Advancing Obstructive Airway Disease Treatment: Dual PDE3/4 Inhibition as a Therapeutic Strategy
Abstract
1. Introduction
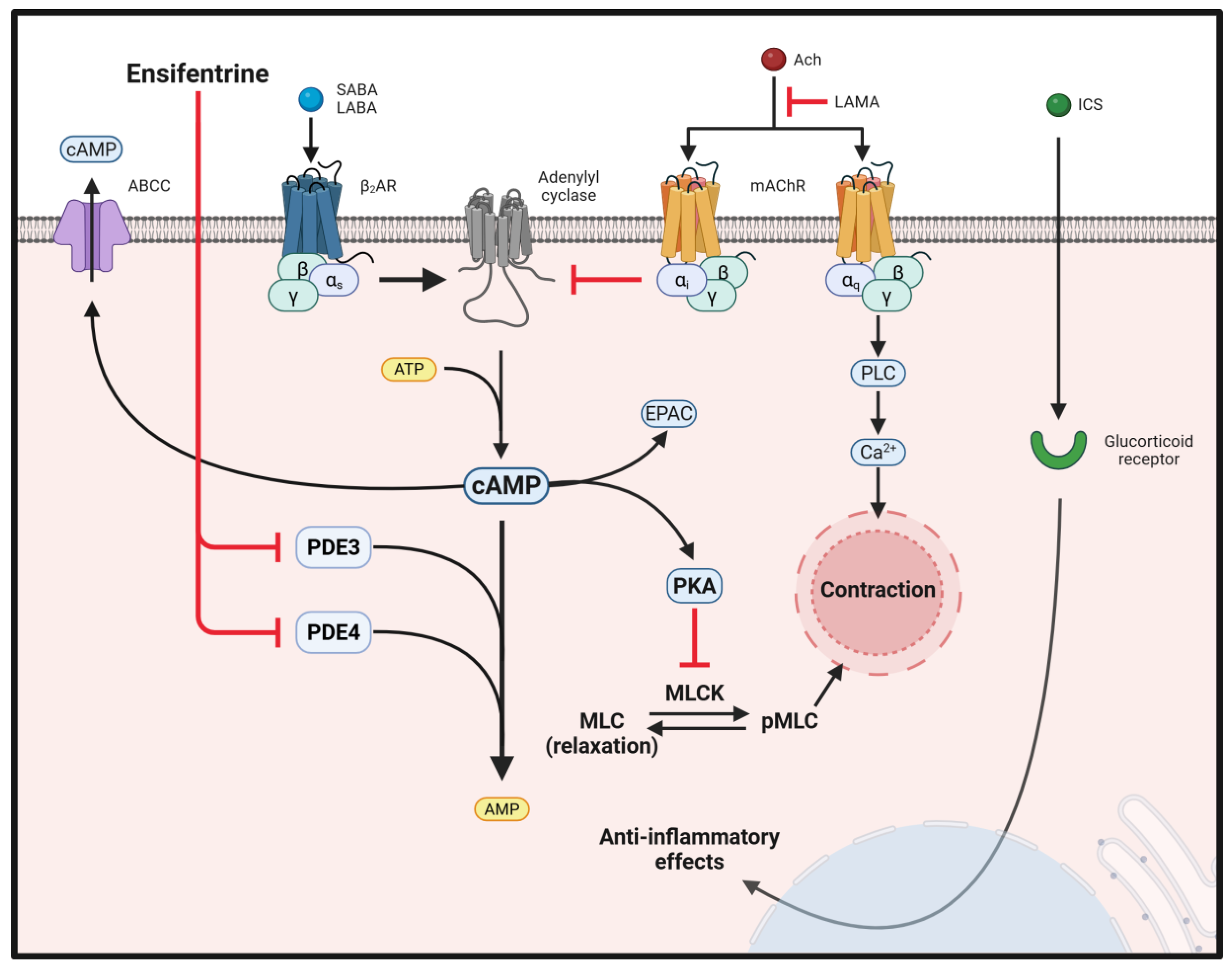
2. Cyclic AMP and PDEs
| Cell Type | PDE Isoforms | Notes on Relevant Cellular Effects of Inhibition | Ref |
|---|---|---|---|
| Airway Epithelium | PDE1 | Inhibition blockaded lipopolysaccharide-endotoxin (LPS)-mediated biosynthesis of interleukin (IL)-6. | [58,59,60,61,62,63] |
| PDE3 | Inhibition of PDE3 leads to activation of cystic fibrosis transmembrane conductance regulator (CFTR). Blocking PDE3 activity also abolished the effect of LPS on IL-6 and attenuated TNF-α production. | [58,62,64] | |
| PDE4 | Inhibition induces repressive effect on IL-6, IL-8 production and a dual, biphasic (excitatory/inhibitory) effect on TNF-α secretion. Also induces increased production of PGE2. | [58,59,60,61,62,65] | |
| PDE7 | PDE7 inhibition synergizes with PDE4 inhibition to suppress inflammatory signaling. | [60,66,67] | |
| Airway Smooth Muscle (ASM) | PDE1 | Inhibition increases ciliary beat frequency and angle in lung airway cell. | [31,68] |
| PDE3 | Blocking PDE3 activity promotes bronchodilation by relaxing smooth muscle. | [66,69,70] | |
| PDE4 | Inhibition reduces ASM hyperreactivity and inflammation by stopping pro-inflammatory signaling. | [31,69] | |
| PDE7 | Airway reactivity and contractility are decreased after PDE7 inhibition. | [66,71] | |
| PDE8 | Inhibition of PDE isoform enhances isoproterenol induced reduction in cell proliferation. | [31] | |
| Goblet Cells | PDE4 | Inhibition reduces mucus hypersecretion by downregulating MUC5AC expression. | [72] |
| Submucosal Glands | PDE3 | PDE3 inhibition augments CFTR-dependent submucosal gland secretion. | [73,74] |
| PDE4 | Inhibition of PDE4 stimulates elevated saliva production. | [75] | |
| Eosinophils | PDE4 | Functions such as the release of inflammatory granule constituents, chemotaxis, cytokines and superoxide generation are inhibited by blocking PDE4 | [65,66,76,77] |
| PDE7 | [66] | ||
| Neutrophils | PDE3 | Inhibition reduces neutrophil chemotaxis and activation. | [76,77,78,79] |
| PDE4 | Inhibition suppresses neutrophil degranulation and function (Leukotriene B4 and reactive oxygen species (ROS) synthesis). | [65,76,78] | |
| Macrophages | PDE1 | Inhibition decreases macrophage-mediated inflammation and oxidative stress. | [50,66,76,79,80] |
| PDE3 | Inhibition can play an anti-inflammatory role in allergic airway inflammation. | [36] | |
| PDE4 | PDE4 inhibitors reduce generation of pro-inflammatory cytokine, TNF-α, from macrophages in the presence of PDE3 inhibitor. Inhibition also potentiates chemokine expression elicited by forskolin or Prostaglandin E2 (PGE2). | [61,76,81] | |
| PDE7 | May work in concert with PDE4 inhibition to further suppress macrophage-driven inflammation. | [66,82] | |
| T lymphocytes | PDE3 | Inhibition affects T cell activation and proliferation, potentially modulating immune responses in COPD. | [65,66,76,78,83] |
| PDE4 | Inhibition suppresses T cell proliferation, activation and cytokine production (IL-4, IL-5, and IFN-γ synthesis), reducing airway inflammation. | [76] | |
| PDE7 | PDE7 inhibition decreases proliferation, IL-12 expression, and acts synergistically with PDE4 inhibition. | [66,69,78,84] | |
| PDE8 | Inhibition suppresses attachment of T effector cells to endothelial cells. | [81] |
3. Ensifentrine, a Dual PDE3 and 4 Inhibitor
4. Clinical Trials
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCC1 | ATP-binding cassette [ABC] subfamily member C1 |
| AC | Adenylyl cyclase |
| Ach | Acetylcholine |
| AHR | Airway Hyperresponsiveness |
| AKAP | A-kinase anchoring protein |
| ASM | Airway smooth muscle |
| β2AR | Beta-2 adrenergic receptor |
| cAMP | 3′,5′-cyclic adenosine monophosphate |
| cGMP | 3′,5′-cyclic guanosine monophosphate |
