Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Quantification of Live Cell Number Using Imaging Analysis System
2.3. BrdU Assay
2.4. Real-Time Polymerase Chain Reaction
2.5. Alkaline Phosphatase Staining
2.6. Western Blot Analysis
2.7. ALP Quantification
2.8. Alizarin Red S Staining
2.9. Calcium Quantification
2.10. Osteocalcin Quantification
2.11. Statistical Analysis
3. Results
3.1. Cell Morphology
3.2. Quantification of Live Cell Number
3.3. BrdU Assay


- (a)
- Representative light microscopy images of stem cells from human exfoliated deciduous teeth (SHED). SHED were isolated and cultured from deciduous teeth of patients according to the method of Gronthos et al. Cell cultures were grown in α-MEM containing 10% FBS, 50 U/mL penicillin, 50 µg/mL streptomycin, and 1 µL/mL amphotericin under 37 °C and 5% CO2 conditions. Individual cells were spindle-shaped, and the plated culture formed a herringbone pattern. Cell morphology did not change with successive passages. Scale bar 100 µm.
- (b)
- Cell proliferation curves obtained by Incucyte® S3 Live-Cell Analysis System. SHED from passages 4 to 6 were seeded in 96-well plates at 5.0 × 103 cells/well and cell proliferative capacity was compared in a 10% FBSα-MEM environment. Cells were photographed and assessed every 2 h for a total of 72 h using an Incucyte® S3 Live-Cell Analysis System. No significant differences were found between passage 4, 5, and 6 cells at any time point (n = 12, Tukey’s HSD test, not significant [NS]).
- (c)
- Representative images obtained at the start of the recording and 72 h later. The yellow areas were recognized as cells by the Incucyte® S3 Live-Cell Analysis System. Scale bar 400 µm.
- (d)
- Quantification of passages 4 to 6 SHED using colorimetric absorbance of 5-bromo-2-deoxyuridine uptake. SHED from passages 4 to 6 were seeded at 5.0 × 103 cells/well and incubated in 10% FBS α-MEM at 37 °C in a 5% CO2 environment until 60% confluence was reached. The cells were assessed using a cell proliferation ELISA and BrdU colorimetric kit. No significant differences were observed between SHED passages 4 to 6 (n = 6, Tukey’s HSD test, NS).
3.4. Evaluation of Gene Expression of Human Full-Length Amelogenin in SHED During Induction of Bone Differentiation
- (a)
- SHED were seeded at 9.6 × 104 cells/well and cultured in 10% FBS αMEM. Upon reaching 80% confluence, the culture was replaced with osteogenesis induction medium, and amelogenin was added at a concentration of 1000 ng/mL. The expression of osteogenesis-related markers was assessed by quantitative real-time polymerase chain reaction, revealing increased expression of all mRNA levels (n = 3, Welch’s t-test, * p < 0.05). On day 14 after the induction of bone differentiation, a significant increase in RUNX2 gene expression was observed. and on day 18 a significant increase was observed in RUNX2 gene expression (n = 3, Welch’s t-test, * p < 0.05).
- (b)
- On day 18 a significant increase was observed in CBFB gene expression (n = 3, Welch’s t-test, ** p < 0.01).
- (c)
- The mRNA expression levels of COL1 in SHED were upregulated significantly (p < 0.01) by the treatment with amelogenin on day 18 (n = 3, Welch’s t-test, ** p < 0.01).
- (d)
- On day 18 a significant increase was observed in BGLAP gene expression. Moreover, a significant increase in BGLAP gene expression was observed on day 21 after the induction of bone differentiation (n = 3, Welch’s t-test, ** p < 0.01, *** p < 0.001).
- (e)
- On day 14 a significant increase was observed in BMP2 gene expression. Moreover, on day 18 a significant increase was observed in BMP2 gene expression (n = 3, Welch’s t-test, * p < 0.05, *** p < 0.001).
- (f)
- A significant increase in BMP4 gene expression was observed on day 18 after the induction of bone differentiation (n = 3, Welch’s t-test, *** p < 0.001).
- (g)
- The expression of NOTCH1 was significantly enhanced by amelogenin in SHED compared with that in the control groups on day 18 (n = 3, Welch’s t-test, ** p < 0.01).
