Immunosuppression of Tumor-Derived Factors Modulated Neutrophils in Upper Tract Urothelial Carcinoma Through Upregulation of Arginase-1 via ApoA1-STAT3 Axis
Abstract
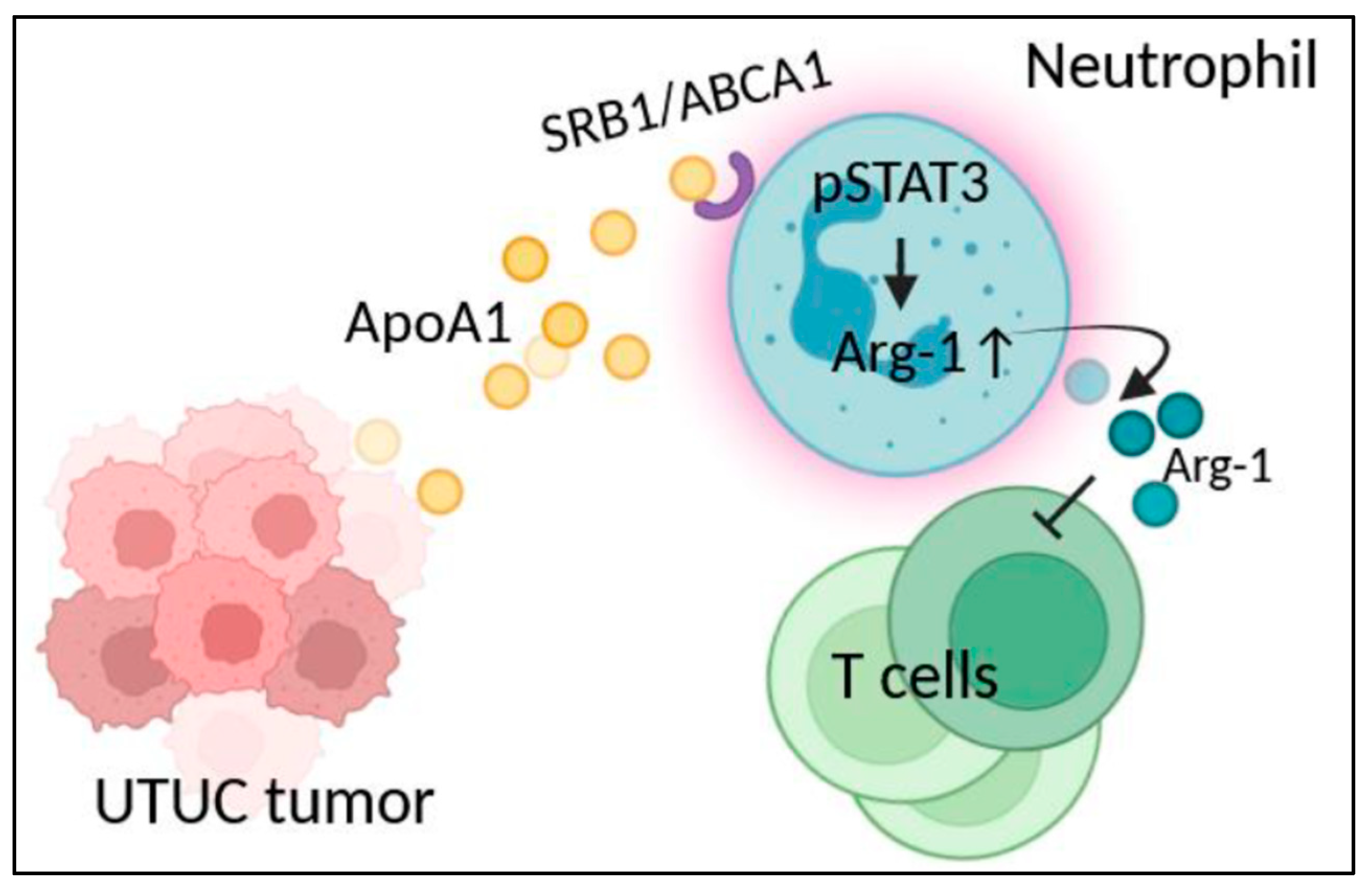
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Tumor Tissue Culture Supernatant (TTCS) and Tumor-Infiltrating Cell Collection
2.3. Protein Array
2.4. In Vitro Neutrophil Culture and Neutrophil/T Cell Coculture
2.5. RNA Extraction and Quantitative Real-Time PCR
2.6. Flow Cytometry
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) and Cytometric Bead Array (CBA)
2.8. Statistical Analysis
3. Results
3.1. UTUC Tumor-Derived Apo-A1 Increased Arginase-1 Expression in Neutrophils
3.2. STAT3 Activation Involved in Arginase-1 Upregulation of Neutrophils by Apo-A1 Signaling
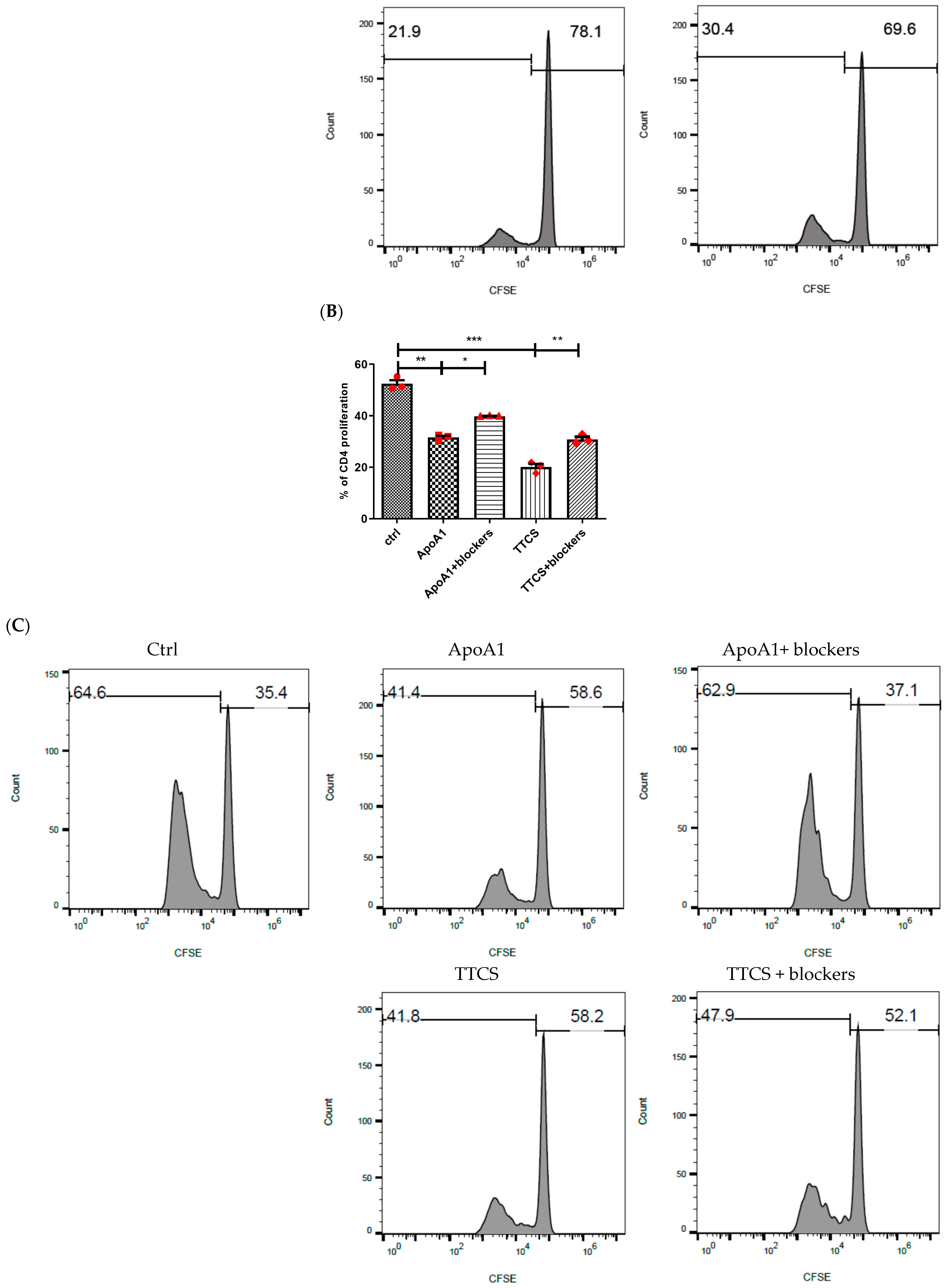
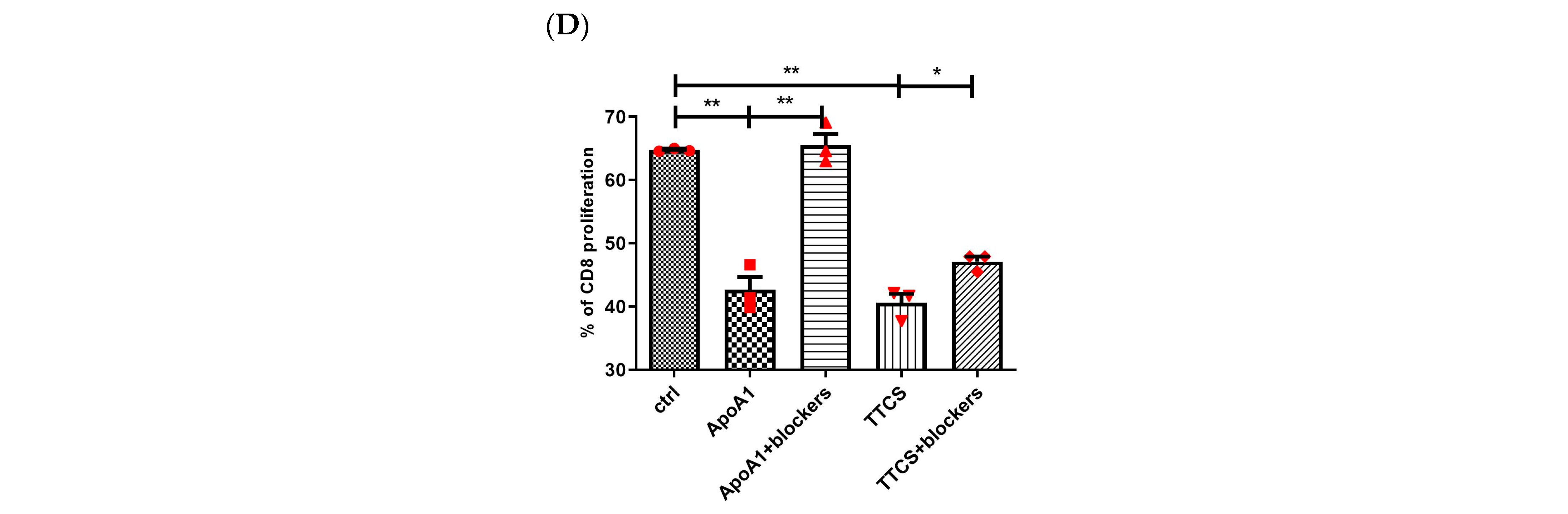
3.3. Apo-A1 Signaling in Neutrophils Involved in UTUC Mediated T Cell Suppression
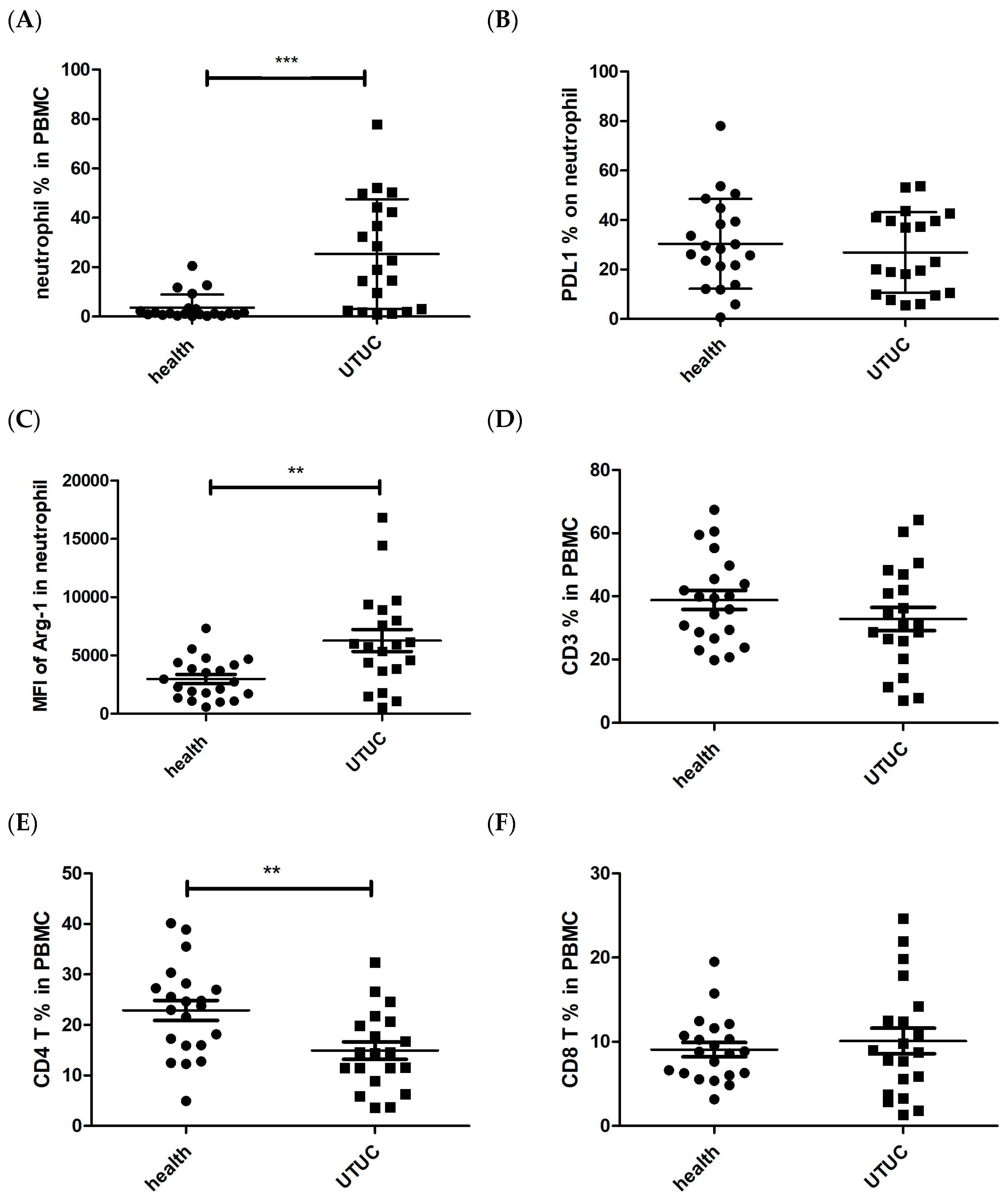
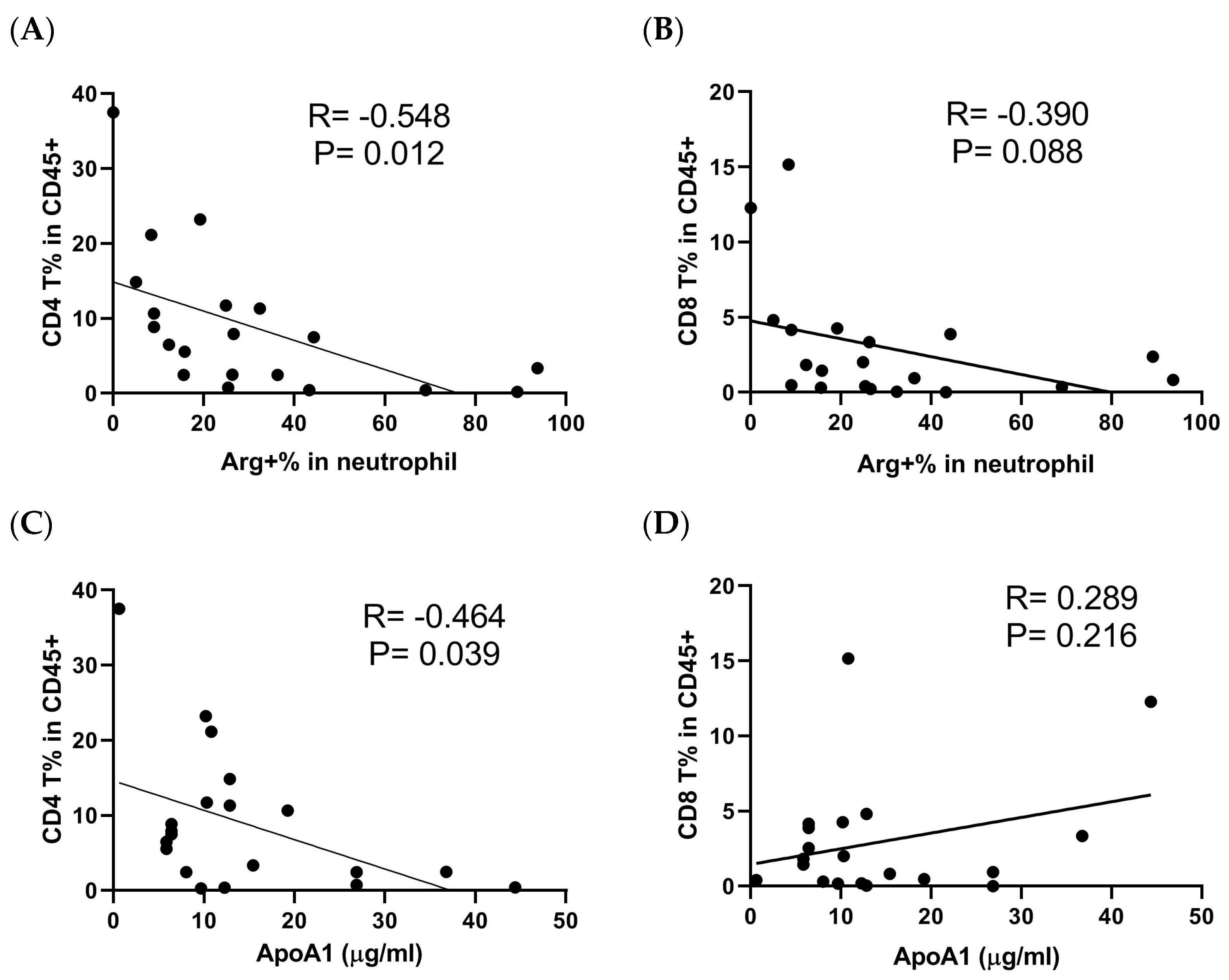
3.4. The Characteristics of Neutrophils in UTUC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP binding cassette transporter A1 |
| Apo-A1 | apolipoprotein A1 |
| CFSE | carboxyfluorescein succinimidyl ester |
| EMMPRIN | extracellular matrix metalloproteinase inducer |
| G-MDSCs | granulocyte myeloid-derived suppressor cells |
| MMP | matrix metalloproteinase |
| NLR | neutrophil-to-lymphocyte ratio |
| PBMCs | peripheral blood mononuclear cells |
| PD-L1 | programmed cell death-ligand 1 |
| pSTAT3 | phosphorylated signal transducer and activator of transcription-3 |
| SRB1 | scavenger receptor class B type 1 |
| TTCS | tumor tissue culture supernatant |
| TAM | tumor-associated macrophage |