| COPD | Chronic obstructive pulmonary disease |
| Epac | Exchange protein activated by cAMP |
| FEV | Forced expiratory volume |
| GPCR | G protein-coupled receptor |
| GRα | Glucocorticoid receptor α |
| HSP | Heat shock protein |
| ICS | Inhaled corticosteroids |
| LABA | Long-Acting Beta-Agonists |
| LAMA | Long-Acting Muscarinic antagonists |
| mAChR | Muscarinic acetylcholine receptor |
| MLC | Myosin light chain |
| MLCK | Myosin light chain kinase |
| PDE | Phosphodiesterase |
| PKA | Protein kinase A |
| PLC | Phospholipase C |
| ROS | Reactive oxygen species |
| SABA | Short-Acting Beta-Agonists |
References
- Kumar, R.; Khan, M.I.; Panwar, A.; Vashist, B.; Rai, S.K.; Kumar, A. PDE4 Inhibitors and their Potential Combinations for the Treatment of Chronic Obstructive Pulmonary Disease: A Narrative Review. Open Respir. Med. J. 2024, 18, e18743064340418. [Google Scholar] [CrossRef] [PubMed]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.S.; Miller, J.D.; Marton, J.P.; Caloyeras, J.P.; Russell, M.W.; Menzin, J. Assessment of the economic burden of COPD in the U.S.: A review and synthesis of the literature. COPD 2006, 3, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Giembycz, M.A.; Kaur, M.; Leigh, R.; Newton, R. A Holy Grail of asthma management: Toward understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br. J. Pharmacol. 2008, 153, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Pathophysiology of asthma. Br. J. Clin. Pharmacol. 1996, 42, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Panettieri, R.A. Effects of Corticosteroids on Structural Cells in Asthma and Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2004, 1, 231–234. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Petite, S.E. Role of Long-Acting Muscarinic Antagonist/Long-Acting beta(2)-Agonist Therapy in Chronic Obstructive Pulmonary Disease. Ann. Pharmacother. 2017, 51, 696–705. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Ferguson, G.T. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir. Res. 2013, 14, 49. [Google Scholar] [CrossRef]
- Calzetta, L.; Ritondo, B.L.; de Marco, P.; Cazzola, M.; Rogliani, P. Evaluating triple ICS/LABA/LAMA therapies for COPD patients: A network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies. Expert. Rev. Respir. Med. 2021, 15, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Vandemheen, K.; Fergusson, D.; Fitzgerald, M.; Maltais, F.; Bourbeau, J.; Goldstein, R.; McIvor, A.; Balter, M.; O’Donnell, D. The Canadian Optimal Therapy of COPD Trial: Design, Organization and Patient Recruitment. Can. Respir. J. 2004, 11, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Martinez, F.D.; Hackshaw, A.; Rabe, K.F.; Sterk, P.J.; Djukanovic, R. Safety of long-acting β-agonists: Urgent need to clear the air remains. Eur. Respir. J. 2009, 33, 3–5. [Google Scholar] [CrossRef]
- Martinez, F.D. Safety of Long-Acting Beta-Agonists—An Urgent Need to Clear the Air. N. Engl. J. Med. 2005, 353, 2637–2639. [Google Scholar] [CrossRef]
- Patel, R.; Naqvi, S.A.; Griffiths, C.; Bloom, C.I. Systemic adverse effects from inhaled corticosteroid use in asthma: A systematic review. BMJ Open Respir. Res. 2020, 7, e000756. [Google Scholar] [CrossRef]
- Al Matni, M.Y.; Meliton, L.; Dudek, S.M.; Letsiou, E. Dual Inhibition of Phosphodiesterase 3 and 4 Enzymes by Ensifentrine Protects against MRSA-Induced Lung Endothelial and Epithelial Dysfunction. Cells 2024, 13, 1750. [Google Scholar] [CrossRef]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. cAMP regulation of airway smooth muscle function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef]
- Sherpa, R.T.; Moshal, K.S.; Agarwal, S.R.; Ostrom, R.S.; Harvey, R.D. Role of protein kinase A and A kinase anchoring proteins in buffering and compartmentation of cAMP signalling in human airway smooth muscle cells. Br. J. Pharmacol. 2024, 181, 2622–2635. [Google Scholar] [CrossRef]
