, L.; Ho, K.K. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Transl. 2017, 9, 89–103. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes Derived from IL-10-Treated Dendritic Cells Can Suppress Inflammation and Collagen-Induced Arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef]
- Medina, J.; Pérez-Baos, S.; Naredo, E.; López-Reyes, A.; Herrero-Beaumont, G.; Largo, R. AB0075 Intraarterial injection of human adipose-derived mesenchymal stem cells (HAD-MSCS) attenuates inflammation in acute arthritis model. Ann. Rheum. Dis. 2018, 77, 1236. [Google Scholar] [CrossRef]
- Baharlou, R.; Ahmadi-Vasmehjani, A.; Faraji, F.; Atashzar, M.R.; Khoubyari, M.; Ahi, S.; Erfanian, S.; Navabi, S.-S. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: Regulatory effects on peripheral blood mononuclear cells activation. Int. Immunopharmacol. 2017, 47, 59–69. [Google Scholar] [CrossRef]
- Caplan, A.I. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J. Orthop. Res. 2017, 35, 1151–1159. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Mitchell, M.I.; Khalil, M.; Ben-Dov, I.Z.; Alverez-Perez, J.; Illsley, N.P.; Zamudio, S.; Al-Khan, A.; Loudig, O. Customizing EV-CATCHER to Purify Placental Extracellular Vesicles from Maternal Plasma to Detect Placental Pathologies. Int. J. Mol. Sci. 2024, 25, 5102. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Duan, J.; Xiong, N.; Qian, N.; Zhang, R.; Yang, L.; Yu, S.; Wang, F. Repeated intra-articular injections of umbilical cord-derived mesenchymal stem cells for knee osteoarthritis: A phase I., single-arm study. BMC Musculoskelet. Disord. 2023, 24, 488. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2009, 1176, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Muthu, S.; Patil, S.C.; Jeyaraman, N.; Jeyaraman, M.; Gangadaran, P.; Rajendran, R.L.; Oh, E.J.; Khanna, M.; Chung, H.Y.; Ahn, B.-C. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: A meta-analysis. World J. Orthop. 2023, 14, 23–41. [Google Scholar] [CrossRef]
- Kariminekoo, S.; Movassaghpour, A.; Rahimzadeh, A.; Talebi, M.; Shamsasenjan, K.; Akbarzadeh, A. Implications of mesen-chymal stem cells in regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2016, 44, 749–757. [Google Scholar] [CrossRef]
- Vadhan, A.; Gupta, T.; Hsu, W.-L. Mesenchymal Stem Cell-Derived Exosomes as a Treatment Option for Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 9149. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; van Dalen, S.; ter Huurne, M.; Roth, J.; Vogl, T.; Noël, D.; Jorgensen, C.; van den Berg, Q.B.; van de Loo, F.A. Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthr. Cartil. 2014, 22, 1158–1166. [Google Scholar]
- Kuroda, K.; Kabata, T.; Hayashi, K.; Maeda, T.; Kajino, Y.; Iwai, S.; Fujita, K.; Hasegawa, K.; Inoue, D.; Sugimoto, N.; et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet. Disord. 2015, 16, 236. [Google Scholar] [CrossRef]
- Jin, R.; Shen, M.; Yu, L.; Wang, X.; Lin, X. Adipose-Derived Stem Cells Suppress Inflammation Induced by IL-1β through Down-Regulation of P2X7R Mediated by miR-373 in Chondrocytes of Osteoarthritis. Mol. Cells 2017, 40, 222–229. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Cheng, G.; Yuan, X.; Wu, Y.; Wu, H.; Wang, Q.; Chen, J.; Kuai, J.; Chang, Y.; et al. Attenuation of experimental osteoarthritis with human adipose-derived mesenchymal stem cell therapy: Inhibition of the pyroptosis in chondrocytes. Inflamm. Res. 2022, 72, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, D.-Y.; Noh, H.J.; Lee, S.Y.; Yoo, J.A.; Won, S.J.; Jeon, Y.S.; Baek, J.H.; Ryu, D.J. Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis. Cells 2024, 13, 2046. [Google Scholar] [CrossRef] [PubMed]
