Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting
Abstract
1. Introduction
2. Spheroids and Organoids
3. Vascularization Strategies
3.1. Internal Induction
3.2. External Induction
3.2.1. Medium Composition
3.2.2. Hydrogel
4. Applications of Vascularized Spheroids and Organoids Using 3D Bioprinting
4.1. Bone
4.2. Liver
4.3. Cardiac Tissue
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | Adipose-derived stem cells |
| MSCs | Mesenchymal stem cells |
| BM-MSCs | Bone marrow mesenchymal stem cells |
| BdECM | Bone-derived extracellular matrix |
| b-TCP | Β-tricalcium phosphate |
| CVDs | Cardiovascular diseases |
| ECM | Extracellular matrix |
| DPSCs | Dental pulp stem cells |
| EVCs | Early vascular cells |
| ECs | Endothelial cells |
| ECFC | Endothelial colony–forming cell |
| EPCs | Endothelial progenitor cells |
| ESC | Embryonic stem cells |
| FBS | Fetal bovine serum |
| FGF-2 | Fibroblast growth factors |
| FBs | Fibroblasts |
| FRESH | Freeform reversible embedding of suspended hydrogels |
| GelMA | Gelatin methacryloyl |
| GPCs | Gingiva-derived progenitor cells |
| HMW | High molecular weight |
| hPSCs | Human pluripotent stem cells |
| HLFs | Human lung fibroblasts |
| HPL | Human platelet lysate |
| hTMSCs | Human turbinate mesenchymal stem cells |
| HUVECs | Human umbilical vein endothelial cells |
| HA | Hyaluronic acid |
| HIFs | Hypoxia induced factor |
| iPSC | Induced pluripotent stem cells |
| LPCs | Liver progenitor cells |
| LSECs | Liver sinusoidal endothelial cells |
| MVF | Microvascular fragments |
| MI | Myocardial infarction |
| PDGFR | Platelet-derived growth factor receptor |
| PDGF | Platelet-derived growth factor |
| PLGA | Poly (lactic-co-glycolic acid) copolymer |
| PEG | Polyethylene glycol |
| SMCs | Smooth muscle cells |
| SLA | Stereolithography |
| TGF-b | Transforming growth factor beta |
| UC-MSC | Umbilical cord mesenchymal stem cells |
| VEGF | Vascular endothelial growth factor |
| CM | Cardiomyocytes |
References
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Coffin, J.D.; Harrison, J.; Schwartz, S.; Heimark, R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev. Biol. 1991, 148, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Benmeridja, L.; De Moor, L.; De Maere, E.; Vanlauwe, F.; Ryx, M.; Tytgat, L.; Vercruysse, C.; Dubruel, P.; Van Vlierberghe, S.; Blondeel, P.; et al. High-throughput fabrication of vascularized adipose microtissues for 3D bioprinting. J. Tissue Eng. Regen. Med. 2020, 14, 840–854. [Google Scholar] [CrossRef]
- De Moor, L.; Smet, J.; Plovyt, M.; Bekaert, B.; Vercruysse, C.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Engineering microvasculature by 3D bioprinting of prevascularized spheroids in photo-crosslinkable gelatin. Biofabrication 2021, 13, 045021. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A novel modality in disease modeling. Bio-Design Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Heydari, Z.; Zarkesh, I.; Ghanian, M.-H.; Aghdaei, M.H.; Kotova, S.; Zahmatkesh, E.; Farzaneh, Z.; Piryaei, A.; Akbarzadeh, I.; Shpichka, A.; et al. Biofabrication of size-controlled liver microtissues incorporated with ECM-derived microparticles to prolong hepatocyte function. Bio-Design Manuf. 2021, 4, 790–805. [Google Scholar] [CrossRef]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.K.; Salmeron-Sanchez, M.; Stevens, M.M. Void-free 3D Bioprinting for In-situ Endothelialization and Microfluidic Perfusion. Adv. Funct. Mater. 2020, 30, 1908349. [Google Scholar] [CrossRef]
- Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S. Prospects for 3D bioprinting of organoids. Biodes. Manuf. 2021, 4, 627–640. [Google Scholar] [CrossRef]
- Sivakumar, H.; Devarasetty, M.; Kram, D.E.; Strowd, R.E.; Skardal, A. Multi-Cell Type Glioblastoma Tumor Spheroids for Evaluating Sub-Population-Specific Drug Response. Front. Bioeng. Biotechnol. 2020, 8, 538663. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; De Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, L.A.; Rodriguez-Salvador, M. Uncovering 3D bioprinting research trends: A keyword network mapping analysis. Int. J. Bioprint. 2018, 4, 147. [Google Scholar] [CrossRef] [PubMed]
- Browning, A.P.; A Sharp, J.; Murphy, R.J.; Gunasingh, G.; Lawson, B.; Burrage, K.; Haass, N.K.; Simpson, M. Quantitative analysis of tumour spheroid structure. eLife 2021, 10, e73020. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.R.C.; Oancea, A.D.; Stüllein, C.; Régnier-Vigouroux, A. Evaluation of Consistency in Spheroid Invasion Assays. Sci. Rep. 2016, 6, 28375. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Spheroids as vascularization units: From angiogenesis research to tissue engineering applications. Biotechnol. Adv. 2017, 35, 782–791. [Google Scholar] [CrossRef]
- Boutin, M.E.; Kramer, L.L.; Livi, L.L.; Brown, T.; Moore, C.; Hoffman-Kim, D. A three-dimensional neural spheroid model for capillary-like network formation. J. Neurosci. Methods 2018, 299, 55–63. [Google Scholar] [CrossRef]
- Kim, E.M.; Bin Lee, Y.; Kim, S.-J.; Park, J.; Lee, J.; Kim, S.W.; Park, H.; Shin, H. Fabrication of core-shell spheroids as building blocks for engineering 3D complex vascularized tissue. Acta Biomater. 2019, 100, 158–172. [Google Scholar] [CrossRef]
- Strobel, H.A.; Moss, S.M.; Hoying, J.B. Methods for vascularization and perfusion of tissue organoids. Mamm. Genome 2022, 33, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Marino, F.; Salerno, L.; Cianflone, E.; Molinaro, C.; Salerno, N.; De Angelis, A.; Viglietto, G.; Urbanek, K.; Torella, D. From Spheroids to Organoids: The Next Generation of Model Systems of Human Cardiac Regeneration in a Dish. Int. J. Mol. Sci. 2021, 22, 13180. [Google Scholar] [CrossRef]
- Laurent, J.; Blin, G.; Chatelain, F.; Vanneaux, V.; Fuchs, A.; Larghero, J.; Théry, M. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat. Biomed. Eng. 2017, 1, 939–956. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, Z.; Kamm, R.D. Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature. Lab Chip 2021, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Tuffin, J.; Chesor, M.; Kuzmuk, V.; Johnson, T.; Satchell, S.C.; Welsh, G.I.; Saleem, M.A. GlomSpheres as a 3D co-culture spheroid model of the kidney glomerulus for rapid drug-screening. Commun. Biol. 2021, 4, 1351. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Di Cio, S.; Marhuenda, E.; Haddrick, M.; Gautrot, J.E. Vascularised cardiac spheroids-on-a-chip for testing the toxicity of therapeutics. Sci. Rep. 2024, 14, 3370. [Google Scholar] [CrossRef]
- Harrison, S.P.; Siller, R.; Tanaka, Y.; Chollet, M.E.; de la Morena-Barrio, M.E.; Xiang, Y.; Patterson, B.; Andersen, E.; Bravo-Pérez, C.; Kempf, H.; et al. Scalable production of tissue-like vascularized liver organoids from human PSCs. Exp. Mol. Med. 2023, 55, 2005–2024. [Google Scholar] [CrossRef]