- (h)
- A significant increase in NOTCH2 gene expression was observed on day 18 after the induction of bone differentiation (n = 3, Welch’s t-test, *** p < 0.001).
- (i)
- The mRNA expression levels of NES in SHED were upregulated significantly (p < 0.001) by the treatment with 1000 ng/mL amelogenin on day 18 (n = 3, Welch’s t-test, *** p < 0.001).
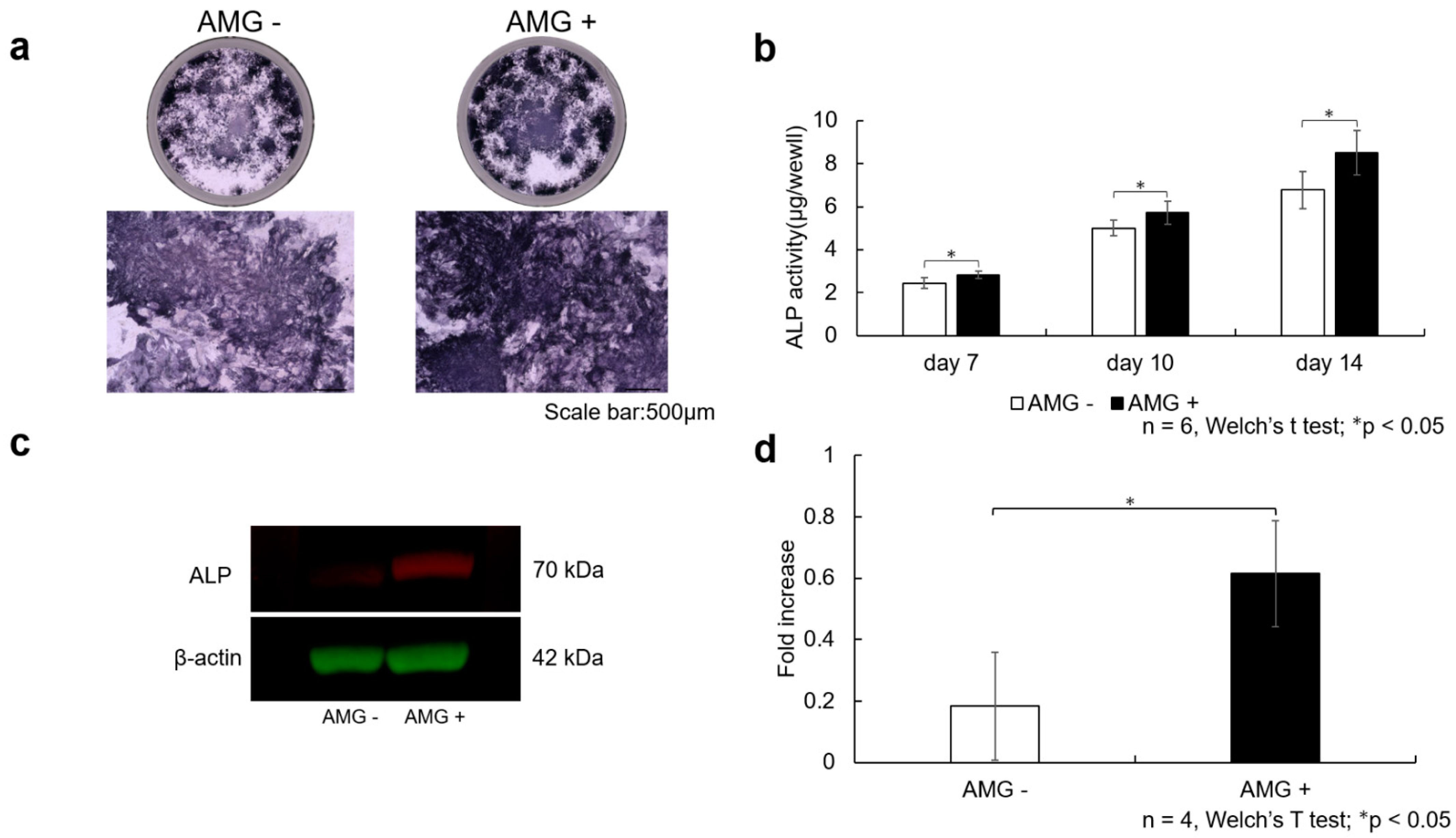
3.5. Evaluation of ALP Staining
3.6. Evaluation of ALP Activity
3.7. Evaluation of Western Blot Analysis
- (a)
- SHED were seeded at 3.8 × 104 cells/well and cultured in 10% FBS αMEM. After reaching 80% confluence, the culture was replaced with osteogenesis induction medium, and amelogenin was added at a concentration of 1000 ng/mL. Scale bar is shown at 500 µm. The amelogenin-treated group demonstrated more enhanced ALP staining than the control group.