- Hosono, Y.; Kuwasawa, A.; Toyoda, E.; Nihei, K.; Sato, S.; Watanabe, M.; Sato, M. Multiple intra-articular injections with adipose-derived stem cells for knee osteoarthritis cause severe arthritis with anti-histone H2B antibody production. Regen. Ther. 2023, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, K.-I.; Yoon, W.K.; Song, S.-J.; Jin, W. Intra-articular Injection of Mesenchymal Stem Cells After High Tibial Osteotomy in Osteoarthritic Knee: Two-Year Follow-up of Randomized Control Trial. STEM CELLS Transl. Med. 2022, 11, 572–585. [Google Scholar] [CrossRef]
- Hatano, M.; Ishikura, H.; Terao, T.; Kasai, T.; Yamagami, R.; Higuchi, J.; Yoshida, S.; Arino, Y.; Murakami, R.; Sato, M.; et al. Intra-articular administration of autologous adipose-derived stem cells in hip osteoarthritis: Longitudinal treatment trajectories and prognostic factors. Regen. Ther. 2025, 29, 217–226. [Google Scholar] [CrossRef]
- Chen, C.F.; Hu, C.C.; Wu, C.T.; Wu, H.H.; Chang, C.S.; Hung, Y.P.; Tsai, C.-C.; Chang, Y. Treatment of knee osteoarthritis with intra-articular injec-tion of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: A phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res. Ther. 2021, 12, 562. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hung, Y.-P.; Chen, C.-Y.; Chen, Y.-T.; Tsai, T.-C.; Yang, J.-J.; Wu, C.-C. ELIXCYTE®, an Allogenic Adipose-Derived Stem Cell Product, Mitigates Osteoarthritis by Reducing Inflammation and Preventing Cartilage Degradation In Vitro. Curr. Issues Mol. Biol. 2024, 46, 8395–8406. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of ex-tracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Clua-Ferré, L.; Suau, R.; Vañó-Segarra, I.; Ginés, I.; Serena, C.; Manyé, J. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease. Clin. Transl. Med. 2024, 14, e70075. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Q.; Zhang, Q.; Tian, W.; Chen, T.; Liu, Z. Therapeutic potential of adipose-derived stem cell extracellular vesicles: From inflammation regulation to tissue repair. Stem Cell Res. Ther. 2024, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 108–120. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [CrossRef]
- López-Armada, M.; Caramés, B.; Lires-Deán, M.; Cillero-Pastor, B.; Ruiz-Romero, C.; Galdo, F.; Blanco, F. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr. Cartil. 2006, 14, 660–669. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. In Seminars in Arthritis and Rheumatism; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Liu, Q.; Wu, J.; Wang, H.; Jia, Z.; Li, G. Human Infrapatellar Fat Pad Mesenchymal Stem Cell–derived Extracellular Vesicles Purified by Anion Exchange Chromatography Suppress Osteoarthritis Progression in a Mouse Model. Clin. Orthop. Relat. Res. 2024, 482, 1246–1262. [Google Scholar] [CrossRef]
- Ragni, E.; Colombini, A.; Viganò, M.; Libonati, F.; Orfei, C.P.; Zagra, L.; de Girolamo, L. Cartilage Protective and Immunomodulatory Features of Osteoarthritis Synovial Fluid-Treated Adipose-Derived Mesenchymal Stem Cells Secreted Factors and Extracellular Vesicles-Embedded miRNAs. Cells 2021, 10, 1072. [Google Scholar] [CrossRef]
- Samal, J.R.; Rangasami, V.K.; Samanta, S.; Varghese, O.P.; Oommen, O.P. Discrepancies on the role of oxygen gradient and cul-ture condition on mesenchymal stem cell fate. Adv. Healthc. Mater. 2021, 10, 2002058. [Google Scholar] [CrossRef]
- Chang, L.-H.; Wu, S.-C.; Chen, C.-H.; Chen, J.-W.; Huang, W.-C.; Wu, C.-W.; Lin, Y.-S.; Chen, Y.-J.; Chang, J.-K.; Ho, M.-L. Exosomes Derived from Hypoxia-Cultured Human Adipose Stem Cells Alleviate Articular Chondrocyte Inflammaging and Post-Traumatic Osteoarthritis Progression. Int. J. Mol. Sci. 2023, 24, 13414. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Orfei, C.P.; De Luca, P.; Mondadori, C.; Viganò, M.; Colombini, A.; de Girolamo, L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: The example of joint disease. Stem Cell Res. Ther. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.H.; Kim, H.K.; Yang, S.; Park, J.H.; Jo, D.; Cho, Y.W.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duan, J.; Lin, W.; Liu, J. Exosomal miR-93-5p regulated the progression of osteoarthritis by targeting ADAMTS9. Open Med. 2023, 18, 20230668. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; del Caz, M.D.P.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef]