- Kim, W.J.; Jang, C.H.; Kim, G.H. Bone tissue engineering supported by bioprinted cell constructs with endothelial cell spheroids. Theranostics 2022, 12, 5404–5417. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Kang, K.T. Two-Cell Spheroid Angiogenesis Assay System Using Both Endothelial Colony Forming Cells and Mesenchymal Stem Cells. Biomol. Ther. 2018, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Au, P.; Daheron, L.M.; Duda, D.G.; Cohen, K.S.; Tyrrell, J.A.; Lanning, R.M.; Fukumura, D.; Scadden, D.T.; Jain, R.K. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 2008, 111, 1302–1305. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; Khan, Z.A.; Picard, A.; Wu, X.; Paruchuri, S.; Bischoff, J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 2007, 109, 4761–4768. [Google Scholar] [CrossRef]
- Menéndez, A.B.-C.; Du, Z.; Bosch, T.P.P.v.D.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Pekozer, G.G.; Kose, G.T.; Hasirci, V. Influence of co-culture on osteogenesis and angiogenesis of bone marrow mesenchymal stem cells and aortic endothelial cells. Microvasc. Res. 2016, 108, 1–9. [Google Scholar] [CrossRef]
- Eckermann, C.W.; Lehle, K.; Schmid, S.A.; Wheatley, D.N.; Kunz-Schughart, L.A. Characterization and modulation of fibroblast/endothelial cell co-cultures for the in vitro preformation of three-dimensional tubular networks. Cell Biol. Int. 2011, 35, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Rashad, A.; Nymark, E.H.; Suliman, S.; de Lange Davies, C.; Stavropoulos, A.; Bolstad, A.I.; Mustafa, K. Spheroid Coculture of Human Gingiva-Derived Progenitor Cells with Endothelial Cells in Modified Platelet Lysate Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 739225. [Google Scholar] [CrossRef]
- Marshall, J.; Barnes, A.; Genever, P. Analysis of the Intrinsic Self-Organising Properties of Mesenchymal Stromal Cells in Three-Dimensional Co-Culture Models with Endothelial Cells. Bioengineering 2018, 5, 92. [Google Scholar] [CrossRef]
- Whisler, J.A.; Chen, M.B.; Kamm, R.D. Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng. Part C Methods 2014, 20, 543–552. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, V.L.; Henriet, E.; Linville, R.M.; Wong, A.D.; Searson, P.C.; Ewald, A.J. A Tissue-Engineered 3D Microvessel Model Reveals the Dynamics of Mosaic Vessel Formation in Breast Cancer. Cancer Res. 2020, 80, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, S.; Fanai, M.L.; Wang, J.; Cai, J.; Feng, J. 3D co-culture model of endothelial colony-forming cells (ECFCs) reverses late passage adipose-derived stem cell senescence for wound healing. Stem Cell Res. Ther. 2020, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; He, Y.; Li, Y.; He, X. Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway. Stem Cell Res. Ther. 2020, 11, 537. [Google Scholar] [CrossRef]
- Dingle, A.M.; Yap, K.K.; Gerrand, Y.-W.; Taylor, C.J.; Keramidaris, E.; Lokmic, Z.; Kong, A.M.; Peters, H.L.; Morrison, W.A.; Mitchell, G.M. Characterization of isolated liver sinusoidal endothelial cells for liver bioengineering. Angiogenesis 2018, 21, 581–597. [Google Scholar] [CrossRef]
- Yap, K.K.; Gerrand, Y.W.; Dingle, A.M.; Yeoh, G.C.; Morrison, W.A.; Mitchell, G.M. Liver sinusoidal endothelial cells promote the differentiation and survival of mouse vascularised hepatobiliary organoids. Biomaterials 2020, 251, 120091. [Google Scholar] [CrossRef]
- Sang, X.; Xu, J.; Wang, Y.; Li, J.; Xu, J.; Chen, X.; Shi, X.; Wu, F. Generation of vascularized pancreatic progenitors through co-differentiation of endoderm and mesoderm from human pluripotent stem cells. Stem Cell Res. Ther. 2024, 15, 502. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Rockel, A.; Alt, Y.; Kern, A.; Ergün, S. Generation of Vascularized Neural Organoids by Co-culturing with Mesodermal Progenitor Cells. STAR Protoc. 2020, 1, 100041. [Google Scholar] [CrossRef]