- (b)
- ALP activity was also assessed using the pNpp Alkaline Phosphatase Kit, revealing increased ALP activity at 7, 10, and 14 days after the start of osteogenesis. Data are expressed as the absorbance (405 nm). A more significant enhancement in ALP activity was observed in the amelogenin-treated group than in the control group (n = 6, Welch’s t-test; * p < 0.05).
- (c)
- After SHED were stimulated for 14 days in bone differentiation induction medium supplemented with amelogenin (1000 ng/mL), total protein was collected, and Western blot analysis was performed to confirm ALP protein expression. β-actin was used as the loading control. Increased ALP protein levels were observed in the amelogenin-treated group compared with the control group.
- (d)
- The obtained bands were quantified using ImageJ software (n = 4, Welch’s t-test; * p < 0.05).
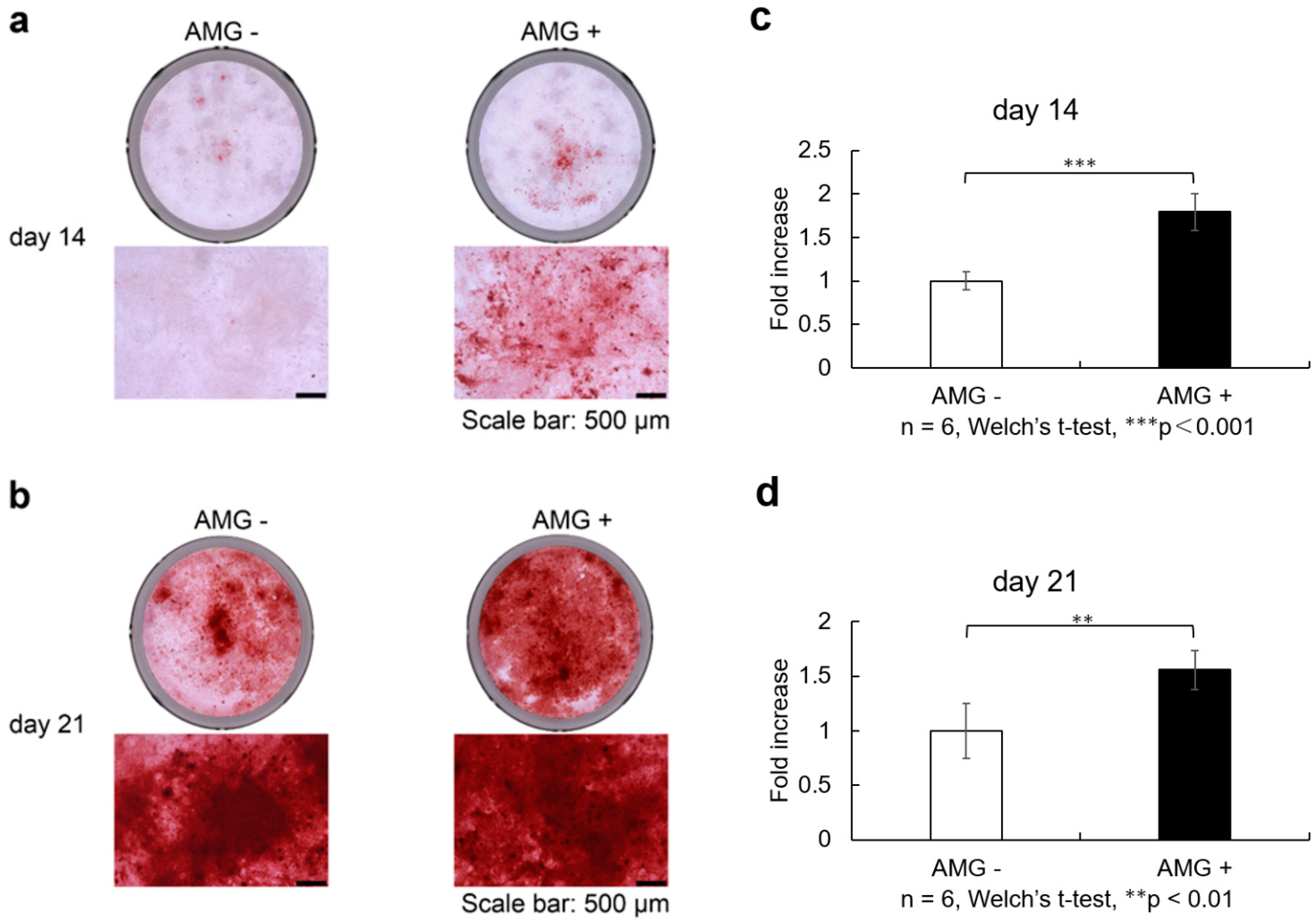
3.8. Evaluation of Alizarin Red S Staining
- (a)
- SHED was seeded and cultured in 10% FBS αMEM. Upon reaching 80% confluence, the culture was replaced with osteogenesis induction medium, and amelogenin was added at a concentration of 1000 ng/ml. Alizarin red S staining was performed at 14 and 21 days of culture in the bone differentiation induction medium. Scale bars are shown at 500 µm. On days 14 after osteoblast differentiation, staining was more enhanced in the amelogenin-treated group than in the control group.
- (b)
- On days 21 after osteoblast differentiation, staining was more enhanced in the amelogenin-treated group than in the control group.
- (c)
- Staining was more enhanced in the amelogenin-treated group than in the control group. Calcification nodule lysate was added, and the absorbance of the resulting coloured material was measured. Calcification was more evident in the amelogenin-treated group than in the control group. (n = 6, Welch’s t-test, *** p < 0.001).
- (d)
- On days 21 after osteoblast differentiation, calcification was more evident in the amelogenin-treated group than in the control group (n = 6, Welch’s t-test, ** p < 0.01).
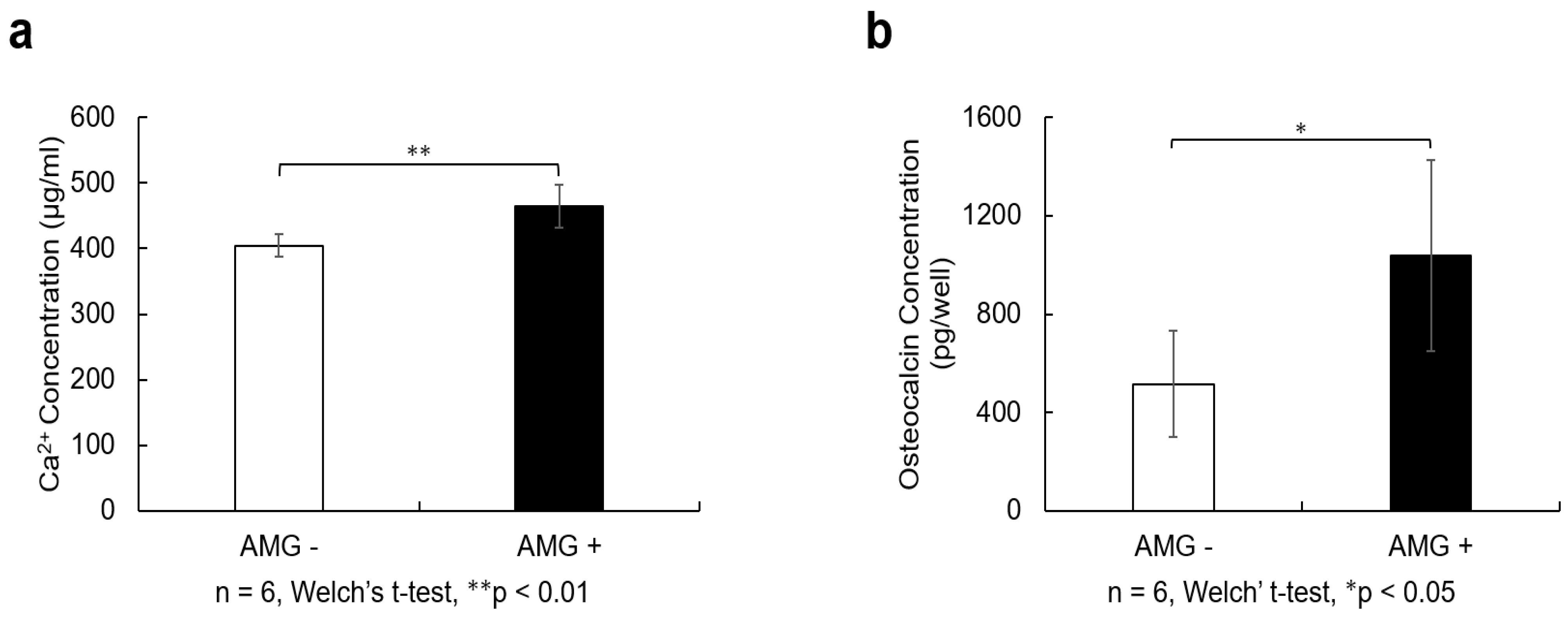
3.9. Evaluation of Calcium Quantification
3.10. Evaluation of Osteocalcin Quantification
- (a)
- SHED were seeded at 9.6 × 104 cells/well and cultured in 10% FBS αMEM. After reaching 80% confluence, the culture was replaced with osteogenesis induction medium, and amelogenin was added at a concentration of 1000 ng/mL. Twenty-one days after the start of osteoblast differentiation induction, 10% formic acid solution was added to cells and shaken at 4 °C for 8 h. The resulting lysate was used for calcium quantification. The amelogenin-treated group showed higher calcium content than the control group (n = 6, Welch’s t-test; ** p < 0.01).