- Zaccolo, M. Spatial control of cAMP signalling in health and disease. Curr. Opin. Pharmacol. 2011, 11, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Spina, D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr. Opin. Pharmacol. 2012, 12, 275–286. [Google Scholar] [CrossRef]
- Morgan, S.J.; Deshpande, D.A.; Tiegs, B.C.; Misior, A.M.; Yan, H.; Hershfeld, A.V.; Rich, T.C.; Panettieri, R.A.; An, S.S.; Penn, R.B. beta-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J. Biol. Chem. 2014, 289, 23065–23074. [Google Scholar] [CrossRef] [PubMed]
- Oguma, T.; Kume, H.; Ito, S.; Takeda, N.; Honjo, H.; Kodama, I.; Shimokata, K.; Kamiya, K. Involvement of reduced sensitivity to Ca2+ in β-adrenergic action on airway smooth muscle. Clin. Exp. Allergy 2006, 36, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Endou, K.; Iizuka, K.; Yoshii, A.; Tsukagoshi, H.; Ishizuka, T.; Dobashi, K.; Nakazawa, T.; Mori, M. 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L641–L648. [Google Scholar] [CrossRef]
- Komalavilas, P.; Penn, R.B.; Flynn, C.R.; Thresher, J.; Lopes, L.B.; Furnish, E.J.; Guo, M.; Pallero, M.A.; Murphy-Ullrich, J.E.; Brophy, C.M. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L69–L78. [Google Scholar] [CrossRef]
- Joskova, M.; Mokry, J.; Franova, S. Respiratory Cilia as a Therapeutic Target of Phosphodiesterase Inhibitors. Front. Pharmacol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Abbott-Banner, K.H.; Page, C.P. Dual PDE3/4 and PDE4 inhibitors: Novel treatments for COPD and other inflammatory airway diseases. Basic. Clin. Pharmacol. Toxicol. 2014, 114, 365–376. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Smith, K.H.; Koziol-White, C.J.; Li, F.; Kazarian, A.G.; Corpuz, M.L.; Shumyatcher, M.; Ehlert, F.J.; Himes, B.E.; Panettieri, R.A., Jr.; et al. PDE8 Is Expressed in Human Airway Smooth Muscle and Selectively Regulates cAMP Signaling by beta(2)-Adrenergic Receptors and Adenylyl Cyclase 6. Am. J. Respir. Cell Mol. Biol. 2018, 58, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Ora, J.; Cavalli, F.; Rogliani, P.; Cazzola, M. New Avenues for Phosphodiesterase Inhibitors in Asthma. J. Exp. Pharmacol. 2021, 13, 291–302. [Google Scholar] [CrossRef]
- Page, C.P. Phosphodiesterase inhibitors for the treatment of asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 2014, 165, 152–164. [Google Scholar] [CrossRef] [PubMed]
- KleinJan, A. Airway inflammation in asthma: Key players beyond the Th2 pathway. Curr. Opin. Pulm. Med. 2016, 22, 46–52. [Google Scholar] [CrossRef]
- Shakur, Y.; Holst, L.S.; Landstrom, T.R.; Movsesian, M.; Degerman, E.; Manganiello, V. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 66, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Beute, J.; Lukkes, M.; Koekoek, E.P.; Nastiti, H.; Ganesh, K.; de Bruijn, M.J.; Hockman, S.; van Nimwegen, M.; Braunstahl, G.J.; Boon, L.; et al. A pathophysiological role of PDE3 in allergic airway inflammation. JCI Insight 2018, 3, e94888. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Rabe, K.F.; Tenor, H.; Dent, G.; Schudt, C.; Liebig, S.; Magnussen, H. Phosphodiesterase isozymes modulating inherent tone in human airways: Identification and characterization. Am. J. Physiol. 1993, 264, L458–L464. [Google Scholar] [CrossRef]
- Spina, D.; Page, C.P. Xanthines and Phosphodiesterase Inhibitors. Handb. Exp. Pharmacol. 2017, 237, 63–91. [Google Scholar] [CrossRef]
- Gantner, F.; Gotz, C.; Gekeler, V.; Schudt, C.; Wendel, A.; Hatzelmann, A. Phosphodiesterase profile of human B lymphocytes from normal and atopic donors and the effects of PDE inhibition on B cell proliferation. Br. J. Pharmacol. 1998, 123, 1031–1038. [Google Scholar] [CrossRef]
