Abstract
Alveolar macrophages (AMs) are immune cells located in the alveoli—the tiny air sacs in the lungs where gas exchange occurs. Their functions are regulated by various epigenetic mechanisms, which are essential for both healthy lung function and disease development. In the lung’s microenvironment, AMs play critical roles in immune surveillance, pathogen clearance, and tissue repair. This review examines how epigenetic regulation influences AM functions and their involvement in lung diseases. Key mechanisms, such as DNA methylation, histone modifications, and non-coding RNAs, regulate gene expression in response to environmental signals. In healthy lungs, these modifications enable AMs to quickly respond to inhaled threats. However, when these processes malfunction, they could contribute to diseases such as pulmonary fibrosis, COPD, and pulmonary hypertension. By exploring how epigenetic changes affect AM polarization, plasticity, and immune responses, we can gain deeper insights into their role in lung diseases and open new avenues for treating and preventing respiratory conditions. Ultimately, understanding the epigenetic mechanisms within AMs enhances our knowledge of lung immunology and offers potential for innovative interventions to restore lung health and prevent respiratory diseases.
1. Introduction
Macrophages are essential components of the innate immune system, functioning as phagocytes that utilize pattern recognition receptors to detect and eliminate pathogens and debris [1,2]. In the lungs, two primary populations of macrophages exist: alveolar resident macrophages (AMs) and interstitial macrophages (IMs) [3]. AMs are mainly located in the alveoli, where they serve as the first line of defense, protecting the respiratory system from inhaled pathogens and particles to maintain lung homeostasis [4,5]. In contrast, IMs reside in the tissue surrounding the alveoli, playing a critical role in tissue repair during injury, infection, or inflammation [6,7] (Figure 1). These macrophages can adopt either pro-inflammatory or anti-inflammatory phenotypes depending on the signals they receive, which is reflected in the expression of markers such as CD11c, CD11b, and various colony-stimulating factor receptor subtypes [8].
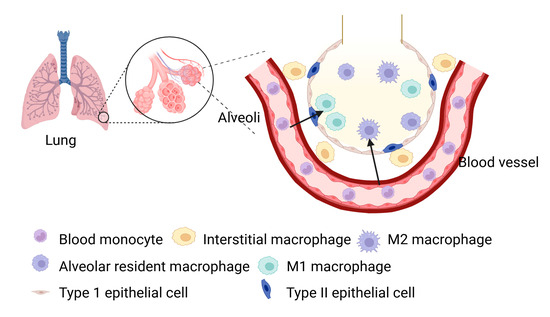
Figure 1.
Anatomic localization of various macrophages within the alveoli of normal lung.
2. Alveolar Macrophages in Mice
During embryonic development, resident AMs originate from the yolk sac (YS) and fetal liver (FL), establishing a population of self-renewing cells [9,10]. In contrast, IMs have a more complex origin, arising from both YS-derived macrophages and bone marrow (BM)-derived monocytes. The development of resident AMs in mice is a fascinating process that occurs in multiple stages, spanning both embryonic and postnatal phases. During early embryogenesis, around embryonic day 9.5, YS-derived macrophages are among the first immune cells to colonize the developing lungs [11,12]. These macrophages play a critical role in the early establishment of the immune system within the lung tissue. As development progresses, around embryonic day 12.5, fetal liver-derived monocytes contribute to the growing AM population [13] (Figure 2). These monocytes migrate to the lungs, where they differentiate into mature AMs. The coordination of various signaling pathways and transcription factors, such as PU.1, is crucial in guiding these developmental processes [8,14]. After birth, the lungs continue to undergo significant changes, and the AM population becomes more firmly established. By approximately postnatal day 10, a well-defined and functional AM population emerges from both YS and FL precursors. The migration, seeding, and differentiation of these macrophages are primarily regulated by the colony-stimulating factor 1 receptor (CSF-1R) and sustained through the recruitment of circulating monocytes via the CCL2/CCR2 axis [15]. These macrophages exhibit a remarkable capacity for replenishment by circulating monocytes and are categorized into three distinct subpopulations: IM1, IM2, and IM3, based on specific surface markers [10,16,17]. Once established, AMs play an essential role in immune surveillance within the lung tissue [12].
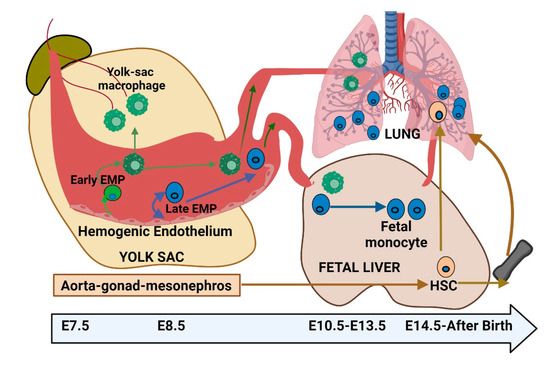
Figure 2.
Embryonic development of lung alveolar macrophages—insights into origin and differentiation.