- (b)
- Cell culture supernatants were collected from the cells 21 days after the induction of osteoblast differentiation and centrifuged at 2000× g for 10 min. The supernatant was collected and quantified using an Osteocalcin SimpleStep ELISA® Kit (Abcam, Cambridge, MA, USA). The amelogenin-treated group showed a higher amount of osteocalcin than the control group (n = 6, Welch’s t-test; * p < 0.05).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CLP | Cleft palate |
| MSCs | Mesenchymal stem cells |
| SHED | Stem cells from human exfoliated deciduous teeth |
| EMD | Enamel matrix derivative |
| PBS | Phosphate-buffered saline |
| α-MEM | Minimal essential medium eagle |
| FBS | Fetal bovine serum |
| ALP | Alkaline phosphatase |
| VEGF | Vascular endothelial growth factor |
References
- Schroeder, J.E.; Mosheiff, R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.S.; Lehman, J.A. Evaluation of alveolar bone grafting: A survey of ACPA teams. Cleft Palate Craniofac. J. 2005, 42, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Dissaux, C.; Ruffenach, L.; Bruant-Rodier, C.; George, D.; Bodin, F.; Rémond, Y. Cleft alveolar bone graft materials: Literature review. Cleft Palate Craniofac. J. 2022, 59, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Tournier, P.; Guicheux, J.; Paré, A.; Veziers, J.; Barbeito, A.; Bardonnet, R.; Corre, P.; Geoffroy, V.; Weiss, P.; Gaudin, A. An extrudable partially demineralized allogeneic bone paste exhibits a similar bone healing capacity as the “gold standard” bone graft. Front. Bioeng. Biotechnol. 2021, 9, 658853. [Google Scholar] [CrossRef]
- Raposo-Amaral, C.A.; Denadai, R.; Chammas, D.Z.; Marques, F.F.; Pinho, A.S.; Roberto, W.M.; Buzzo, C.L.; Raposo-Amaral, C.E. Cleft patient-reported postoperative donor site pain following alveolar autologous iliac crest bone grafting: Comparing two minimally invasive harvesting techniques. J. Craniofac. Surg. 2015, 26, 2099–2103. [Google Scholar] [CrossRef]
- Faour, O.; Dimitriou, R.; Cousins, C.A.; Giannoudis, P.V. The use of bone graft substitutes in large cancellous voids: Any specific needs? Injury 2011, 42 (Suppl. S2), S87–S90. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in growth factor delivery for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Seong, J.M.; Kim, B.C.; Park, J.H.; Kwon, I.K.; Mantalaris, A.; Hwang, Y.S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001. [Google Scholar] [CrossRef]