- Billington, C.K.; Le Jeune, I.R.; Young, K.W.; Hall, I.P. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Ibrahim, P.N.; Gillette, S.; Bollag, G. Phosphodiesterase-4 as a potential drug target. Expert. Opin. Ther. Targets 2005, 9, 1283–1305. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Chervinsky, P.; Busse, W.; Ohta, K.; Bardin, P.; Bredenbroker, D.; Bateman, E.D. Roflumilast for asthma: Efficacy findings in placebo-controlled studies. Pulm. Pharmacol. Ther. 2015, 35, S20–S27. [Google Scholar] [CrossRef]
- Kawamatawong, T. Roles of roflumilast, a selective phosphodiesterase 4 inhibitor, in airway diseases. J. Thorac. Dis. 2017, 9, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.T.; Lakshmi, S.P.; Banno, A.; Reddy, R.C. Glucocorticoid Receptor alpha Mediates Roflumilast’s Ability to Restore Dexamethasone Sensitivity in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 125–134. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cooke, G.E.; Kowey, P.R.; Calverley, P.M.A.; Bredenbröker, D.; Goehring, U.-M.; Zhu, H.; Lakkis, H.; Mosberg, H.; Rowe, P.; et al. Cardiovascular Safety in Patients Receiving Roflumilast for the Treatment of COPD. Chest 2013, 144, 758–765. [Google Scholar] [CrossRef]
- Wouters, E.F.M.; Bredenbröker, D.; Teichmann, P.; Brose, M.; Rabe, K.F.; Fabbri, L.M.; Göke, B. Effect of the Phosphodiesterase 4 Inhibitor Roflumilast on Glucose Metabolism in Patients with Treatment-Naive, Newly Diagnosed Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, E1720–E1725. [Google Scholar] [CrossRef]
- Fan, T.; Wang, W.; Wang, Y.; Zeng, M.; Liu, Y.; Zhu, S.; Yang, L. PDE4 inhibitors: Potential protective effects in inflammation and vascular diseases. Front. Pharmacol. 2024, 15, 1407871. [Google Scholar] [CrossRef]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef]
- Giembycz, M.A. Phosphodiesterase-4: Selective and Dual-Specificity Inhibitors for the Therapy of Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 2005, 2, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Movsesian, M.A.; Kukreja, R.C. Phosphodiesterase Inhibition in Heart Failure; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–249. [Google Scholar] [CrossRef]
- Turner, M.J.; Dauletbaev, N.; Lands, L.C.; Hanrahan, J.W. The Phosphodiesterase Inhibitor Ensifentrine Reduces Production of Proinflammatory Mediators in Well Differentiated Bronchial Epithelial Cells by Inhibiting PDE4. J. Pharmacol. Exp. Ther. 2020, 375, 414–429. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Oxford, A.W.; Comer, M.B.; Seeds, E.A.; Page, C.P. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one]. J. Pharmacol. Exp. Ther. 2006, 318, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F.; Rheault, T.; Macdonald-Berko, M.; Bengtsson, T.; Rickard, K. Ensifentrine as a Novel, Inhaled Treatment for Patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Anzueto, A.; Barjaktarevic, I.Z.; Siler, T.M.; Rheault, T.; Bengtsson, T.; Rickard, K.; Sciurba, F. Ensifentrine, a Novel Phosphodiesterase 3 and 4 Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease: Randomized, Double-Blind, Placebo-controlled, Multicenter Phase III Trials (the ENHANCE Trials). Am. J. Respir. Crit. Care Med. 2023, 208, 406–416. [Google Scholar] [CrossRef]
- Faruqi, M.A.; Khan, M.; Mannino, D. Perspectives on Ensifentrine and Its Therapeutic Potential in the Treatment of COPD: Evidence to Date. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 11–16. [Google Scholar] [CrossRef]
- Haddad, J.J.; Land, S.C.; Tarnow-Mordi, W.O.; Zembala, M.; Kowalczyk, D.; Lauterbach, R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J. Pharmacol. Exp. Ther. 2002, 300, 559–566. [Google Scholar] [CrossRef]
- Essayan, D.M. Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem. Pharmacol. 1999, 57, 965–973. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Jahn, H.U.; Seybold, J.; Neurohr, C.; Barnes, P.J.; Hippenstiel, S.; Kraemer, H.J.; Suttorp, N. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 292–302. [Google Scholar] [CrossRef]