3. Alveolar Macrophages in Humans
Unlike in mice, understanding the origin and development of human lung AMs, particularly in the context of lung diseases, is limited due to the impracticality of invasive in vivo experiments. However, valuable insights have emerged from studies involving BM and lung transplantations. Allogeneic BM transplantation depletes host AMs and results in rapid turnover and mixed host–donor chimerism, highlighting the role of donor hematopoiesis in replenishing AMs. In contrast, lung transplantation preserves lung macrophages, allowing donor-derived AMs in persistence [12,18]. The varying extent of donor AM persistence suggests that human AMs are primarily maintained through local self-renewal in the steady-state lung. During lung injury with macrophage depletion, monocytes replace resident AMs. The presence of AMs in certain leukemias, despite depleted blood monocytes, suggests that AMs may be independent of circulating monocytes under steady-state conditions [12]. However, the embryonic origin of human AMs in the developing lung remains uncertain, and the specific progenitors responsible for their development, whether they are embryonic or adult, are still elusive.
All lung macrophages, as essential components of the pulmonary immune system, undergo complex epigenetic changes that carefully regulate their functions in both health and disease [19,20]. Epigenetics plays a critical role in shaping the behavior of lung macrophages, which display remarkable plasticity, allowing them to adapt their responses to a variety of environmental cues and precisely adjust their epigenetic marks [20,21,22]. This review explores the complex role of epigenetics in regulating gene expression, plasticity, and adaptability in AMs, highlighting how mechanisms such as DNA methylation, histone modifications, non-coding RNAs, and epigenetic inheritance are crucial for modulating AM responses to environmental signals, both in normal lung homeostasis and disease states. By examining current research, it also aims to provide a comprehensive overview of how these mechanisms influence AM function and identify potential therapeutic strategies for manipulating AM activity in lung diseases.
4. Epigenetic Modification in Lung Macrophages
Epigenetics controls gene expression without altering the DNA sequence, influencing cell identity through chromatin structure and DNA accessibility [23,24,25]. While once considered stable, epigenetic markers are now known to change in response to development and environmental signals [26]. These changes involve mechanisms like DNA methylation, histone modifications, and non-coding RNAs, all of which regulate gene expression by affecting protein–DNA interactions and mRNA stability.
4.1. DNA Methylation
DNA methylation is a crucial epigenetic modification that regulates gene expression in lung macrophages [27], influencing their immune roles [28,29]. This process involves adding methyl groups to specific cytosine residues in DNA, typically at CpG dinucleotides. DNA methylation is catalyzed by DNA methyltransferases (Dnmts), including Dnmt1, Dnmt2, Dnmt3a, Dnmt3b, and Dnmt3l [30,31]. Additionally, the methylated cytosine can be converted to 5-hydroxymethylcytosine by Tet methylcytosine dioxygenases (Tets), such as Tet1, Tet2, and Tet3 (Figure 3). The primary function of DNA methylation in lung macrophages is to fine-tune their response to various stimuli, crucially regulating immune functions [32]. Typically, DNA methylation leads to gene repression by blocking transcription factor binding and RNA polymerase activity when a methyl group is added to a CpG site in the gene’s promoter region [33]. This inhibition is essential for silencing genes that are not needed under certain conditions [34]. During infection, immune genes may undergo demethylation, enabling their rapid expression and enhancing the immune response [29]. Conversely, during immune quiescence, these genes may be remethylated to maintain their silenced state [35].
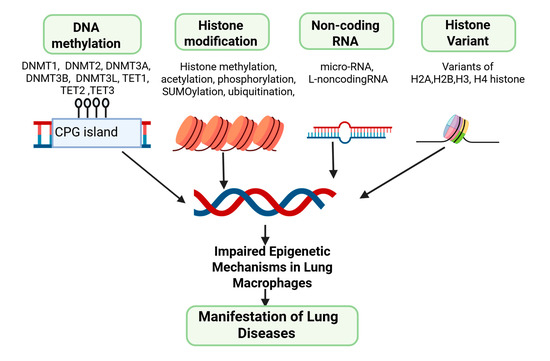
Figure 3.
Illustrative role of epigenetic mechanisms within lung macrophages for manifesting lung disease.
Furthermore, DNA methylation provides lung macrophages with a form of epigenetic memory, marking previous exposures to pathogens or environmental factors [30,36]. This memory shapes their response when encountering the same stimulus again, allowing for a more effective reaction to recurring infections [30]. External factors such as diet, pollutants, and oxidative stress can alter DNA methylation patterns in lung macrophages [37]. As macrophages age, their DNA methylation patterns shift, impacting their functions and response to infections and diseases [38]. These age-related changes are particularly significant in conditions like idiopathic pulmonary fibrosis (IPF), where aging is a known risk factor [38]. Alterations in DNA methylation at cytosine–guanine dinucleotides affect chromatin accessibility, transcription factor binding, and gene expression in AMs. As one of the most stable epigenetic markers, DNA methylation serves as a measurable indicator of how development and disease influence the epigenome.