- Zha, K.; Tian, Y.; Panayi, A.C.; Mi, B.; Liu, G. Recent advances in enhancement strategies for osteogenic differentiation of mesenchymal stem cells in bone tissue engineering. Front. Cell Dev. Biol. 2022, 10, 824812. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Gopal, S.; Masood, H.; Vivek, P.; Deb, K. Regenerative potential of dental pulp mesenchymal stem cells harvested from high caries patient’s teeth. J. Stem Cells 2013, 8, 25–41. [Google Scholar] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Hiraki, T.; Rikitake, K.; Tsuka, Y.; Abe, T.; Tanimoto, K. Success rates in isolating mesenchymal stem cells from permanent and deciduous teeth. Sci. Rep. 2019, 9, 16764. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Xia, Q.; Meng, M.; Ye, Z.; Tang, Z.; Feng, H.; Chen, X.; Chen, H.; Zeng, X.; et al. Enamel matrix derivative (EMD) enhances the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs). Bioengineered 2021, 12, 7033–7045. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Gronthos, S.; Menicanin, D.; Marino, V.; Bartold, P.M. Effect of coating Straumann Bone Ceramic with Emdogain on mesenchymal stromal cell hard tissue formation. Clin. Oral. Investig. 2012, 16, 867–878. [Google Scholar] [CrossRef]
- Sculean, A.; Windisch, P.; Döri, F.; Keglevich, T.; Molnár, B.; Gera, I. Emdogain in regenerative periodontal therapy. A review of the literature. Fogorv. Sz. 2007, 100, 211–219. [Google Scholar]
- Tanimoto, K.; Huang, Y.C.; Tanne, Y.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Ozaki, N.; Sasamoto, T.; Yoshimi, Y.; Kato, Y.; et al. Amelogenin enhances the osteogenic differentiation of mesenchymal stem cells derived from bone marrow. Cells Tissues Organs 2012, 196, 411–419. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Vesper, K.; Hyzy, S.L.; Almaguer-Flores, A.; Boyan, B.D.; Schwartz, Z. Role of the N-terminal peptide of amelogenin on osteoblastic differentiation of human mesenchymal stem cells. Eur. Cells Mater. 2014, 28, 1–10; discussion 10. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Ando, K.; Hirose, N.; Tanne, Y.; Sumi, K.; Tanimoto, K. The C-terminus of the amelogenin peptide influences the proliferation of human bone marrow mesenchymal stem cells. J. Periodontol. 2018, 89, 496–505. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tanimoto, K.; Tanne, Y.; Kamiya, T.; Kunimatsu, R.; Michida, M.; Yoshioka, M.; Yoshimi, Y.; Kato, Y.; Tanne, K. Effects of human full-length amelogenin on the proliferation of human mesenchymal stem cells derived from bone marrow. Cell Tissue Res. 2010, 342, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, K.M.M.; Zhai, H.; Zhu, L.; Horst, J.A.; Sitlin, M.; Nguyen, M.; Wagner, M.; Simpliciano, C.; Milder, M.; Chen, C.-L.; et al. Amyloid-like ribbons of amelogenins in enamel mineralization. Sci. Rep. 2016, 6, 23105. [Google Scholar] [CrossRef] [PubMed]