- Brown, D.M.; Hutchison, L.; Donaldson, K.; MacKenzie, S.J.; Dick, C.A.; Stone, V. The effect of oxidative stress on macrophages and lung epithelial cells: The role of phosphodiesterases 1 and 4. Toxicol. Lett. 2007, 168, 1–6. [Google Scholar] [CrossRef]
- Wright, L.C.; Seybold, J.; Robichaud, A.; Adcock, I.M.; Barnes, P.J. Phosphodiesterase expression in human epithelial cells. Am. J. Physiol. 1998, 275, L694–L700. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, H.; Hosogi, S.; Ikeuchi, Y.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Nakano, T.; Sano, K.I.; Inui, T.; Marunaka, Y.; et al. A low [Ca(2+)](i)-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflug. Arch. 2017, 469, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Kelley, T.J.; al-Nakkash, L.; Drumm, M.L. CFTR-mediated chloride permeability is regulated by type III phosphodiesterases in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1995, 13, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Spina, D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 391–414. [Google Scholar] [CrossRef]
- Smith, S.J.; Brookes-Fazakerley, S.; Donnelly, L.E.; Barnes, P.J.; Barnette, M.S.; Giembycz, M.A. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L279–L289. [Google Scholar] [CrossRef]
- Fortin, M.; D’Anjou, H.; Higgins, M.E.; Gougeon, J.; Aube, P.; Moktefi, K.; Mouissi, S.; Seguin, S.; Seguin, R.; Renzi, P.M.; et al. A multi-target antisense approach against PDE4 and PDE7 reduces smoke-induced lung inflammation in mice. Respir. Res. 2009, 10, 39. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Pullamsetti, S.S.; Kwapiszewska, G.; Dumitrascu, R.; Tian, X.; Weissmann, N.; Ghofrani, H.A.; Kaulen, C.; Dunkern, T.; Schudt, C.; et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: Target for reverse-remodeling therapy. Circulation 2007, 115, 2331–2339. [Google Scholar] [CrossRef]
- Singh, D.; Lea, S.; Mathioudakis, A.G. Inhaled Phosphodiesterase Inhibitors for the Treatment of Chronic Obstructive Pulmonary Disease. Drugs 2021, 81, 1821–1830. [Google Scholar] [CrossRef]
- Zuo, H.; Han, B.; Poppinga, W.J.; Ringnalda, L.; Kistemaker, L.E.M.; Halayko, A.J.; Gosens, R.; Nikolaev, V.O.; Schmidt, M. Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells. Br. J. Pharmacol. 2018, 175, 2988–3006. [Google Scholar] [CrossRef]
- Mokry, J.; Joskova, M.; Mokra, D.; Christensen, I.; Nosalova, G. Effects of selective inhibition of PDE4 and PDE7 on airway reactivity and cough in healthy and ovalbumin-sensitized guinea pigs. Adv. Exp. Med. Biol. 2013, 756, 57–64. [Google Scholar] [CrossRef]
- Mata, M.; Sarria, B.; Buenestado, A.; Cortijo, J.; Cerda, M.; Morcillo, E.J. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax 2005, 60, 144–152. [Google Scholar] [CrossRef]
- Penmatsa, H.; Zhang, W.; Yarlagadda, S.; Li, C.; Conoley, V.G.; Yue, J.; Bahouth, S.W.; Buddington, R.K.; Zhang, G.; Nelson, D.J.; et al. Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol. Biol. Cell 2010, 21, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Sugatani, T.; Manganiello, V.C.; Shimizu, K.; Tagawa, T. Expression of phosphodiesterase 3 in rat submandibular gland cell lines. Arch. Oral Biol. 2001, 46, 453–457. [Google Scholar] [CrossRef]
- Boyd, A.; Aragon, I.V.; Abou Saleh, L.; Southers, D.; Richter, W. The cAMP-phosphodiesterase 4 (PDE4) controls beta-adrenoceptor- and CFTR-dependent saliva secretion in mice. Biochem. J. 2021, 478, 1891–1906. [Google Scholar] [CrossRef] [PubMed]