- Bina, V.; Brancato, A.M.; Caliogna, L.; Berni, M.; Gastaldi, G.; Mosconi, M.; Pasta, G.; Grassi, F.A.; Jannelli, E. Mesenchymal Stem Cells and Secretome as a New Possible Approach to Treat Cartilage Damage: An In Vitro Study. Biomolecules 2024, 14, 1068. [Google Scholar] [CrossRef]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef]
- Caramés, B.; Taniguchi, N.; Otsuki, S.; Blanco, F.; Lotz, M. 188 AUTOPHAGY IS A PROTECTIVE MECHANISM IN NORMAL CARTILAGE AND ITS AGING-RELATED LOSS IS LINKED WITH CELL DEATH AND OSTEOARTHRITIS. Osteoarthr. Cartil. 2009, 17, S109. [Google Scholar] [CrossRef]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 2021, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Na, Y.; Han, C.; Ren, Y.; Liu, M.; Ma, P.; Bai, R. Exosomal miR-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting FEZ2. Int. Immunopharmacol. 2023, 120, 110315. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Z.; Xie, Z.; Sun, X.; Li, C.; Chen, Y.; Xu, J.; Pi, G. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis 2022, 28, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xiu, G.; Wang, J.; Wen, Y.; Lu, J.; Wu, B.; Wang, G.; Yang, D.; Ling, B.; Du, D.; et al. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnol. 2023, 21, 341. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Pu, G.; Wu, J.; Qin, F. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J. Orthop. Surg. Res. 2022, 17, 567. [Google Scholar] [CrossRef]
- Yin, Z.; Qin, C.; Pan, S.; Shi, C.; Wu, G.; Feng, Y.; Zhang, J.; Yu, Z.; Liang, B.; Gui, G. Injectable hyperbranched PEG crosslinked hyaluronan hydrogel micro-particles containing mir-99a-3p modified subcutaneous ADSCs-derived exosomes was beneficial for long-term treatment of osteo-arthritis. Mater Today Bio 2023, 23, 100813. [Google Scholar]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; De Girolamo, L. Interaction with hyaluronan matrix and miRNA cargo as contributors for in vitro potential of mesenchymal stem cell-derived extracellular vesicles in a model of human osteoarthritic synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D Printing of Microenvironment-Specific Bioinspired and Exosome-Reinforced Hydrogel Scaffolds for Efficient Cartilage and Subchondral Bone Regeneration. Adv. Sci. 2023, 10, e2303650. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Xiang, W.; Gong, Y.; Feng, D.; Fang, S.; Wu, Y.; Liu, Z.; Li, Y.; Chen, R.; et al. Engineering exosomes derived from TNF-α preconditioned IPFP-MSCs enhance both yield and therapeutic efficacy for osteoarthritis. J. Nanobiotechnol. 2024, 22, 555. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; del Caz, M.D.P.; Castejón, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef]
- Cavallo, C.; Merli, G.; Borzì, R.M.; Zini, N.; D’adamo, S.; Guescini, M.; Grigolo, B.; Di Martino, A.; Santi, S.; Filardo, G. Small Extracellular Vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-κB dependent inflammatory/catabolic environment of osteoarthritis. Sci. Rep. 2021, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Merli, G.; Zini, N.; D’adamo, S.; Cattini, L.; Guescini, M.; Grigolo, B.; Di Martino, A.; Santi, S.; Borzì, R.M.; et al. Small Extracellular Vesicles from Inflamed Adipose Derived Stromal Cells Enhance the NF-κB-Dependent Inflammatory/Catabolic Environment of Osteoarthritis. Stem Cells Int. 2022, 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, A.; Lu, L.; Pan, Z.; Ma, M.; Luo, S.; Liu, Z.; Yang, L.; Cai, J.; Yin, F. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem. Pharmacol. 2022, 206, 115343. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tang, C.; Deng, M.; Yuan, J.; Fan, Y.; Gao, S.; Feng, Y.; Yang, J.; Chen, C. Tropoelastin-Pretreated Exosomes from Adipose-Derived Stem Cells Improve the Synthesis of Cartilage Matrix and Alleviate Osteoarthritis. J. Funct. Biomater. 2023, 14, 203. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, Y.; Deng, M.; Li, D.; Wang, X.; Zheng, H.; Xu, Y.; Li, W.; Zhang, W. Fat extract promotes angiogenesis in a murine model of limb ischemia: A novel cell-free therapeutic strategy. Stem Cell Res. Ther. 2018, 9, 294. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, Z.; Yu, Q.; Zheng, H.; Xu, Y.; Deng, M.; Wang, X.; Zhang, L.; Zhang, W.; Li, W. Fat Extract Improves Random Pattern Skin Flap Survival in a Rat Model. Aesthetic Surg. J. 2019, 39, NP504–NP514. [Google Scholar] [CrossRef]
- Deng, M.; Wang, X.; Yu, Z.; Cai, Y.; Liu, W.; Zhou, G.; Wang, X.; Yu, Z.; Zhang, W.J. Cell-free fat extract promotes tissue regeneration in a tissue expansion model. Stem Cell Res. Ther. 2020, 11, 50. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.; Zhou, X.; Duan, J.; Hu, Y.; Zhang, W.; Liu, Q.; Xu, B.; Zhang, A. Cell-free fat extract improves ovarian function and fertility in mice with premature ovarian insufficiency. Stem Cell Res. Ther. 2022, 13, 320. [Google Scholar] [CrossRef]
- Cai, Y.; Jia, Z.; Zhang, Y.; Kang, B.; Chen, C.; Liu, W.; Zhang, W. Cell-free fat extract restores hair loss: A novel therapeutic strategy for androgenetic alopecia. Stem Cell Res. Ther. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, B.; Dong, Y.; Fan, M.; Li, W.; Zhang, W. Annexin A5 Derived from Cell-free Fat Extract Attenuates Osteoarthritis via Macrophage Regulation. Int. J. Biol. Sci. 2024, 20, 2994–3007. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult hu-man adipose tissue. Cytom. Part A J. Int. Soc. Adv. Cytom. 2010, 77, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Condé-Green, A.; Rodriguez, R.L.; Slezak, S.; Singh, D.P.; Goldberg, N.H.; McLenithan, J. Comparison between Stromal Vascular Cells’ Isolation with Enzymatic Digestion and Mechanical Processing of Aspirated Adipose Tissue. Plast. Reconstr. Surg. 2014, 134, 54. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Nagase, T.; Koshima, I.; et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef]
- Markarian, C.F.; Frey, G.Z.; Silveira, M.D.; Milani, A.R.; Ely, P.B.; Horn, A.P.; Nardi, N.B.; Camassola, M. Isolation of adipose-derived stem cells: A compar-ison among different methods. Biotechnol. Lett. 2014, 36, 693–702. [Google Scholar] [CrossRef]
- Raposio, E.; Caruana, G.; Bonomini, S.; Libondi, G. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014, 133, 1406–1409. [Google Scholar] [CrossRef]
- Solodeev, I.; Meilik, B.; Gur, E.; Shani, N. A Closed-system Technology for Mechanical Isolation of High Quantities of Stromal Vascular Fraction from Fat for Immediate Clinical Use. Plast. Reconstr. Surg.—Glob. Open 2023, 11, e5096. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast can-cer cells. Proc. Natl. Acad. Sci. USA. 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of Pancreatic Cancer Stem Cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Chen, H.; Zhang, S.; Wen, J.-C.; Zheng, J.-K.; Chen, Q.; Li, W.-Y.; Wang, P.-P.; Ma, L.; Huang, T.-H.; et al. Several types of soft tissue sarcomas originate from the malignant transformation of adipose tissue-derived stem cells. Mol. Med. Rep. 2010, 3, 441–448. [Google Scholar] [CrossRef]