In lung macrophages, DNA methylation is modified in chronic airway diseases, driven by both genetic and environmental factors [39]. An extensive epigenome-wide analysis has identified 95 differentially methylated CpGs in lung macrophages across different lung regions, from the upper to lower lobes [28]. This finding suggests that regional variations in macrophage metabolism may contribute to distinct immune responses in different parts of the lung [28,34]. The anatomical differences in the lung influencing DNA methylation patterns in AMs highlights the role of the lung microenvironment in shaping AM development [29]. Understanding these epigenetic processes is crucial for advancing lung immunology and developing targeted treatments for lung diseases. However, despite recent progress in understanding the ontogeny of lung macrophages, our knowledge of the epigenetic mechanisms driving the transition from monocytes to macrophages in the lung and how diseases impact these processes remains limited.
4.2. Histone Modification
Histone modifications involve a class of proteins that play a fundamental role in the packaging of DNA into chromatin and the regulation of gene expression [40]. These processes are crucial epigenetic mechanisms that influence the function and responses of lung macrophages [41]. Histones play a central role in packaging DNA into chromatin and regulating gene expression [40,42] (Figure 3). Modifications of histones including methylation, acetylation, phosphorylation, ubiquitylation, and sumoylation involve covalent changes to histone proteins, significantly affecting gene expression. By altering the chromatin structure and interacting with histone modifiers, these modifications affect various biological processes.
Histone acetylation and deacetylation are vital processes in gene regulation, typically catalyzed by enzymes with histone acetyltransferase (HAT) or histone deacetylase (HDAC) activity. Acetylation involves the transfer of an acetyl group from acetyl coenzyme A to a target molecule, while deacetylation is the reverse process, removing the acetyl group [43]. These modifications are particularly important in macrophages, where specific histone methylation patterns help prevent excessive inflammation by restricting access to pro-inflammatory genes. In general, histone H3 can be acetylated at lys 9, 14, 18, 23, and 56, methylated at arginine 2 and lys 4, 9, 27, 36, and 79, and phosphorylated at ser 10, 28, and Thr 3, 11. Similarly, histone H4 undergoes acetylation at lys 5, 8, 12, and 16, methylation at arginine 3 and lys20, and phosphorylation at Ser 141 and 142. These modifications are essential for regulating transcription, chromatin packaging, and DNA repair. In lung macrophages, these modifications play a pivotal role in activating immune response genes when encountering pathogens [44]. For instance, histone acetylation is associated with enhanced gene expression [45]. Conversely, histone methylation can lead to chromatin condensation, suppressing gene expression [44].
Among the various HDAC enzymes, HDAC3 deficiency in AMs leads to severe mitochondrial oxidative dysfunction and cell death by impairing Pparγ expression, including its downstream signaling pathways [46]. Interestingly, HDAC3-deficient macrophages alleviate LPS-induced acute lung injury [46] and reduce pyroptosis in murine lung tissues by decreasing histone acetylation. These findings suggest that histone modifications play a significant role in lung injury [42,46,47,48,49]. In summary, histone modifications play a pivotal role in governing the function and responses of AMs, with far-reaching implications for lung health and disease susceptibility. Understanding these mechanisms is essential for not only advancing our knowledge of lung immunology but also developing targeted interventions for respiratory conditions.
4.3. Non-Coding RNAs
Non-coding RNAs (ncRNAs) are microRNAs and long non-coding RNAs (lncRNAs) that modulate gene expression by interacting with the epigenetic machinery [50,51]. NcRNAs are involved in regulating gene expression and chromatin structure, thereby impacting the functions and responses of these immune cells [50,52]. Additionally, emerging evidence suggests that lncRNAs play a role in regulating macrophage polarization, exerting consequential effects on the manifestation of respiratory diseases [53,54]. MicroRNAs are short RNA molecules that bind to mRNAs, leading to mRNA degradation or translational repression [55]. In AMs, specific microRNAs including miRNA-124, miRNA-155, and miRNA-223 can target genes involved in immune responses and inflammation, effectively shaping the immune function of these cells [56]. Moreover, these microRNAs play a crucial role in regulating lung inflammation and directing macrophage polarization [57,58]. Elevated expression of miRNA-124 suppresses M1 macrophage activity, whereas miRNA-155 promotes M1 polarization. Conversely, depletion of miRNA-223 leads to M1 polarization [59]. Certainly, the nuanced regulatory role of microRNAs is evident in both lung inflammation and macrophage functions.
Likewise, lncRNAs are longer RNA molecules that interact with chromatin and epigenetic modifiers, affecting chromatin structure and gene accessibility [60]. They either enhance or repress gene expression, depending on the context. In AMs, lncRNAs may be involved in regulating immune genes and determining the macrophage’s regulatory response to various stimuli. These non-coding RNAs also contribute to epigenetic memory in AMs [53] and exert a lasting impact on the cell’s ability to respond to recurring challenges by influencing the expression of genes involved in immune responses [54] (Figure 3). Understanding the role of non-coding RNAs in AMs, epigenetic regulation is essential for unraveling the intricacies of lung immunology and developing targeted interventions for respiratory conditions.
4.4. Histone Variants
Histone variants are specialized variants of histone proteins with unique amino acid sequences, playing integral roles in orchestrating chromatin structure and gene expression. Unlike the canonical histones, these specialized histone proteins are incorporated into nucleosomes by replacing canonical histones and contribute to the structural and functional diversity of chromatin.