- Giraudo, M.V.; Di Francesco, D.; Catoira, M.C.; Cotella, D.; Fusaro, L.; Boccafoschi, F. Angiogenic Potential in Biological Hydrogels. Biomedicines 2020, 8, 436. [Google Scholar] [CrossRef]
- Nuutila, K.; Samandari, M.; Endo, Y.; Zhang, Y.; Quint, J.; Schmidt, T.A.; Tamayol, A.; Sinha, I. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact. Mater. 2021, 8, 296–308. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, S.; Chen, J.; Jian, T.; Wang, X.; Zhu, X.; Dong, Y.; Fan, G. Photo-Crosslinked Pro-Angiogenic Hydrogel Dressing for Wound Healing. Int. J. Mol. Sci. 2024, 25, 9948. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, V.; Mittermayr, R.; Hartinger, J.; Martino, M.M.; Lorentz, K.M.; Wolbank, S.; Hofmann, A.; Largo, R.A.; Marschall, J.S.; Groppa, E.; et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. 2014, 111, 6952–6957. [Google Scholar] [CrossRef] [PubMed]
- Gorkun, A.A.; Revokatova, D.P.; Zurina, I.M.; Nikishin, D.A.; Bikmulina, P.Y.; Timashev, P.S.; Shpichka, A.I.; Kosheleva, N.V.; Kolokoltsova, T.D.; Saburina, I.N. The Duo of Osteogenic and Angiogenic Differentiation in ADSC-Derived Spheroids. Front. Cell Dev. Biol. 2021, 9, 572727. [Google Scholar] [CrossRef]
- Jia, T.; Jacquet, T.; Dalonneau, F.; Coudert, P.; Vaganay, E.; Exbrayat-Héritier, C.; Vollaire, J.; Josserand, V.; Ruggiero, F.; Coll, J.-L.; et al. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Murakami, M.; Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008, 15, 215. [Google Scholar] [CrossRef]
- Lange, S.; Heger, J.; Euler, G.; Wartenberg, M.; Piper, H.M.; Sauer, H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc. Res. 2009, 81, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Rolny, C.; Nilsson, I.; Magnusson, P.; Armulik, A.; Jakobsson, L.; Wentzel, P.; Lindblom, P.; Norlin, J.; Betsholtz, C.; Heuchel, R.; et al. Platelet-derived growth factor receptor-β promotes early endothelial cell differentiation. Blood 2006, 108, 1877–1886. [Google Scholar] [CrossRef]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Ashimori, A.; Higashijima, F.; Ogata, T.; Sakuma, A.; Hamada, W.; Sunada, J.; Aoki, R.; Mikuni, M.; Hayashi, K.; Wakuta, M.; et al. HIF-1α-dependent upregulation of angiogenic factors by mechanical stimulation in retinal pigment epithelial cells. Dis. Model. Mech. 2024, 17, dmm050640. [Google Scholar] [CrossRef]
- Moriya, J.; Wu, X.; Zavala-Solorio, J.; Ross, J.; Liang, X.H.; Ferrara, N. Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice. J. Vasc. Surg. 2014, 59, 1402–1409.e4. [Google Scholar] [CrossRef]
- Wlodarczyk, J.; Leng, A.; Abadchi, S.N.; Shababi, N.; Mokhtari-Esbuie, F.; Gheshlaghi, S.; Ravari, M.R.; Pippenger, E.K.; Afrasiabi, A.; Ha, J.; et al. Transfection of hypoxia-inducible factor-1α mRNA upregulates the expression of genes encoding angiogenic growth factors. Sci. Rep. 2024, 14, 6738. [Google Scholar] [CrossRef] [PubMed]
- Bauman, E.; Feijão, T.; Carvalho, D.T.O.; Granja, P.L.; Barrias, C.C. Xeno-free pre-vascularized spheroids for therapeutic applications. Sci. Rep. 2018, 8, 230. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Z.-Y.; Han, Z.-Y.; Yuan, Y.; Shao, Y.; Feng, X.-Q.; Weitz, D.A. Regulation of cell attachment, spreading, and migration by hydrogel substrates with independently tunable mesh size. Acta Biomater. 2022, 141, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Gorkun, A.A.; Shpichka, A.I.; Zurina, I.; Koroleva, A.V.; Kosheleva, N.V.; A Nikishin, D.; Butnaru, D.; Timashev, P.S.; Repin, V.S.; Saburina, I. Angiogenic potential of spheroids from umbilical cord and adipose-derived multipotent mesenchymal stromal cells within fibrin gel. Biomed. Mater. 