- Hatzelmann, A.; Schudt, C. Anti-Inflammatory and Immunomodulatory Potential of the Novel PDE4 Inhibitor Roflumilast in Vitro. J. Pharmacol. Exp. Ther. 2001, 297, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Parkkonen, J.; Hasala, H.; Moilanen, E.; Giembycz, M.A.; Kankaanranta, H. Phosphodiesterase 4 inhibitors delay human eosinophil and neutrophil apoptosis in the absence and presence of salbutamol. Pulm. Pharmacol. Ther. 2008, 21, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Leport, M.; Holand, T.; Vos, T.; Morgan, M.; Fink, M.; Pruniaux, M.P.; Berthelier, C.; O’Connor, B.J.; Bertrand, C.; et al. Phosphodiesterase (PDE) 7 in inflammatory cells from patients with asthma and COPD. Pulm. Pharmacol. Ther. 2007, 20, 60–68. [Google Scholar] [CrossRef]
- Barber, R.; Baillie, G.S.; Bergmann, R.; Shepherd, M.C.; Sepper, R.; Houslay, M.D.; Heeke, G.V. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L332–L343. [Google Scholar] [CrossRef]
- Hwang, T.L.; Tang, M.C.; Kuo, L.M.; Chang, W.D.; Chung, P.J.; Chang, Y.W.; Fang, Y.C. YC-1 potentiates cAMP-induced CREB activation and nitric oxide production in alveolar macrophages. Toxicol. Appl. Pharmacol. 2012, 260, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Vang, A.G.; Ben-Sasson, S.Z.; Dong, H.; Kream, B.; DeNinno, M.P.; Claffey, M.M.; Housley, W.; Clark, R.B.; Epstein, P.M.; Brocke, S. PDE8 regulates rapid Teff cell adhesion and proliferation independent of ICER. PLoS ONE 2010, 5, e12011. [Google Scholar] [CrossRef]
- Smith, S.J.; Cieslinski, L.B.; Newton, R.; Donnelly, L.E.; Fenwick, P.S.; Nicholson, A.G.; Barnes, P.J.; Barnette, M.S.; Giembycz, M.A. Discovery of BRL 50481 [3-(N,N-dimethylsulfonamido)-4-methyl-nitrobenzene], a selective inhibitor of phosphodiesterase 7: In vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes. Mol. Pharmacol. 2004, 66, 1679–1689. [Google Scholar] [CrossRef]
- Giembycz, M.A.; Corrigan, C.J.; Seybold, J.; Newton, R.; Barnes, P.J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: Role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 1996, 118, 1945–1958. [Google Scholar] [CrossRef] [PubMed]
- Glavas, N.A.; Ostenson, C.; Schaefer, J.B.; Vasta, V.; Beavo, J.A. T cell activation up-regulates cyclic nucleotide phosphodiesterases 8A1 and 7A3. Proc. Natl. Acad. Sci. USA 2001, 98, 6319–6324. [Google Scholar] [CrossRef]
- Urbanova, A.; Kertys, M.; Simekova, M.; Mikolka, P.; Kosutova, P.; Mokra, D.; Mokry, J. Bronchodilator and Anti-Inflammatory Action of Theophylline in a Model of Ovalbumin-Induced Allergic Inflammation. Adv. Exp. Med. Biol. 2016, 935, 53–62. [Google Scholar] [CrossRef]
- Mann, J.S.; Holgate, S.T. Specific antagonism of adenosine-induced bronchoconstriction in asthma by oral theophylline. Br. J. Clin. Pharmacol. 1985, 19, 685–692. [Google Scholar] [CrossRef]
- Spears, M.; Donnelly, I.; Jolly, L.; Brannigan, M.; Ito, K.; McSharry, C.; Lafferty, J.; Chaudhuri, R.; Braganza, G.; Adcock, I.M.; et al. Effect of low-dose theophylline plus beclometasone on lung function in smokers with asthma: A pilot study. Eur. Respir. J. 2009, 33, 1010–1017. [Google Scholar] [CrossRef]
- Journey, J.D.; Bentley, T.P. Theophylline Toxicity. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Calzetta, L.; Cazzola, M.; Gholamalishahi, S.; Rogliani, P. The novel inhaled dual PDE3 and PDE4 inhibitor ensifentrine for the treatment of COPD: A systematic review and meta-analysis protocol on trough FEV1 and exacerbation according to PRISMA statement. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Turner, M.J.; Matthes, E.; Billet, A.; Ferguson, A.J.; Thomas, D.Y.; Randell, S.H.; Ostrowski, L.E.; Abbott-Banner, K.; Hanrahan, J.W. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L59–L70. [Google Scholar] [CrossRef] [PubMed]
- Spina, D.; Ferlenga, P.; Biasini, I.; Moriggi, E.; Marchini, F.; Semeraro, C.; Page, C.P. The effect duration of selective phosphodiesterase inhibitors in the guinea pig. Life Sci. 1998, 62, 953–965. [Google Scholar] [CrossRef]
- Venkatasamy, R.; Spina, D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br. J. Pharmacol. 2016, 173, 2335–2351. [Google Scholar] [CrossRef]