Histones are categorized into two primary groups: canonical histones and histone variants [61]. Canonical histones are the core histones that form the structural backbone of the nucleosome, which is the basic repeating unit of chromatin. There are five major types of core canonical histones notably known for their individual function, including histone 2A (H2A) for stabilizing the nucleosome structure, histone 2B (H2B) for nucleosome stability, centrally located histone 3 (H3) for regulating gene expression through post-translation modification, and histone 4 (H4) for interacting with H3-mediated stabilization in the nucleosome structure, including its role in various post-translational modifications [61,62]. H2A, H2B, H3, and H4 form the nucleosome octamer while canonical linker histone 1 (H1) binds with the DNA between nucleosomes, promoting higher-order chromatin structure.
Among these variants, several have distinct isoforms, each with specific functions. In the H2A family, H2A.Z encompasses H2A.Z.1 and H2A.Z.2, both associated with gene activation by promoting an open chromatin structure. Meanwhile, H2A.X includes H2A.X.1 and H2A.X.2, critical for DNA repair by becoming phosphorylated at DNA double-strand breaks [62,63]. In the H2B category, H2B.Z.1 and H2B.Z.2 are associated with active gene transcription and nucleosome stability. Among the H3 variants, H3.3 comprises H3.3A and H3.3B, linked to gene regulation and epigenetic memory [61,62,63]. CENP-A, another H3 variant, has multiple isoforms crucial for centromere formation [64]. In the H4 family, various isoforms of H4K20me1 are involved in gene silencing and heterochromatin formation. Each of these histone variants and their isoforms adds depth to the regulation of chromatin structure and gene expression, playing vital roles in various biological processes and genomic stability [63] (Figure 3). Nevertheless, a defined role for any histone variant specifically designed to comprehend its impact on AMs has not been established. Our current research is directed towards uncovering this aspect. Hence, understanding the complexities of these histone variants and their isoforms is essential for unraveling the intricacies of epigenetic regulation in AMs.
Overall, the interconnectedness of epigenetic mechanisms in AMs reveals a sophisticated regulatory network that enables these immune cells to finely tune their responses to the constantly changing lung environment. A foundational aspect of this integration lies in the interplay between DNA methylation and histone modifications [26,65,66]. DNA methylation, often associated with stable gene silencing, can act for specific protein complexes; for instance, MBD proteins not only recognize methylated DNA but also recruit histone-modifying enzymes such as HDACs, thereby linking DNA methylation to chromatin compaction and transcriptional repression [67,68]. Conversely, certain histone modifications can influence DNA methylation. For example, H3K4me3, commonly found at actively transcribed gene promoters, can inhibit the binding and activity of DNMTs, maintaining these regions in a transcriptionally permissive state [69]. Likewise, the co-occurrence of histone acetylation, typically linked to gene activation, with certain histone methylation marks can establish a unique chromatin environment that either enhances or represses gene expression, depending on the precise modifications and their genomic context [70]. Adding further nuance is the emerging field of metabolic epigenetics, which highlights how fluctuations in cellular metabolism directly impact epigenetic enzyme activity. Metabolites such as S-adenosylmethionine (SAM), derived from methionine metabolism, serve as essential methyl donors for DNMTs and some HMTs, meaning changes in methionine availability can significantly alter methylation landscapes [71]. Similarly, acetyl-CoA, a central metabolite in energy production, provides the acetyl group for HATs, so shifts in glucose and fatty acid metabolism can dynamically influence histone acetylation and gene expression [72].
These interconnected mechanisms become particularly relevant in various disease states [73], including pulmonary fibrosis [65]. In AMs, when transitioning toward a profibrotic (M2-like) phenotype, aberrant DNA methylation patterns may silence genes involved in inflammation resolution or extracellular matrix (ECM) degradation while simultaneously fostering a chromatin landscape that is receptive to activating epigenetic marks [74]. Moreover, the heightened glycolytic metabolism characteristic of pro-inflammatory and profibrotic macrophages leads to increased production of lactate, which serves as the substrate for histone lactylation [75,76], a recently discovered modification that promotes the expression of genes implicated in ECM production and inflammatory signaling. It is plausible that specific DNA methylation patterns may prime certain genomic regions for activation by lactylation, or that lactylation itself stabilizes transcriptional programs initiated by DNA methylation changes. Understanding these synergistic interactions, where metabolic reprogramming fuels epigenetic modifications that reinforce pathological phenotypes, offers a promising avenue for therapeutic intervention. By targeting and disrupting these interconnected pathways, it may be possible to reprogram AMs toward a more inflammation-resolving, fibrosis-limiting phenotype, offering potential treatments for pulmonary fibrosis and other lung diseases.