- Vowden, P.; Romanelli, M.; Peter, R.; Boström, A.; Josefsson, A.; Stege, H. The effect of amelogenins (Xelma) on hard-to-heal venous leg ulcers. Wound Repair. Regen. 2006, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Hiraki, T.; Abe, T.; Tsuka, Y.; Hiraki, A.; Tanimoto, K. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Rikitake, K.; Yoshimi, Y.; Putranti, N.A.R.; Hayashi, Y.; Tanimoto, K. Bone Differentiation Ability of CD146-Positive Stem Cells from Human Exfoliated Deciduous Teeth. Int. J. Mol. Sci. 2023, 24, 4048. [Google Scholar] [CrossRef]
- Keating, J.F.; Simpson, A.H.R.W.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38 (Suppl. S1), S75–S80. [Google Scholar] [CrossRef]
- Al-Ahmady, H.H.; Abd Elazeem, A.F.; Bellah Ahmed, N.E.M.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with nano hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Craniomaxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef]
- Pradel, W.; Lauer, G. Tissue-engineered bone grafts for osteoplasty in patients with cleft alveolus. Ann. Anat. 2012, 194, 545–548. [Google Scholar] [CrossRef]
- Neuhuber, B.; Swanger, S.A.; Howard, L.; Mackay, A.; Fischer, I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp. Hematol. 2008, 36, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Briquet, A.; Dubois, S.; Bekaert, S.; Dolhet, M.; Beguin, Y.; Gothot, A. Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages. Haematologica 2010, 95, 47–56. [Google Scholar] [CrossRef]
- Hu, J.C.C.; Chan, H.C.; Simmer, S.G.; Seymen, F.; Richardson, A.S.; Hu, Y.; Milkovich, R.N.; Estrella, N.M.; Yildirim, M.; Bayram, M.; et al. Amelogenesis imperfecta in two families with defined AMELX deletions in ARHGAP6. PLoS ONE 2012, 7, e52052. [Google Scholar] [CrossRef]
- Oliveira, F.V.; Dionísio, T.J.; Neves, L.T.; Machado, M.A.A.M.; Santos, C.F.; Oliveira, T.M. Amelogenin gene influence on enamel defects of cleft lip and palate patients. Braz. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Bai, Y.; Li, P.; Yin, G.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y. BMP-2, VEGF and bFGF synergistically promote the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Biotechnol. Lett. 2013, 35, 301–308. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef]
- Dreyer, C.H.; Kjaergaard, K.; Ding, M.; Qin, L. Vascular endothelial growth factor for in vivo bone formation: A systematic review. J. Orthop. Transl. 2020, 24, 46–57. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, Y.H.; Li, J.C.; Li, Q.; Ye, D.; Zhang, X.X.; Li, J.T. Vascular endothelial growth factor protein and gene delivery by novel nanomaterials for promoting liver regeneration after partial hepatectomy. World J. Gastroenterol. 2023, 29, 3748–3757. [Google Scholar] [CrossRef]