| UTUC | upper tract urothelial carcinoma |
References
- Lin, Q.; Wang, X.; Hu, Y. The opportunities and challenges in immunotherapy: Insights from the regulation of PD-L1 in cancer cells. Cancer Lett. 2023, 569, 216318. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Li, H.; van der Merwe, P.A.; Sivakumar, S. Biomarkers of response to PD-1 pathway blockade. Br. J. Cancer 2022, 126, 1663–1675. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, D.; Sun, K.; Wang, L.; Zhang, Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front. Cell Dev. Biol. 2020, 8, 672. [Google Scholar] [CrossRef]
- Leow, J.J.; Martin-Doyle, W.; Fay, A.P.; Choueiri, T.K.; Chang, S.L.; Bellmunt, J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur. Urol. 2014, 66, 529–541. [Google Scholar] [CrossRef]
- Robinson, B.D.; Vlachostergios, P.J.; Bhinder, B.; Liu, W.; Li, K.; Moss, T.J.; Bareja, R.; Park, K.; Tavassoli, P.; Cyrta, J.; et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat. Commun. 2019, 10, 2977. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Marchioni, M.; Primiceri, G.; Ingrosso, M.; Filograna, R.; Castellan, P.; De Francesco, P.; Schips, L. The Clinical Use of the Neutrophil to Lymphocyte Ratio (NLR) in Urothelial Cancer: A Systematic Review. Clin. Genitourin. Cancer 2016, 14, 473–484. [Google Scholar] [CrossRef]
- Shao, Y.; Li, W.; Wang, D.; Wu, B. Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2020, 18, 273. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, K.; Suekane, S.; Ueda, K.; Nakiri, M.; Matsuo, M.; Igawa, T. High postoperative neutrophil-to-lymphocyte ratio as a poor prognostic marker in patients with upper tract urothelial carcinoma. Oncol. Lett. 2019, 17, 5241–5250. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Janisch, F.; Mostafaei, H.; Lysenko, I.; Kimura, S.; Egawa, S.; Shariat, S.F. Prognostic value of preoperative blood-based biomarkers in upper tract urothelial carcinoma treated with nephroureterectomy: A systematic review and meta-analysis. Urol. Oncol. 2020, 38, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Chen, J.; Gao, C.; Lin, Z.; Xie, X.; Dai, H. Brief review on the roles of neutrophils in cancer development. J. Leukoc. Biol. 2021, 109, 407–413. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Mantovani, A.; Marone, G. Roles of neutrophils in cancer growth and progression. J. Leukoc. Biol. 2018, 103, 457–464. [Google Scholar] [CrossRef]
- Wu, W.C.; Sun, H.W.; Chen, H.T.; Liang, J.; Yu, X.J.; Wu, C.; Wang, Z.; Zheng, L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl. Acad. Sci. USA 2014, 111, 4221–4226. [Google Scholar] [CrossRef]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The role of lipids in cancer progression and metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Yang, M.; Wang, B.; Hou, W.; Yu, H.; Zhou, B.; Zhong, W.; Liu, Z.; Li, J.; Zeng, H.; Liu, C.; et al. Negative Effects of Stromal Neutrophils on T Cells Reduce Survival in Resectable Urothelial Carcinoma of the Bladder. Front. Immunol. 2022, 13, 827457. [Google Scholar] [CrossRef]
- Kim, G.T.; Kim, E.Y.; Shin, S.H.; Lee, H.; Lee, S.H.; Sohn, K.Y.; Kim, J.W. Improving anticancer effect of aPD-L1 through lowering neutrophil infiltration by PLAG in tumor implanted with MB49 mouse urothelial carcinoma. BMC Cancer 2022, 22, 727. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Fox, G.C.; Xiang, J.; Kwakwa, K.A.; Davis, J.L.; Belle, J.I.; Lee, W.C.; Wong, W.H.; Fontana, F.; et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J. Clin. Invest. 2021, 131, e145296. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef]