5. Epigenetic Regulation of Macrophages in Lung Diseases
Macrophage expansion and repopulation in response to injury, infection, or inflammation are tightly regulated and depend on the nature of the stimulus [3]. During acute inflammation or severe infection, AMs are temporarily lost [77]. For example, in mice, sterile inflammation induces the transient loss of AMs, followed by the expansion of IMs within lung tissue [78]. This IM expansion is blocked in the absence of CCR2, emphasizing the importance of monocyte recruitment in this process. In contrast, during the resolution of inflammation, the repopulation of AMs primarily depends on local proliferation [3]. However, in more severe inflammatory conditions, such as lung fibrosis, resident AMs are often replaced by monocyte-derived macrophages [6]. It remains unclear whether this replacement is due to severe inflammation affecting the self-renewal of AMs or is driven by structural changes. Furthermore, classical monocytes can migrate into the airways in response to injury or infection [3,21]. There is also evidence suggesting that monocyte-derived macrophages in the interstitial space can migrate to the airways. However, the extent to which these alternative differentiation pathways influence the fate and function of macrophages remains uncertain, highlighting the need for further research into the complex dynamics of macrophage responses in different inflammatory contexts.
Resident AMs showed remarkable plasticity, shifting from pro-inflammatory (M1) to anti-inflammatory/tissue-repair (M2) phenotypes, and they are largely controlled by epigenetic mechanisms that alter gene expression without changing the DNA sequence [47,77,79,80]. These epigenetic changes in lung macrophages modulate inflammation, immune tolerance, tissue remodeling, and disease progression in various lung diseases [47,80]. Understanding this process sheds light on how the epigenetic regulation of AMs contributes to the pathogenesis of conditions such as pulmonary fibrosis, chronic obstructive pulmonary disease, lung cancer, and other respiratory disease conditions.
5.1. Lung Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease characterized by the scarring of lung tissue, which leads to impaired lung function [81]. Although the exact cause of IPF is unknown, factors such as genetics, environmental exposures, and abnormal immune responses are believed to contribute to its development [82,83]. Additionally, macrophages play a key role in IPF by becoming activated in response to lung injury. Once activated, they adopt profibrotic functions by secreting growth factors (such as TGF-β and PDGF), proteases, and chemokines that recruit fibroblasts and induce extracellular matrix deposition, contributing to lung scarring [84,85]. Their involvement in pulmonary fibrosis includes the depletion of resident AMs, increase in IMs, and initiation and sustainment of the inflammatory response within lung tissue [86,87]. Indeed, resident AMs participate in resolving inflammation and aiding tissue repair through the phagocytosis of cellular debris and apoptotic cells in response to lung injury [87,88]. Dysregulated macrophage activity, along with their interactions with fibroblasts, exacerbates chronic inflammation and excessive tissue repair, both of which drive disease progression.
5.1.1. DNA Methylation
Recent high-resolution studies have mapped the DNA methylome of AMs in IPF patients [29]. McErlean et al. demonstrated that the AMs of IPF patients exhibit distinct methylation signatures compared with healthy controls, suggesting widespread epigenetic reprogramming during fibrosis [29,32]. Many of the methylated altered CpG sites in IPF AMs are in intronic or intergenic regions associated with open chromatin. This suggests a functional link between methylation changes and gene regulation [26]. Interestingly, while IPF is an age-related disease and global DNA methylation generally correlates with age [89,90], IPF AMs did not show a methylation pattern like accelerated aging [29]. Instead, specific pathways were affected, with IPF AMs including differential methylation in genes related to lipid and glucose metabolism [29,32]. This aligns with the concept of macrophage immunometabolism in fibrosis, where changes in the methylation of key metabolic regulators can push AMs toward a profibrotic metabolic state, such as Warburg-like glycolysis, which supports fibrotic activation [29]. Overall, DNA methylation profiles contribute to the altered phenotype of AMs in fibrosis.
Macrophages display diverse phenotypes in pulmonary fibrosis, with M1 macrophages being linked to inflammation and fibrosis [86] and M2 macrophages contributing to tissue repair and anti-inflammatory functions [91]. The balance between M1 and M2 macrophages plays a key role in fibrosis progression [91]. Research has highlighted the role of monocyte-derived M2 macrophages in bleomycin-induced fibrosis, where they replace resident AMs, provoke inflammatory responses, and contribute to lung fibrosis by releasing profibrotic factors [87]. The role of IMs in lung fibrosis is gradually becoming clearer, though their precise function remains unclear. Both IMs and resident AMs are present in clinical and preclinical models of radiation-induced lung fibrosis [3,10]. Interestingly, depleting IMs using a colony-stimulating factor receptor-1 neutralizing antibody effectively reduces fibrosis in vivo, while depletion of tissue-resident AMs does not impact the fibrosis score [87]. AMs during fibrosis often exhibit an M2-like activation state which, as mentioned, is influenced by both DNA methylation and specific histone modifications [92]. While it is well established that DNA methylation contributes to the pathogenesis of IPF, how the expression and activity of DNA methyltransferase family members influences IPF is not entirely understood. In one study, higher DNMT3a and DNMT3b expression were found in patients with IPF relative to levels in normal control subjects [93], but no significant difference was observed in DNMT1 expression, suggesting that DNMTs may be upregulated in IPF [32,90,92,93].