- Eckardt, H.; Ding, M.; Lind, M.; Hansen, E.S.; Christensen, K.S.; Hvid, I. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J. Bone Jt. Surg. Br. 2005, 87, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Rong, S.; Zhongchen, S.; Lan, C. Human amelogenin up-regulates osteogenic gene expression in human bone marrow stroma cells. Biochem. Biophys. Res. Commun. 2011, 408, 437–441. [Google Scholar] [CrossRef]
- Hsia, T.L.; Lin, Z.; Xia, Y.; Shu, R.; Xie, Y. A photoresponsive recombinant human amelogenin-loaded hyaluronic acid hydrogel promotes bone regeneration. J. Periodontal Res. 2024, 59, 589–598. [Google Scholar] [CrossRef]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef]
- Komori, T. Requisite roles of Runx2 and Cbfb in skeletal development. J. Bone Miner. Metab. 2003, 21, 193–197. [Google Scholar] [CrossRef]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Majidinia, M.; Alizadeh, E.; Yousefi, B.; Akbarzadeh, M.; Mihanfar, A.; Rahmati-Yamchi, M.; Zarghami, N. Co-inhibition of Notch and NF-κB signaling pathway decreases proliferation through downregulating IκB-α and Hes-1 expression in human ovarian cancer OVCAR-3 cells. Drug Res. 2017, 67, 13–19. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, G.; Qiu, S.B.; He, F.; Tan, Y.H.; Chen, Q. Effect of Notch ligand Delta1-RNA interference by lentivirus on proliferation and differentiation of human dental pulp stem cells. Zhonghua Kou Qiang Yi Xue Za Zhi 2011, 46, 730–734. [Google Scholar]
- Sawangmake, C.; Nowwarote, N.; Pavasant, P.; Chansiripornchai, P.; Osathanon, T. A feasibility study of an in vitro differentiation potential toward insulin-producing cells by dental tissue-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 452, 581–587. [Google Scholar] [CrossRef]
- Zhu, F.; Sweetwyne, M.T.; Hankenson, K.D. PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells 2013, 31, 1181–1192. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Roméas, A.; Lendahl, U.; Sharpe, P.T.; Farges, J.C. Notch2 protein distribution in human teeth under normal and pathological conditions. Exp. Cell Res. 2003, 282, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, S.C.; Tong, J.; Hu, Y.; Zhang, Y.Q.; Yu, Q. Activation and dynamic expression of Notch signalling in dental pulp cells after injury in vitro and in vivo. Int. Endod. J. 2016, 49, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Smith, A.J.; Lesot, H. Designing new treatment strategies in vital pulp therapy. J. Dent. 2000, 28, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Nakatomi, M.; Ohshima, H. Expression patterns of nestin and dentin sialoprotein during dentinogenesis in mice. Biomed. Res. 2012, 33, 119–132. [Google Scholar] [CrossRef]