- Calzetta, L.; Page, C.P.; Spina, D.; Cazzola, M.; Rogliani, P.; Facciolo, F.; Matera, M.G. Effect of the Mixed Phosphodiesterase 3/4 Inhibitor RPL554 on Human Isolated Bronchial Smooth Muscle Tone. J. Pharmacol. Exp. Ther. 2013, 346, 414–423. [Google Scholar] [CrossRef]
- Evans, T.W.; Rogers, D.F.; Aursudkij, B.; Chung, K.F.; Barnes, P.J. Regional and time-dependent effects of inflammatory mediators on airway microvascular permeability in the guinea pig. Clin. Sci. 1989, 76, 479–485. [Google Scholar] [CrossRef]
- Franciosi, L.G.; Diamant, Z.; Banner, K.H.; Zuiker, R.; Morelli, N.; Kamerling, I.M.C.; De Kam, M.L.; Burggraaf, J.; Cohen, A.F.; Cazzola, M.; et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: Findings from four clinical trials. Lancet Respir. Med. 2013, 1, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Abbott-Banner, K.; Bengtsson, T.; Newman, K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur. Respir. J. 2018, 52, 1801074. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.; Pauwels, R.A.; Hurd, S.S.; Committee, G.S. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary updated 2003. COPD 2004, 1, 105–141, discussion 103–104. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C. An inhaled “bifunctional” dual PDE3/4 inhibitor provides additional short-term improvements in lung function compared to existing classes of bronchodilator: Implications for future treatment of COPD. Eur. Respir. J. 2018, 52, 1801675. [Google Scholar] [CrossRef]
- Singh, D.; Martinez, F.J.; Watz, H.; Bengtsson, T.; Maurer, B.T. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir. Res. 2020, 21, 47. [Google Scholar] [CrossRef]
- Pleasants, R.A.; Hess, D.R. Aerosol Delivery Devices for Obstructive Lung Diseases. Respir. Care 2018, 63, 708–733. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir. Care 2005, 50, 1313–1321, discussion 1321-1312. [Google Scholar]
- Tashkin, D.P. A review of nebulized drug delivery in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2585–2596. [Google Scholar] [CrossRef]
- Keam, S.J. Ensifentrine: First Approval. Drugs 2024, 84, 1157–1163. [Google Scholar] [CrossRef]
- Donohue, J.F. Minimal clinically important differences in COPD lung function. COPD 2005, 2, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Beeh, K.M.; Chapman, K.R.; Decramer, M.; Mahler, D.A.; Wedzicha, J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014, 189, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Jones, P. Quality of life, symptoms and pulmonary function in asthma: Long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Nedocromil Sodium Quality of Life Study Group. Eur. Respir. J. 1994, 7, 55–62. [Google Scholar] [CrossRef]
- Siler, T.M.; Fogarty, C.M.; Rheault, T.; Bengtsson, T.; Ann Rickard, K. Pooled safety results over 24 weeks from the ENHANCE program with Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor. Chest 2023, 164, A4981–A4983. [Google Scholar] [CrossRef]
- Xiong, D.J.P.; Martin, J.G.; Lauzon, A.M. Airway smooth muscle function in asthma. Front. Physiol. 2022, 13, 993406. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, B.; Kim, J.; Ke, Y.; Li, Y.; Zhang, C.O.; Promnares, K.; Tanaka, K.A.; Konstantin; Karki, P.; Birukova, A.A. Mechanisms of pulmonary endothelial permeability and inflammation caused by extracellular histone subunits H3 and H4. FASEB J. 2022, 36, e22470. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Lam, H.; Jude, J.A.; Karmacharya, N.; Kan, M.; Jester, W.; Koziol-White, C.; Himes, B.E.; Chupp, G.L.; An, S.S.; et al. Inhibition of ABCC1 Decreases cAMP Egress and Promotes Human Airway Smooth Muscle Cell Relaxation. Am. J. Respir. Cell Mol. Biol. 2022, 66, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Lu, T.W.; Stolerman, L.M.; Tenner, B.; Yang, J.R.; Zhang, J.F.; Falcke, M.; Rangamani, P.; Taylor, S.S.; Mehta, S.; et al. Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 2020, 182, 1531–1544.E15. [Google Scholar] [CrossRef] [PubMed]