In fibrotic animal models, the number of M2 macrophages, including the expression of profibrotic markers, were increased in myeloid lineage-specific DNMT3B-deficient mice challenged with bleomycin compared with controls [94]. This increased M2 polarization in myeloid DNMT3B-deficient mice was accompanied by increased pulmonary fibrosis, which suggests that macrophage DNMT3B inhibits the development of pulmonary fibrosis by restricting alternative macrophage polarization [92]. Given the importance of macrophages in the development of pulmonary fibrosis and the role of DNMT3B in macrophage polarization, myeloid DNMT3B limits the development of pulmonary fibrosis by limiting M2 macrophage polarization potentially by methylation of the promoter of the gene encoding Arg1 [29,87,93]. Profiling these DNA methylation from healthy subjects and IPF patients showed that aberrant macrophage polarization played a crucial role in developing IPF [29,30,93]. During the development of IPF, DNMTs are associated with inappropriate gene expression activation, resulting in the opposite roles of M1/M2 polarization [95]. These data suggest that DNA methylation mediated by different DNMTs may contribute to IPF pathogenesis [96]. The study showed that DNMT3B is crucial for chromosomal stability through DNA methylation and influences macrophage polarization, impacting the progression of IPF [97].
In the epigenetics domain, methyl-CpG-binding domain (MBD) proteins, particularly MBD2, regulate gene expression by binding to methylated DNA [96,98]. Increased MBD2 expression in IPF macrophages contributes to fibrosis, while its deficiency reduces collagen deposition and improves the fibrotic response through PI3K/Akt inhibition [99]. Similarly, the methyl-CpG-binding protein 2 (MECP2) influences profibrotic macrophage polarization, and targeting it with siRNA liposomes reduces fibrosis in mice [86]. Though DNA methylation changes are seen in IPF lungs, their exact role in macrophages remains unclear, with DNA methyltransferases playing a complex role in regulating M2 macrophage polarization in fibrosis [29].
5.1.2. Histone Modification
In IPF, certain histone deacetylases are upregulated in the lung, potentially contributing to the disease. Notably, HDAC6 expression is increased in IPF lung tissues, and inhibiting HDAC6 has been shown to attenuate fibrosis [100,101]. HDAC6 deacetylates non-histone proteins like α-tubulin, and inhibiting it with compounds like trichostatin or tobramycin reduces TGF-β signaling and improves bleomycin-induced fibrosis in mice, suggesting that aberrant deacetylation in IPF AMs promotes fibrogenesis by enabling persistent activation of TGF-β target genes or by suppressing antifibrotic genes [100,102]. Conversely, histone acetylation marks, such as H3K4me3 and H3K9ac, are enriched in fibrotic macrophages [103]. A murine study showed that treating fibrotic lungs with the HDAC inhibitor valproic acid increased these marks at the IL-6 promoter, boosting an IL-6 response that paradoxically attenuated fibrosis by shifting macrophages away from a profibrotic M2 state [104,105,106]. Changes in histone modifications, particularly a reduction in acetylation, play a significant role in the development of fibrosis in IPF [107]. Various studies have indicated a deficiency in histone H3 and H4 acetylation at the promoter regions of genes associated with antifibrotic effects or apoptosis, such as cyclooxygenase 2, CXC chemokine gamma, IFN-y-inducible protein, and Fas, in fibroblasts from IPF patients [108,109]. These findings underscore the involvement of histone modification changes within macrophages in the pathogenesis of IPF [109]. Despite the success of histone deacetylase inhibitors in addressing fibrotic responses [110], the specific role of macrophage-specific histone modifications in lung fibrosis remains unclear. Additionally, profibrotic macrophages are linked to histone lactylation, driven by elevated lactate levels in fibrotic tissue [111]. Lactate produced by glycolytic macrophages and damaged cells can lactylate histone lysines and activate genes like Arg1 and VEGFA, promoting tissue remodeling [112]. While lactylation was first noted in tumor macrophages [112,113], it likely occurs in IPF [114], with ongoing research exploring this novel epigenetic mark.
The epigenomic landscape of macrophages plays a key role in fibrosis [32]. Resident AMs and monocyte-derived AMs, which accumulate during injury, have distinct chromatin states, and both contribute to IPF [32]. In fibrosis, monocyte-derived AMs exhibit an epigenetic profile focused on tissue repair, with open chromatin at loci for TGF-β, PDGF, and scavenger receptors, and closed chromatin at pro-inflammatory cytokine loci [85,115]. This promotes fibroblast and epithelial interactions for matrix deposition instead of inflammation. Chromatin remodelers like BRG1 of the SWI/SNF complex, which normally activate antifibrotic genes, are altered in fibrosis, leading to reduced PPAR-γ expression in AMs [116]. Without PPAR-γ, AMs release more TGF-β and fail to resolve fibrosis. These changes in chromatin configuration are central to macrophage-driven fibrosis [116].