- Marti, I.L.A.A.; Reith, W. Arginine-dependent immune responses. Cell Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef] [PubMed]
- Vonwirth, V.; Bulbul, Y.; Werner, A.; Echchannaoui, H.; Windschmitt, J.; Habermeier, A.; Ioannidis, S.; Shin, N.; Conradi, R.; Bros, M.; et al. Inhibition of Arginase 1 Liberates Potent T Cell Immunostimulatory Activity of Human Neutrophil Granulocytes. Front. Immunol. 2020, 11, 617699. [Google Scholar] [CrossRef]
- Ettel, P.; Weichhart, T. Not just sugar: Metabolic control of neutrophil development and effector functions. J. Leukoc. Biol. 2024, 116, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e1374. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulou, S.; Valadez-Cosmes, P.; Mihalic, Z.N.; Schicho, R.; Kargl, J. Tumor-Mediated Neutrophil Polarization and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 3218. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Cancer-related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018, 285, 4316–4342. [Google Scholar] [CrossRef]
- Tello-Montoliu, A.; Patel, J.V.; Lip, G.Y. Angiogenin: A review of the pathophysiology and potential clinical applications. J. Thromb. Haemost. 2006, 4, 1864–1874. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.L.; Lou, B.; Ma, J.; Wu, M.P. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci. 2005, 77, 325–335. [Google Scholar] [CrossRef]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef]
- Sanson, M.; Distel, E.; Fisher, E.A. HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process. PLoS ONE 2013, 8, e74676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Yin, K.; Fu, Y.C.; Tang, C.K. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol. Med. 2012, 18, 149–158. [Google Scholar] [CrossRef]