2018, 13, 044108. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, F.; Wan, X.; Liu, S.; Fu, L.; Mo, J.; Meng, X.; Mo, Z. Hyaluronic Acid Facilitates Angiogenesis of Endothelial Colony Forming Cell Combining with Mesenchymal Stem Cell via CD44/MicroRNA-139-5p Pathway. Front. Bioeng. Biotechnol. 2022, 10, 794037. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C.; Wang, X.; He, T.; Li, L.; Liang, X.; Wang, L.; Song, L.; Wei, Y.; Wu, Q.; et al. Hyaluronic Acid Oligosaccharides Improve Myocardial Function Reconstruction and Angiogenesis against Myocardial Infarction by Regulation of Macrophages. Theranostics 2019, 9, 1980–1992. [Google Scholar] [CrossRef]
- Karam, J.; Singer, B.J.; Miwa, H.; Chen, L.H.; Maran, K.; Hasani, M.; Garza, S.; Onyekwere, B.; Yeh, H.-C.; Li, S.; et al. Molecular weight of hyaluronic acid crosslinked into biomaterial scaffolds affects angiogenic potential. Acta Biomater. 2023, 169, 228–242. [Google Scholar] [CrossRef]
- Fortunato, T.M.; Beltrami, C.; Emanueli, C.; De Bank, P.A.; Pula, G. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: Tissue engineering implications. Sci. Rep. 2016, 6, 25326. [Google Scholar] [CrossRef]
- Radermacher, C.; Rohde, A.; Kucikas, V.; Buhl, E.M.; Wein, S.; Jonigk, D.; Jahnen-Dechent, W.; Neuss, S. Various Hydrogel Types as a Potential In Vitro Angiogenesis Model. Gels 2024, 10, 820. [Google Scholar] [CrossRef]
- Wei, Z.; Lei, M.; Wang, Y.; Xie, Y.; Xie, X.; Lan, D.; Jia, Y.; Liu, J.; Ma, Y.; Cheng, B.; et al. Hydrogels with tunable mechanical plasticity regulate endothelial cell outgrowth in vasculogenesis and angiogenesis. Nat. Commun. 2023, 14, 8307. [Google Scholar] [CrossRef]
- Shpichka, A.; Osipova, D.; Efremov, Y.; Bikmulina, P.; Kosheleva, N.; Lipina, M.; Bezrukov, E.A.; Sukhanov, R.B.; Solovieva, A.B.; Vosough, M.; et al. Fibrin-based Bioinks: New Tricks from an Old Dog. Int. J. Bioprinting 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A Collagen–Chitosan Hydrogel for Endothelial Differentiation and Angiogenesis. Tissue Eng. Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef]
- De Moor, L.; Merovci, I.; Baetens, S.; Verstraeten, J.; Kowalska, P.; Krysko, D.V.; De Vos, W.H.; Declercq, H. High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication 2018, 10, 035009. [Google Scholar] [CrossRef]
- Guye, P.; Ebrahimkhani, M.R.; Kipniss, N.; Velazquez, J.J.; Schoenfeld, E.; Kiani, S.; Griffith, L.G.; Weiss, R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun. 2016, 7, 10243. [Google Scholar] [CrossRef]
- Kang, D.; Hong, G.; An, S.; Jang, I.; Yun, W.; Shim, J.; Jin, S. Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small 2020, 16, e1905505. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Zhang, R.-R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Watson, C.L.; Mahe, M.M.; Múnera, J.; Howell, J.C.; Sundaram, N.; Poling, H.M.; I Schweitzer, J.; E Vallance, J.; Mayhew, C.N.; Sun, Y.; et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014, 20, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Maier, B.; Baillie, G.J.; Ferguson, C.; Parton, R.G.; Wolvetang, E.J.; Roost, M.S.; Chuva de Sousa Lopes, S.M.; et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015, 526, 564–568. [Google Scholar] [CrossRef]
- Berg, C.W.v.D.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; Berg, B.M.v.D.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J.; et al. Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Rep. 2018, 10, 751–765. [Google Scholar] [CrossRef]