- Bahammam, L.A.; Alsharqawi, W.; Bahammam, H.A.; Mounir, M. Histological evaluation of pulpal response and dentin bridge formation after direct pulp capping using recombinant amelogenin and mineral trioxide aggregate (MTA). Curēus 2024, 16, e54560. [Google Scholar] [CrossRef]
- Peng, X.; Han, S.; Wang, K.; Ding, L.; Liu, Z.; Zhang, L. Evaluating the potential of an amelogenin-derived peptide in tertiary dentin formation. Regen. Biomater. 2021, 8, rbab004. [Google Scholar] [CrossRef]
- Wang, W.; Han, Z.C. Heterogeneity of human mesenchymal stromal/stem cells. Adv. Exp. Med. Biol. 2019, 1123, 165–177. [Google Scholar] [CrossRef]
- Johnson, K.W.; Dooner, M.; Quesenberry, P.J. Fluorescence activated cell sorting: A window on the stem cell. Curr. Pharm. Biotechnol. 2007, 8, 133–139. [Google Scholar] [CrossRef]

| Gene | Sequence (5′ → 3′) | |
|---|---|---|
| GAPDH | Forward | ATG GCC TTC CGT GTT CCT |
| Reverse | CCC AAG ATG CCC TTC AGT | |
| RUNX2 | Forward | CAC TGG CGC TGC AAC AAG A |
| Reverse | CAT TCC GGA GCT CAG CAG AAT AA | |
| CBFB | Forward | AGA AGC AAG TTC GAG AAC GAG |
| Reverse | CCT GAA GCC CGT GTA CTT AAT CT | |
| COL1 | Forward | CCC GGG TTT CAG AGA CAA CTT C |
| Reverse | TCC ACA TGC TTT ATT CCA GCA ATC | |
| BMP2 | Forward | CTG GCT GAT CAT CTG AAC TCC ACT A |
| Reverse | TCG GGA CAC AGC ATG CCT TA | |
| BMP4 | Forward | AGA TCC ACA GCA CTG GTC TTG AGT A |
| Reverse | TCT CAG GGA TGC TGC TGA GG | |
| BGLAP | Forward | GAC TGT GAC GAG TTG GCT GA |
| Reverse | GAA GAG GAA AGA AGG GTG CC | |
| NOTCH1 | Forward | GAG GCG TGG CAG ACT ATG C |
| Reverse | CTT GTA CTC CGT CAG CGT GA | |
| NOTCH2 | Forward | CAA CCG CAA TGG AGG CTA TG |
| Reverse | GCG AAG GCA CAA TCA TCA ATG TT | |
| NES | Forward | CAA CAG CGA CGG AGG TCT C |
| Reverse | GCC TCT ACG CTC TCT TCT TTG A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirabae, A.; Kunimatsu, R.; Yoshimi, Y.; Rikitake, K.; Ogashira, S.; Nakatani, A.; Sakata, S.; Tanimoto, K. Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED. Cells 2025, 14, 657. https://doi.org/10.3390/cells14090657
Hirabae A, Kunimatsu R, Yoshimi Y, Rikitake K, Ogashira S, Nakatani A, Sakata S, Tanimoto K. Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED. Cells. 2025; 14(9):657. https://doi.org/10.3390/cells14090657
Chicago/Turabian StyleHirabae, Akira, Ryo Kunimatsu, Yuki Yoshimi, Kodai Rikitake, Shintaro Ogashira, Ayaka Nakatani, Shuzo Sakata, and Kotaro Tanimoto. 2025. "Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED" Cells 14, no. 9: 657. https://doi.org/10.3390/cells14090657
APA StyleHirabae, A., Kunimatsu, R., Yoshimi, Y., Rikitake, K., Ogashira, S., Nakatani, A., Sakata, S., & Tanimoto, K. (2025). Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED. Cells, 14(9), 657. https://doi.org/10.3390/cells14090657