- Vasquez, M.; Simoes, I.; Consuegra-Fernandez, M.; Aranda, F.; Lozano, F.; Berraondo, P. Exploiting scavenger receptors in cancer immunotherapy: Lessons from CD5 and SR-B1. Eur. J. Immunol. 2017, 47, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Baekkevold, E.S.; Brandau, S.; Bujko, A.; Cassatella, M.A.; Dorhoi, A.; Krieg, C.; Lin, A.; Lore, K.; Marini, O.; et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. 2019, 68, 687–697. [Google Scholar] [CrossRef]
- Guc, E.; Pollard, J.W. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021, 54, 885–902. [Google Scholar] [CrossRef]
- Mandelli, G.E.; Missale, F.; Bresciani, D.; Gatta, L.B.; Scapini, P.; Caveggion, E.; Roca, E.; Bugatti, M.; Monti, M.; Cristinelli, L.; et al. Tumor Infiltrating Neutrophils Are Enriched in Basal-Type Urothelial Bladder Cancer. Cells 2020, 9, 291. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e813. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Kirschmeier, P.; Koyama, S.; Zhu, M.; Li, Y.Y.; Naito, Y.; Wu, M.; Malladi, V.S.; Huang, W.; Walker, W.; et al. Suppression of Myeloid Cell Arginase Activity leads to Therapeutic Response in a NSCLC Mouse Model by Activating Anti-Tumor Immunity. J. Immunother. Cancer 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yi, J.; Li, W.; Zheng, X.; Liu, J.; Wang, J.; Du, G. Apolipoproteins and cancer. Cancer Med. 2019, 8, 7032–7043. [Google Scholar] [CrossRef]
- Acta OncolVanarsa, K.; Castillo, J.; Wang, L.; Lee, K.H.; Pedroza, C.; Lotan, Y.; Mohan, C. Comprehensive proteomics and platform validation of urinary biomarkers for bladder cancer diagnosis and staging. BMC Med. 2023, 21, 133. [Google Scholar]