- Song, L.; Yuan, X.; Jones, Z.; Griffin, K.; Zhou, Y.; Ma, T.; Li, Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019, 9, 5977. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Kim, M.H.; Hayes, D.J.; Ozbolat, I.T. miRNA induced co-differentiation and cross-talk of adipose tissue-derived progenitor cells for 3D heterotypic pre-vascularized bone formation. Biofabrication 2021, 13, 044107. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.F.D.; Lindsay, C.D.; Roth, J.G.; LeSavage, B.L.; Seymour, A.J.; Krajina, B.A.; Ribeiro, R.; Costa, P.F.; Blaeser, A.; Heilshorn, S.C. Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-on-Chip Platforms. Front. Bioeng. Biotechnol. 2020, 8, 374. [Google Scholar] [CrossRef]
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef]
- Horder, H.; Lasheras, M.G.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and Differentiation of Adipose-Derived Stromal Cell Spheroids for a 3D Breast Cancer-Adipose Tissue Model. Cells 2021, 10, 803. [Google Scholar] [CrossRef]
- Swaminathan, S.; Hamid, Q.; Sun, W.; Clyne, A. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 2019, 11, 025003. [Google Scholar] [CrossRef]
- Wrublewsky, S.; Schultz, J.; Ammo, T.; Bickelmann, C.; Metzger, W.; Später, T.; Pohlemann, T.; Menger, M.D.; Laschke, M.W. Biofabrication of prevascularized spheroids for bone tissue engineering by fusion of microvascular fragments with osteoblasts. Front. Bioeng. Biotechnol. 2024, 12, 1436519. [Google Scholar] [CrossRef]
- Ayan, B.; Heo, D.N.; Zhang, Z.; Dey, M.; Povilianskas, A.; Drapaca, C.; Ozbolat, I.T. Aspiration-assisted bioprinting for precise positioning of biologics. Sci. Adv. 2020, 6, eaaw5111. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.-C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, B.; Liang, Z.; Wang, Y.; Ren, S.; Huang, Y.; Li, C.; Sun, H.; Li, Y.; Liu, L.; et al. Liver bioprinting within a novel support medium with functionalized spheroids, hepatic vein structures, and enhanced post-transplantation vascularization. Biomaterials 2024, 311, 122681. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Mei, Y. Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef]
- Polonchuk, L.; Surija, L.; Lee, M.H.; Sharma, P.; Ming, C.L.C.; Richter, F.; Ben-Sefer, E.; Rad, M.A.; Sarmast, H.M.S.; Al Shamery, W.; et al. Towards engineering heart tissues from bioprinted cardiac spheroids. Biofabrication 2021, 13, 045009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Mei, T.; Cao, H.; Hu, Y.; Jia, W.; Wang, J.; Zhang, Z.; Wang, Z.; Le, W.; et al. hESCs-Derived Early Vascular Cell Spheroids for Cardiac Tissue Vascular Engineering and Myocardial Infarction Treatment. Adv. Sci. 2022, 9, 2104299. [Google Scholar] [CrossRef]
- Watson, E.C.; Adams, R.H. Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb. Perspect. Med. 2018, 8, a031559. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Grayson, W.L.; Park, M.; Hutton, D.; Zhou, B.; Guo, X.E.; Niklason, L.; Sousa, R.A.; Reis, R.L.; Vunjak-Novakovic, G. In Vitro Model of Vascularized Bone: Synergizing Vascular Development and Osteogenesis. PLoS ONE 2011, 6, e28352. [Google Scholar] [CrossRef]