5.1.3. Non-Coding RNA
Next, non-coding RNA regulation of AMs has emerged as a key driver of pulmonary fibrosis. A notable example is miR-33, which is upregulated in AMs from IPF patients and regulates lipid metabolism [117]. Elevated miR-33 suppresses genes involved in cholesterol efflux, fatty acid oxidation, and mitochondrial maintenance, promoting a dysfunctional, profibrotic macrophage phenotype. Deleting miR-33 in mice protected against lung fibrosis, enhancing autophagy and mitochondrial health while reducing inflammatory cytokines. Inhibition of miR-33 also reduced fibrosis in vivo [117]. These findings highlight miR-33 as an important regulator of macrophage immunometabolism in fibrosis. Other microRNAs, such as miR-21, miR-199a-5p, and let-7d, also play roles in fibrotic AM regulation. let-7c mitigates M1 phenotype and promotes the M2 phenotype [118]. MiR-21 and miR-155 are profibrotic, while let-7i, miR-107, mir-126, miR-140, and miR-511 are antifibrotic [95]. MiR-142-5p increased in IPF and MiR-155 exacerbates pathogenic PF, while miR-140 protects against radiation-induced fibrosis. Non-coding RNAs modulate macrophage plasticity in fibrotic diseases, emphasizing the role of macrophage-derived microRNAs in PF [54,95].
Deciphering the complex epigenetic terrain of lung fibrosis is vital for pinpointing potential therapeutic targets. The reversibility of epigenetic modifications offers promise for drug development in fibrotic diseases. However, continued research is necessary to fully comprehend the intricate interplay of epigenetic mechanisms in AMs and their connection to lung fibrosis.
5.2. Chronic Obstructive Pulmonary Diseases
Progressive respiratory disease COPD is characterized by persistent airflow limitation and difficulties in breathing, incorporating chronic bronchitis and emphysema [119,120]. Among various etiological factors, cigarette smoking is a major cause for COPD occurring and induces a persistent epigenetic reprogramming of AMs [109]. The pathogenesis of COPD involves chronic airway inflammation, damage from oxidative stress, and an imbalance in protease/antiprotease mechanisms, with immunomodulatory disorders contributing to its development. Various immune cells, such as macrophages, neutrophils, and dendritic cells, play crucial roles in the immune functions associated with COPD [121]. Studies indicate a significant increase in macrophage numbers in COPD-affected areas, correlating with disease severity [17,19,121]. AMs in COPD are replenished through local proliferation and blood monocyte recruitment [122]. Recent research identified four macrophage phenotypes in COPD: non-polarized M0, iNOS-positive inflammatory M1, arginase-positive anti-inflammatory M2, and a dual positive-M1-M2 type, with a dominance of pro-inflammatory M1 macrophages in the small airways, while M2 macrophages, including subtypes M2a, M2b, M2c, and M2d, possess anti-inflammatory properties, promoting Th2-type immunity [121,123].
5.2.1. DNA Methylation
Cigarette smoke causes genome-wide DNA methylation changes in AMs [100]. Notably, smokers’ AMs exhibit altered 5-methylcytosine and 5-hydroxymethylcytosine patterns at thousands of loci, leading to aberrant gene expression. Isolated DNA of AMs from heavy smokers and never smokers showed 25,000 loci of methylation status, indicating differential methylated genes ranges from immune system to inflammatory pathways, indicating a crucial role of methylation with CpG methylation associated with COPD disease occurrence and severity [27,124]. Changes in these DNA methylation patterns in COPD patients affect pro-inflammatory gene promoters in alveolar cells [124]. Among numerous genes, sphingosine-1-phosphate receptor 5 (S1PR5) encodes a G protein-coupled receptor and binds the lipid-signaling molecule sphingosine 1-phosphate in AMs and is known for regulating lung function and efferocytosis process [125,126]. Despite this, the increased expression of S1PR5 in AMs of COPD patients showed reduced methylation associated with defective efferocytosis processes. Furthermore, several genes of lung macrophages, including HSH2D, SNX10, CLIP4, and TYKZ, are among 95 CpG loci with significant differences of methylation in COPD patients [127].
Furthermore, an epigenome-wide study of bronchoalveolar lavage cells found numerous differentially methylated CpG sites in smokers and COPD patients, including genes in the aryl hydrocarbon receptor pathway. These studies reveal differentially methylated genes, such as SERPINA1 and AHRR, associated with COPD. Aberrant DNA methylation in genes like GABRB1 and TNFAIP2 is also observed in smokers and COPD [128,129]. Hyper- and hypomethylation in various genes are linked to COPD development, lung function decline, and metabolic differences in lung macrophages [38,130]. Overexpression of DNA methyltransferases correlate with abnormal DNA methylation, and exposure to cigarette smoke condensates affects their expression. Additionally, altered DNA methylation is observed in genes related to extracellular matrix proteins that support cells in tissue [131]. Hypomethylation of HDAC6 is reported, leading to HDAC activity and epithelial dysfunction. DNA methylation patterns in COPD patients in alveolar epithelial cells and AMs may affect the promoters of pro-inflammatory genes, potentially influencing macrophage function within the lungs [30,33].