- Chang, C.C.; Chang, C.B.; Chen, C.J.; Tung, C.L.; Hung, C.F.; Lai, W.H.; Shen, C.H.; Tsai, C.Y.; Lai, Y.Y.; Lee, M.Y.; et al. Increased Apolipoprotein A1 Expression Correlates with Tumor-Associated Neutrophils and T Lymphocytes in Upper Tract Urothelial Carcinoma. Curr. Issues Mol. Biol. 2024, 46, 2155–2165. [Google Scholar] [CrossRef]
- Zeng, W.; Xiong, G.; Hua, L.; Hu, Y.; Guo, X.; Peng, X. APOA1 mRNA and protein in kidney renal clear cell carcinoma correlate with the disease outcome. Sci. Rep. 2022, 12, 12406. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Gui, T.; Yang, Y.; Feng, T.; Tzvetkov, N.T.; Xu, T.; Gai, Z.; Zhou, Y.; Zhang, J.; et al. SR-BI as a target of natural products and its significance in cancer. Semin. Cancer Biol. 2022, 80, 18–38. [Google Scholar] [CrossRef]
- Feng, H.; Wang, M.; Wu, C.; Yu, J.; Wang, D.; Ma, J.; Han, J. High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in lung adenocarcinoma: A STROBE compliant article. Medicine 2018, 97, e0203. [Google Scholar] [CrossRef]
- Xu, G.H.; Lou, N.; Shi, H.C.; Xu, Y.C.; Ruan, H.L.; Xiao, W.; Liu, L.; Li, X.; Xiao, H.B.; Qiu, B.; et al. Up-regulation of SR-BI promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma. BMC Cancer 2018, 18, 88. [Google Scholar] [CrossRef]
- Traughber, C.A.; Opoku, E.; Brubaker, G.; Major, J.; Lu, H.; Lorkowski, S.W.; Neumann, C.; Hardaway, A.; Chung, Y.M.; Gulshan, K.; et al. Uptake of high-density lipoprotein by scavenger receptor class B type 1 is associated with prostate cancer proliferation and tumor progression in mice. J. Biol. Chem. 2020, 295, 8252–8261. [Google Scholar] [CrossRef] [PubMed]