- Hutton, D.L.; Moore, E.M.; Gimble, J.M.; Grayson, W.L. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng. Part A 2013, 19, 2076–2086. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Ali, A.S.; Wu, D.; Bannach-Brown, A.; Dhamrait, D.; Berg, J.; Tolksdorf, B.; Lichtenstein, D.; Dressler, C.; Braeuning, A.; Kurreck, J.; et al. 3D bioprinting of liver models: A systematic scoping review of methods, bioinks, and reporting quality. Mater. Today Bio 2024, 26, 100991. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.-C.; et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2020, 70, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Chai, Y.; Zhuo, C.; Xu, Y.; Xue, T.; Shao, D.; Tao, Y.; Li, M. 3D Printing of a Vascularized Mini-Liver Based on the Size-Dependent Functional Enhancements of Cell Spheroids for Rescue of Liver Failure. Adv. Sci. 2024, 11, e2309899. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Feng, N.L.; Lam, Y.T.; Michael, P.L.; Santos, M.; Wise, S.G. Engineering Vascular Bioreactor Systems to Closely Mimic Physiological Forces In Vitro. Tissue Eng. Part B Rev. 2023, 29, 232–243. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2017, 45, 261–272. [Google Scholar] [CrossRef]
- Matos, R.S.; Maselli, D.; McVey, J.H.; Heiss, C.; Campagnolo, P. 3D Printed Bioreactor Enabling the Pulsatile Culture of Native and Angioplastied Large Arteries. Front. Cardiovasc. Med. 2022, 9, 864580. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Kosheleva, N.V.; Efremov, Y.M.; Shavkuta, B.S.; Zurina, I.M.; Zhang, D.; Zhang, Y.; Minaev, N.V.; Gorkun, A.A.; Wei, S.; Shpichka, A.I.; et al. Cell spheroid fusion: Beyond liquid drops model. Sci. Rep. 2020, 10, 12614. [Google Scholar] [CrossRef] [PubMed]
- Yanbarisov, R.; Efremov, Y.; Kosheleva, N.; Timashev, P.; Vassilevski, Y. Numerical modelling of multicellular spheroid compression: Viscoelastic fluid vs. viscoelastic solid. Mathematics 2021, 9, 2333. [Google Scholar] [CrossRef]
- Sego, T.J.; Kasacheuski, U.; Hauersperger, D.; Tovar, A.; Moldovan, N.I. A heuristic computational model of basic cellular processes and oxygenation during spheroid-dependent biofabrication. Biofabrication 2017, 9, 024104. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Sousa, L.C.; António, C.C.; Silva, S.; Pinto, S.I.S. A review of computational methodologies to predict the fractional flow reserve in coronary arteries with stenosis. J. Biomech. 2015, 178, 112299. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Simakov, S.; Gamilov, T.; Vassilevski, Y.; Wang, Y.; Liang, F. Influence of pressure guidewire on coronary hemodynamics and fractional flow reserve. Phys. Fluids 2025, 37, 031920. [Google Scholar] [CrossRef]
- Isichei, J.C.; Khorsandroo, S.; Desai, S. Cybersecurity and privacy in smart bioprinting. Bioprinting 2023, 36, e00321. [Google Scholar] [CrossRef]
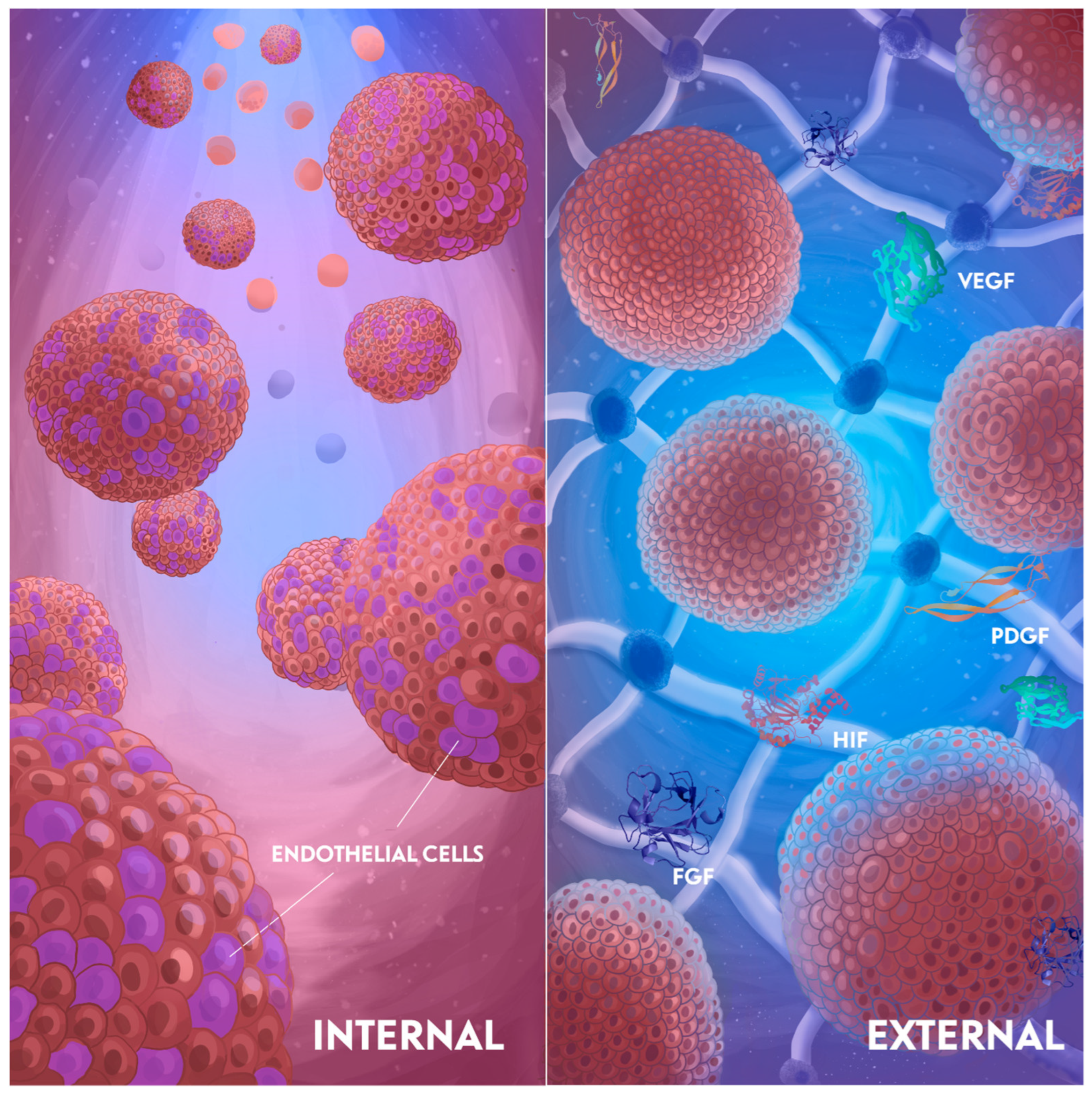


| Medium compounds | VEGFs (VEGF-A) | Direct effect - Stimulate the migration and proliferation of the ECs |
| FGFs (FGF-2) | Indirect effect - Stimulate the proliferation of the ECs by regulation of VEGFR1 alternative splicing - Stimulate PDGF expression | |
| PDGFs (PDGF-C) | Direct effect - Stimulate neovascularization | |
| HIFs | Indirect effect - Stimulate transcription of VEGF and Ang1 | |
| Ang1 | Indirect effect - Stimulate maturation of the new vessels by stabilizing pericytes and SMCs | |
| Hydrogel | Hyaluronic acid | - The mechanism of action is unknown |
| Human platelet lysate | Direct effect - Contains VEGF-A, PDGF-BB and FGF-2 | |
| Fibrin hydrogel | - The mechanism of action is unknown |
| Induction Method | Approach | Cell Types | Biomaterial | Results | Tissue | Ref. |
|---|---|---|---|---|---|---|
| Internal | Co-culture with ECs | ADSCs and HUVECs | BdECM b-TCP | ADSCs in BdECM and b-TCP + ECs spheroids ↑ angiogenic and osteogenic activities ↑ vascularization in rat obliterated mastoid model | Bone | [31] |
| External | miR transfection | ADSCs | - | ADSC differentiated into endothelial and osteogenic progenitors + AAB | Haversian bone canal | [83] |