| Phases | NCT Number | Study Title | No. of Patients Enrolled | Interventions | Start Date | Primary Completion |
|---|---|---|---|---|---|---|
| PHASE1 | NCT02307162 | SAD/MAD Study of a New Formulation of Nebulized RPL554 in Healthy Subjects and COPD Subjects | 112 | Ensifentrine|Placebo | December 2014 | July 2015 |
| PHASE2 | NCT02427165 | Comparison of RPL554 With Placebo and Salbutamol in Asthmatic Patients | 29 | Ensifentrine|Salbutamol|Placebo | April 2015 | November 2015 |
| NCT02542254 | The Effects of RPL554 on Top of Standard COPD Reliever Medications | 36 | Ensifentrine|Salbutamol|Ipratropium|Placebo | October 2015 | December 2015 | |
| NCT03028142 | The Effects of RPL554 in Addition to Tiotropium in COPD Patients | 30 | Ensifentrine|Placeboin addition to tiotropium | January 2017 | August 2017 | |
| NCT02919995 | A Study of RPL554 in Patients With Cystic Fibrosis | 10 | Ensifentrine|Placebo | February 2017 | November 2017 | |
| NCT03443414 | Dose Ranging Study of RPL554 in Chronic Obstructive Pulmonary Disease (COPD) Patients | 405 | Ensifentrine|Placebo | June 2017 | January 2018 | |
| NCT03673670 | Bronchodilator Effect of RPL554 Administered in Addition to Tiotropium/Olodaterol in Patients With COPD | 79 | Ensifentrine|Placebo|Tiotropium/olodaterol (Respimat) | July 2018 | November 2018 | |
| NCT04027439 | Study Evaluating 5 Doses of RPL554 and Placebo in COPD Patients Via a Dry Powder Inhaler | 37 | Ensifentrine|Placebo | December 2018 | May 2019 | |
| NCT04091360 | A Study of RPL554 Drug Administered by Metered Dose Inhaler to Treat Chronic Obstructive Pulmonary Disease | 40 | Ensifentrine|Placebo | April 2019 | December 2020 | |
| NCT03937479 | Study Investigating the Effect of 4 Doses of RPL554 Given in Addition to Tiotropium to Patients With COPD | 416 | Ensifentrine|Placebo in addition to tiotropiuin | May 2019 | November 2019 | |
| NCT04527471 | Pilot Study of Ensifentrine or Placebo Delivered Via pMDI in Hospitalized Patients With COVID-19 | 45 | Ensifentrine|Placebo | September 2020 | February 2021 | |
| NCT05270525 | Effect of Ensifentrine on Sputum Markers of Inflammation in COPD | 50 | Ensifentrine|Placebo | May 2022 | December 2026 | |
| NCT06559150 | A Phase II Study of Ensifentrine in Non-Cystic Fibrosis Bronchiectasis | 180 | Ensifentrine|Placebo | September 2024 | September 2026 | |
| PHASE3 | NCT04542057 | A Phase 3 Trial to Evaluate the Safety and Efficacy of Ensifentrine in Patients With COPD | 790 | Ensifentrine|Placebo | September 2020 | May 2022 |
| NCT04535986 | A Phase 3 Clinical Trial to Evaluate the Safety and Efficacy of Ensifentrine in Patients With COPD | 763 | Ensifentrine|Placebo | September 2020 | September 2022 | |
| NCT06460493 | Effect of Ensifentrine Treatment on CAT Score | 20 | Ensifentrine | June 2024 | November 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherpa, R.T.; Koziol-White, C.J.; Panettieri, R.A., Jr. Advancing Obstructive Airway Disease Treatment: Dual PDE3/4 Inhibition as a Therapeutic Strategy. Cells 2025, 14, 659. https://doi.org/10.3390/cells14090659
Sherpa RT, Koziol-White CJ, Panettieri RA Jr. Advancing Obstructive Airway Disease Treatment: Dual PDE3/4 Inhibition as a Therapeutic Strategy. Cells. 2025; 14(9):659. https://doi.org/10.3390/cells14090659
Chicago/Turabian StyleSherpa, Rinzhin T., Cynthia J. Koziol-White, and Reynold A. Panettieri, Jr. 2025. "Advancing Obstructive Airway Disease Treatment: Dual PDE3/4 Inhibition as a Therapeutic Strategy" Cells 14, no. 9: 659. https://doi.org/10.3390/cells14090659
APA StyleSherpa, R. T., Koziol-White, C. J., & Panettieri, R. A., Jr. (2025). Advancing Obstructive Airway Disease Treatment: Dual PDE3/4 Inhibition as a Therapeutic Strategy. Cells, 14(9), 659. https://doi.org/10.3390/cells14090659