- Grao-Cruces, E.; Lopez-Enriquez, S.; Martin, M.E.; Montserrat-de la Paz, S. High-density lipoproteins and immune response: A review. Int. J. Biol. Macromol. 2022, 195, 117–123. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Duits, D.E.M.; de Visser, K.E. Impact of cancer cell-intrinsic features on neutrophil behavior. Semin. Immunol. 2021, 57, 101546. [Google Scholar] [CrossRef]
- Hossain, D.M.; Pal, S.K.; Moreira, D.; Duttagupta, P.; Zhang, Q.; Won, H.; Jones, J.; D’Apuzzo, M.; Forman, S.; Kortylewski, M. TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients. Clin. Cancer Res. 2015, 21, 3771–3782. [Google Scholar] [CrossRef] [PubMed]
- Clowers, M.J.; Moghaddam, S.J. Cell Type-Specific Roles of STAT3 Signaling in the Pathogenesis and Progression of K-ras Mutant Lung Adenocarcinoma. Cancers 2022, 14, 1785. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Asgari, R.; Vaisi-Raygani, A.; Aleagha, M.S.E.; Mohammadi, P.; Bakhtiari, M.; Arghiani, N. CD147 and MMPs as key factors in physiological and pathological processes. Biomed. Pharmacother. 2023, 157, 113983. [Google Scholar] [CrossRef]
- Xin, X.; Zeng, X.; Gu, H.; Li, M.; Tan, H.; Jin, Z.; Hua, T.; Shi, R.; Wang, H. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32804. [Google Scholar] [CrossRef]
- Wang, C.H.; Dai, J.Y.; Wang, L.; Jia, J.F.; Zheng, Z.H.; Ding, J.; Chen, Z.N.; Zhu, P. Expression of CD147 (EMMPRIN) on neutrophils in rheumatoid arthritis enhances chemotaxis, matrix metalloproteinase production and invasiveness of synoviocytes. J. Cell Mol. Med. 2011, 15, 850–860. [Google Scholar] [CrossRef]







| UTUC Patients (n = 20) | Healthy Subjects (n = 21) | |
|---|---|---|
| Age, year (mean ± SD) | 67.65 ± 12.51 | 69.19 ± 5.14 |
| Gender, n (%) | ||
| Male | 10 (50) | 11 (52.2) |
| Female | 10 (50) | 10 (47.8) |
| Tumor site, n (%) | ||
| Ureter | 12 (60) | |