| Internal | Co-culture with ECs | Adipose tissue-derived microvascular fragments Osteoblasts | Collagen | ↑ sprouts formation ↑ vascularization after in vivo transplantation in the dorsal skinfold chamber model | Bone | [88] |
| Internal | Co-culture with ECs | MSC and HUVECs | - | ↑ osteogenic differentiation and bioequivalent maturation + AAB → osteogenic induction → angiogenic induction | Bone | [89] |
| Internal | Co-culture with ECs | MSC and HUVECs | GelMA | MSCs → osteogenic differentiation + HUVECs spheroid seeding in the inner soft GelMA-based bone marrow | Bone | [90] |
| Internal/External | Co-culture with ECs | iPSC-HEs HUVECs MSCs | Matrigel | ↑ expression of liver markers ↑ albumin after transplantation | Liver | [41] |
| Internal | Co-culture with ECs | Mouse LPCs and LSECs | - | LPCs and LSECs co-culture after 7-day ↑ upregulation of liver-specific genes (albumin, CPS1, CYP3A11) | Liver | [46] |
| External/ Internal | Co-culture with ECs, sacrificial materials | HepG2/C3A and ECs | Alginate Collagen Gelatin | ↑ vascularization ↑ albumin secretion ↑ urea production ↑ cytochrome P450 enzyme activity | Liver | [75] |
| Internal | Co-culture with ECs | Primary mouse hepatocytes ECs line C166 | - | Hepatocytes + ECs spheroids printed on the surface of a preformed polymer network, which were preliminarily populated with ECs spheroids ↑ vascularization ↑ expression of characteristic markers ↑ neovascularization in mice | Liver | [91] |
| Internal | Co-culture with ECs | MSCs, iPSC-HEs (iPSC-derived hepatic endoderm cells) and HUVECs | Matrigel | ↑ integration in host vasculature Secretion of liver proteins after transplantation under cranial window | Liver | [76] |
| External/ Internal | Co culture with ECs | HUVECs, Neonatal rat cardiomyocytes or hiPSC-CM | Alginate GelMA | ↑ sarcomeric α-actinin (protein responsible for contractile function) ↑ connexin-43 (inter-cellular conductive function) Synchronous beating | Cardiac tissue | [82] |
| Internal | Co-culture with ECs | iPSC-CMs, FB, ADSCs, HUVECs | Lumen-like structures, Z-line Spontaneous beating Verapamil and isoproterenol responsiveness | Cardiac tissue | [92] | |
| Internal | Co-culture with ECs | iPSC-CMs, FB, HUVECs | Gelatin (sacrificial bioink) | Sacromeric architecture Contractile activity | Cardiac tissue | [93] |
| External/ Internal | Co-culture with ECs VEGF | FB, ECs, and myocytes | - | Spontaneous contraction | Cardiac tissue | [94] |
| External/Internal | Co-culture with ECs VEGF FGF-2 | hESCs-EVCs CMs | Collagen I Matrigel | Spontaneous beating Organized vascular network Cardiac function recovery after MI in mice | Cardiac tissue | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revokatova, D.; Bikmulina, P.; Heydari, Z.; Solovieva, A.; Vosough, M.; Shpichka, A.; Timashev, P. Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting. Cells 2025, 14, 665. https://doi.org/10.3390/cells14090665
Revokatova D, Bikmulina P, Heydari Z, Solovieva A, Vosough M, Shpichka A, Timashev P. Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting. Cells. 2025; 14(9):665. https://doi.org/10.3390/cells14090665
Chicago/Turabian StyleRevokatova, Daria, Polina Bikmulina, Zahra Heydari, Anna Solovieva, Massoud Vosough, Anastasia Shpichka, and Peter Timashev. 2025. "Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting" Cells 14, no. 9: 665. https://doi.org/10.3390/cells14090665
APA StyleRevokatova, D., Bikmulina, P., Heydari, Z., Solovieva, A., Vosough, M., Shpichka, A., & Timashev, P. (2025). Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting. Cells, 14(9), 665. https://doi.org/10.3390/cells14090665