- Koellensperger, E.; Bonnert, L.-C.; Zoernig, I.; Marmé, F.; Sandmann, S.; Germann, G.; Gramley, F.; Leimer, U. The impact of human adipose tissue-derived stem cells on breast cancer cells: Implications for cell-assisted lipotransfers in breast reconstruction. Stem Cell Res. Ther. 2017, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Chi, Y.; Zhang, Q.; Xu, F.; Yang, Z.; Meng, L.; Yang, S.; Mao, A.; Zhang, J.; et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013, 4, e950. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Human Umbilical Cord-Derived Mesenchymal Stem Cells Do Not Undergo Malignant Transformation during Long-Term Culturing in Serum-Free Medium. PLoS ONE 2014, 9, e98565. [Google Scholar] [CrossRef]
- Bapat, A.; Kalodimou, V.E.; Muthu, S. Regulatory concerns for exosome-and other extracellular vesicle-based diagnostics and medicine products. In Extracellular Vesicles for Therapeutic and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 523–536. [Google Scholar]
- Raposio, E.; Ciliberti, R. Clinical use of adipose-derived stem cells: European legislative issues. Ann. Med. Surg. 2017, 24, 61–64. [Google Scholar] [CrossRef]
- Zocchi, M.L.; Vindigni, V.; Pagani, A.; Pirro, O.; Conti, G.; Sbarbati, A.; Bassetto, F. Regulatory, ethical, and technical considerations on regenerative technologies and adipose-derived mesenchymal stem cells. Eur. J. Plast. Surg. 2019, 42, 531–548. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Hogden, A.; Khanna, A.; Kuah, D. Efficacy of adipose-derived stem cells and stromal vascular fraction for pain relief in Kellgren-Lawrence grade II-III knee osteoarthritis: A systematic review (2019–2024). J. Orthop. 2025, 70, 95–106. [Google Scholar] [CrossRef]
- Lee, H.; Lim, Y.; Lee, S.-H. Rapid-acting pain relief in knee osteoarthritis: Autologous-cultured adipose-derived mesenchymal stem cells outperform stromal vascular fraction: A systematic review and meta-analysis. Stem Cell Res. Ther. 2024, 15, 446. [Google Scholar] [CrossRef]
- Maeda, T.; Sobajima, S.; Matsumoto, T.; Tsubosaka, M.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Comparison of short-term clinical outcomes of intra-articular injection of micro-fragmented adipose tissue and stromal vascular fraction cells for knee osteoarthritis treatment: A retrospective single-center cohort study. Regen. Ther. 2025, 29, 91–99. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, Z.; Yan, J.; Tang, Z.; Zhou, L.; Jin, D.; Jin, Q. Effect of intra-knee injection of autologous adipose stem cells or mesenchymal vascular components on short-term outcomes in patients with knee osteoarthritis: An updated meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2023, 25, 147. [Google Scholar] [CrossRef]
- Kim, K.I.; Kim, M.S.; Kim, J.H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Frac-tions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Con-trolled Trials. Am. J. Sports Med. 2023, 51, 837–848. [Google Scholar] [CrossRef]
- Anil, U.; Markus, D.H.; Hurley, E.T.; Manjunath, A.K.; Alaia, M.J.; Campbell, K.A.; Jazrawi, L.M.; Strauss, E.J. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. Knee 2021, 32, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Huang, Y. Clinical efficacy of cell-free fat extract and its effects on bone marrow edema in patients with early to mid-stage knee osteoarthritis: A clinical trial in comparison with hyaluronic acid. J. Orthop. Surg. Res. 2025, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Murata, D.; Yoshizato, H.; Kashimoto, S.; Nakamura, A.; Morimoto, T.; Nakayama, K. Bio-3D printing of scaffold-free ADSC-derived cartilage constructs comparable to natural cartilage in vitro. J. Orthop. Surg. Res. 2025, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Song, W.; Yan, Z.; Zhai, W.; Ren, L. Gelatin microcarriers as an effective adipose-derived stem cells delivery strategy in osteoarthritis treatment. Int. J. Biol. Macromol. 2024, 283, 137524. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Wang, J. Sequential release of transforming growth factor β1 and fibroblast growth factor 2 from nanofibrous scaffolds induces cartilage differentiation of mouse adipose-derived stem cells. Biointerphases 2024, 19, 041002. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Z.; Ding, Y.; Dai, Y.; Feng, Z.; Li, Z.; Ma, L.; Diao, N.; Guo, A.; Yin, H. A Hybrid Scaffold Induces Chondrogenic Differentiation and Enhances In Vivo Cartilage Regeneration. Tissue Eng. Part A 2025, 31, 219–233. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Nedunchezian, S.; Lin, S.-Y.; Su, Y.-F.; Wu, C.-W.; Wu, S.-C.; Chen, C.-H.; Wang, C.-K. Bilayer osteochondral graft in rabbit xenogeneic transplantation model comprising sintered 3D-printed bioceramic and human adipose-derived stem cells laden biohydrogel. J. Biol. Eng. 2023, 17, 74. [Google Scholar] [CrossRef]


| Time | Country | Patient Number | Position (KL) | Age (Mean, Years) | Follow-Up | Results | Complications | Reference |
|---|---|---|---|---|---|---|---|---|
| 2017 | Japan | 13 (2M/11F) | Knee OA (II-IV) | 74.5 ± 5.4 | 6 months | ↓ WOMAC score (%), VAS | Pain and swelling in injection and fat harvesting sites | [61] |
| 2018 | China | 16 (3M/13F) | Knee OA (II-IV) | 53 ± 10.97 (left) 51 ± 5.95 (right) | 12 months | ↓ VAS, WOMAC score (%) ↑ ROM | Pain in fat harvesting site (25% of patients) Post-injection pain (37.5% of patients) | [51] |
| 2019 | Italy | 20 (9M/11F) | Knee OA (I-IV) | 59.6 ± 10.5 | 18 months | ↓ VAS, WOMAC score (%) | One case of occult swelling in the suprapatellar region Most patients felt “tied knee” | [62] |
| 2019 | Croatia | 20 (15M/5F) | Knee OA (III-IV) | 40–85 | 12 months | ↓ VAS, WOMAC score (%) ↑ KOOS | None | [63] |
| 2019 | Japan | 38 (7M/31F) | Knee OA (II-IV) | 73 ± 9.1 | 6 months | ↓ VAS; ↑ KOOS | Knee joint effusion after injection (8% of patients) Minor complications in fat harvesting site (34% of patients) | [15] |
| 2020 | America | 39 (17M/22F) | Knee OA (II-III) | 59.0 ± 9.9 | 12 months | ↑ WOMAC score (%) | Post-injection pain and swelling (1 patient) | [64] |
| 2020 | Japan | 57 (41M/16F) | Knee OA (II-IV) | 69.4 ± 6.9 | 12 months | ↓ VAS, WOMAC score (%) and ↑ KOOS ↑ MRI: T2 mapping values of lateral femur and tibia ↑ knee extension angle | None | [52] |
| 2020 | Germany | 12 (7M/5F) | Knee OA (III-IV) | 61 (51–80) | 12 months | ↑ KOOS; subjective satisfaction: 67% | Post-injection painless swelling Haematoma and muscle soreness in fat harvesting site | [65] |
| 2022 | China | 47 (18M/29F) | Knee OA (II-III) | 50.83 ± 10.88 | 12 months | ↓ cartilage defect thickness, VAS, WOMAC score (%) | Post-injection pain and swelling | [66] |
| 2022 | Germany | 33 (18M/15F) | Knee OA (I-IV) | 60.58(23–88) | 12 months | ↓ VAS and ↑ KOOS ↑ VR12 psychological scores | None | [57] |
| 2022 | Japan | 38 (18% M/82% F) | Knee OA (II-IV) | 73.6 ± 9 | 24 months | ↓ VAS and ↑ KOOS | Pain, bleeding, induration in fat harvesting site (13 patients) Post-injection pain and swelling (3 knees) | [67] |
| 2022 | Russia | 16 (7M/9F) | Knee OA (II-IV) | 61 (57–64) | 12 months | ↓ VAS and ↑ KOOS | Almost all patients had post-injection site discomfort; some reported painless swelling at the injection site A proportion of 27% of patients had a slight increase in temperature (37.2–37.6 °C) | [68] |
| 2023 | India | 58 | Knee OA (I-III) | 45–85 | 36 months | ↓ VAS and ↑ KOOS | Pain, swelling, and bruising in fat harvesting site (some patients) | [69] |
| 2023 | Japan | Single injection group: 30 (8M/22F) Double injection group: 24 (6M/18F) | Knee OA (II-IV) | Single injection group: 68.8 ± 8.2 Double injection group: 69.1 ± 11.8 | 24 months | ↓ WOMAC score ↑ HKA angle and the mean T2 mapping values | Post-injection pain and swelling 9.3% (single injection group) and 8.3% (double injection group) | [70] |