| Renal pelvic | 11 (55) | |
| Both | 3 (15) | |
| T stage, n (%) | ||
| ≤pT2 | 9 (45) | |
| >pT2 | 11 (55) | |
| Neutrophils, % | * 71.55 ± 9.15 | 63.97 ± 8.40 |
| Lymphocytes, % | 21.59 ± 9.22 | 26.73 ± 8.69 |
| NLR | * 4.46 ± 3.52 | 2.69 ± 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chang, C.-B.; Shen, C.-H.; Lee, M.-Y.; Jou, Y.-C.; Tung, C.-L.; Lai, W.-H.; Hung, C.-F.; Wang, M.; Lai, Y.-Y.; et al. Immunosuppression of Tumor-Derived Factors Modulated Neutrophils in Upper Tract Urothelial Carcinoma Through Upregulation of Arginase-1 via ApoA1-STAT3 Axis. Cells 2025, 14, 660. https://doi.org/10.3390/cells14090660
Chang C-C, Chang C-B, Shen C-H, Lee M-Y, Jou Y-C, Tung C-L, Lai W-H, Hung C-F, Wang M, Lai Y-Y, et al. Immunosuppression of Tumor-Derived Factors Modulated Neutrophils in Upper Tract Urothelial Carcinoma Through Upregulation of Arginase-1 via ApoA1-STAT3 Axis. Cells. 2025; 14(9):660. https://doi.org/10.3390/cells14090660
Chicago/Turabian StyleChang, Chih-Chia, Chia-Bin Chang, Cheng-Huang Shen, Ming-Yang Lee, Yeong-Chin Jou, Chun-Liang Tung, Wei-Hong Lai, Chi-Feng Hung, Meilin Wang, Ya-Yan Lai, and et al. 2025. "Immunosuppression of Tumor-Derived Factors Modulated Neutrophils in Upper Tract Urothelial Carcinoma Through Upregulation of Arginase-1 via ApoA1-STAT3 Axis" Cells 14, no. 9: 660. https://doi.org/10.3390/cells14090660
APA StyleChang, C.-C., Chang, C.-B., Shen, C.-H., Lee, M.-Y., Jou, Y.-C., Tung, C.-L., Lai, W.-H., Hung, C.-F., Wang, M., Lai, Y.-Y., Chen, P.-C., & Wu, S.-F. (2025). Immunosuppression of Tumor-Derived Factors Modulated Neutrophils in Upper Tract Urothelial Carcinoma Through Upregulation of Arginase-1 via ApoA1-STAT3 Axis. Cells, 14(9), 660. https://doi.org/10.3390/cells14090660