| 2023 | Japan | 42 (5M/37F) | Hip OA (II-IV) | 60.2 ± 9.4 | 6 months | ↑ HHS, JHEQ scores and ↓ VAS | Mild hip pain (5 patients (11.9%)) | [60] |
| 2024 | USA | 29 (9M/20F) | Knee OA (II-IV) | 65.6 | 12 months | ↑ KOOS | Mild to moderate post-injection pain or itching (6 patients) Mild to moderate pain, bruising, subcutaneous haematoma or numbness in fat harvesting site (17 patients) | [56] |
| 2025 | Germany | 25 (14M/11F) | Knee OA (IV) | 53–67 | 24 months | ↓ VAS and ↑ KOOS, ADL and QOL scores | None | [58] |
| Time | Disease | Animal | Results | Reference |
|---|---|---|---|---|
| 2008 | Chronic renal dysfunction | Mice | Improvement of glomerulosclerosis, ↓ TGF-β1 and fibronectin mRNA in renal cortex ↓ serum BUN | [84] |
| 2008 | Spinal cord injury | Rat | ↑ βIII microtubule protein; BDNF; GDNF | [85] |
| 2012 | Infarcted myocardium | Mice | ↑ endothelial cells | [81] |
| 2014 | Periodontal tissue loss | Rat | ↑ proliferating cell nuclear antigen; periodontal tissue regeneration | [86] |
| 2014 | Spinal cord injury | Mice | ↑ motor function of the hind limbs; neurotrophic factor; astrocytes and oligodendrocytes | [87] |
| 2015 | Artificial dermal graft | Rat | ↑ capillary infiltration; endothelial cells; thickness of dermal-like tissue | [88] |
| 2015 | Light-aged | Mice | ↑ TGF-β1; collagen I and III ↓ MMP-1 and MMP-3 | [89] |
| 2015 | Glomerulonephritis | Rat | ↑ TSG-6 ↓ macrophage infiltration and IL-6, IL-10, and IL-12β | [90] |
| 2016 | Vesicoureteral reflux | Rat | ↓ ureteral internal/external diameter ratio and connective tissue area in the posterior bladder wall ↓ apoptosis of renal pelvic urinary tract epithelial cells | [91] |
| 2017 | Hypoxic–ischemic encephalopathy | Rat | ↓ brain cell death rate | [92] |
| 2018 | Cerebral infarction | Mice | ↑ Nestin and SOX2; functional recovery | [93] |
| 2018 | Knee cartilage defect | Rat | ↑ Sox9; collagen II (COL2A1) ↑ ICRS score; modified O’Driscol histological score | [77] |
| 2019 | Facial nerve defects | Rat | ↑ number of myelinated fibres; thickness of myelin sheaths in the spinal cord | [94] |
| 2019 | Mandibular bone defect | Rat | ↑ bone regenerationd; bone width | [95] |
| 2020 | Inflammatory bowel disease | Mice | ↑ TRAIL, IDO1, and NOS2 ↓ T-cell proliferation | [96] |
| 2021 | Mandibular defects in osteoporotic | Rat | ↑ ERK1/2 and Smad2 phosphorylation signalling pathways | [97] |
| 2022 | Intra-periodontal bone defects | Rat | ↑ ALP, Runx2, OPN | [98] |
| 2022 | Glomerulonephritis | Mice | ↑ microRNA 23b-3p; TSG-6 mRNA; PGE2 and IL-10 mRNAs; CCL-17 ↓ CD44 mRNA; TNF-α and MCP-1 | [99] |
| 2022 | Periodontal Class II bifurcation defects | Small porcine | ↑ cytoskeletal, periodontal ligament-like fibres and alveolar bone formation | [100] |
| 2022 | Persistent stress urinary incontinence | Rat | ↑ leak point pressure; urethral transverse muscle; smooth muscle | [101] |
| 2023 | Neonatal necrotizing enterocolitis | Rat | ↓ IL-6; CCL-2 | [102] |
| 2024 | OA | Rat | ↑ PTGS2, TNFAIP6, and BMP2; ↓ ADAMTS4 and IL6 in synovial fibroblasts | [82] |
| 2024 | Anorectal sphincter dysfunction | Rat | ↑ MyoD and myogenin genes; mature myocytes | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Felthaus, O.; Prantl, L. Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects. Cells 2025, 14, 669. https://doi.org/10.3390/cells14090669
Zhang H, Felthaus O, Prantl L. Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects. Cells. 2025; 14(9):669. https://doi.org/10.3390/cells14090669
Chicago/Turabian StyleZhang, Hanwen, Oliver Felthaus, and Lukas Prantl. 2025. "Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects" Cells 14, no. 9: 669. https://doi.org/10.3390/cells14090669
APA StyleZhang, H., Felthaus, O., & Prantl, L. (2025). Adipose Tissue-Derived Therapies for Osteoarthritis: Multifaceted Mechanisms and Clinical Prospects. Cells, 14(9), 669. https://doi.org/10.3390/cells14090669