5.2.2. Histone Modification
Furthermore, COPD is strongly associated with an imbalance in histone acetylation homeostasis in AMs [132,133]. Oxidative stress from smoke inactivates histone deacetylases (especially HDAC2) in AMs, shifting the balance toward hyperacetylation of histones on promoters of inflammatory genes [133,134]. This acetylation-skewed state prolongs the transcription of cytokines, matrix metalloproteinases, and other damage mediators in COPD [135,136]. Indeed, HDAC2 is a key enzyme that normally deacetylates the chromatin at pro-inflammatory genes to shut down their expression; studies show that HDAC2 expression/activity is significantly reduced in COPD macrophages, which correlates with uncontrolled inflammation and steroid resistance [100,137]. In AMs from smokers, loss of HDAC2 activity leads to heightened production of IL-8, GM-CSF, and other cytokines despite corticosteroid treatment [138,139], fueling persistent airway inflammation. On the other hand, certain HDACs like HDAC3 can promote pro-inflammatory programs; interestingly, HDAC3 inhibition in COPD models has been suggested to reduce inflammation and favor an anti-inflammatory (M2) shift [100]. Thus, the pattern of histone modifications in COPD AMs is complex but generally favors prolonged inflammation through excess acetylation of inflammatory gene chromatin and selective methylation changes. Likewise, in DNA methylation, histone H3 phosphorylation is a key player in the transcription of NF-κB regulatory genes, crucial for the inflammatory response in COPD. Cigarette smoke induces histone H3 phospho-acetylation on pro-inflammatory gene promoters, emphasizing its importance in NF-κB transcription during exposure to cigarette smoke stimuli [140,141]. It has been known that HDAC is a key molecule in the repression of production of pro-inflammatory cytokines in AMs, and a decrease in HDAC is associated with increased inflammation in COPD and an increase in phosphorylated p38 MAPKs, playing a pivotal role in generating inflammatory cytokines in COPD smokers [142]. Reactive oxygen species, common in COPD, may also heighten inflammation by activating and phosphorylating MAPKs, suggesting smoke-induced COPD activate kinases, leading to histone phosphorylation and subsequent transcription of inflammatory genes, influencing the behavior of AMs. Targeting these kinases could be a potential therapeutic strategy for managing chronic inflammatory responses associated with COPD, specifically in AMs [17,120,121].
Additionally, though histone ubiquitination for H2A (K119) and H2B (K20) are known to play a role in genomic stability and transcriptional regulation, the specific impact on AMs in COPD remains an area requiring further investigation for a comprehensive understanding of the underlying mechanisms [38,48]. On another epigenetic front, histone methylation, involving modifications on arginine and lysine amino acids, has emerged as a crucial regulator of gene expression. Different methylation sites on histones, including H4K20, H3K79, H3K36, H3K27, H3K9, and H3K4, influence biological functions and cellular processes, and its implications in AMs warrant further exploration [143,144]. Furthermore, protein arginine methyltransferases (PRMTs), crucial for arginine methylation, contribute to various cellular processes in AMs, creating an epigenetic signature linked to gene expression. Hypoxic stimuli and oxidative stress, known factors in COPD development, impact PRMT levels in AMs [119,145].
5.2.3. Non-Coding RNA
Next, epigenetic control in COPD AMs is also mediated by ncRNAs. Smoke and oxidant stress can alter miRNA expression profiles in AMs, which in turn modulate inflammatory signaling. In smoker and COPD samples, the proportion of M1 cells was lower compared with M0 and M2 cells [146]. Genes specifically upregulated in M1 cells were primarily associated with inflammation and immunity. Additionally, in M1 macrophages, the expression levels of miR-30a-5p, miR-200c-3p, miR-20b-5p, miR-199b-5p, and miR-301b-3p were lower than those in M0 [146,147]. In M2 macrophages, the expression levels varied, with higher expression in miR-30a-5p and miR-20b-5p and lower expression in miR-200c-3p and miR-301b-3p compared with M1 [148]. This suggests a potential role of microRNA in AMs in the context of COPD. For example, one study found that miR-218 and miR-124 were downregulated in COPD macrophages, leading to overexpression of MMP-2/-9 and enhanced elastin degradation [100]. More directly, miR-146a, an anti-inflammatory microRNA, is often upregulated in endotoxin-tolerant or smoke-exposed macrophages, serving as negative feedback on NF-κB, but its chronic upregulation may contribute to the impaired immune response in COPD [149]. Long non-coding RNAs are less studied in COPD AMs; however, lncRNA MEG3 has been implicated in regulating IL-1β and IL-6 via interaction with DNA methyltransferases in macrophages [150]. Overall, ncRNAs provide an additional layer of epigenetic-like control by targeting mRNAs or chromatin-modifying complexes, ultimately shaping the inflammatory phenotype of COPD AMs.
Recent technological advancements have greatly improved our understanding of AMs in various lung diseases. A key development has been the rise of single-cell omics technologies, such as single-cell RNA sequencing (scRNA-seq), which have revealed the heterogeneity within AM populations and identified disease-specific subtypes, particularly in conditions like COPD [151,152,153]. These studies have shown how distinct AM gene expression profiles are linked to functional dysregulation in COPD, including impaired lipid uptake and reduced angiogenesis [152]. This approach highlighted significant chromatin landscape differences, emphasizing the role of epigenetic regulation in driving macrophage plasticity and dysfunction in COPD. Similarly, a single-cell multi-omics approach was conducted to profile AMs subpopulation in COPD, integrating scRNA-seq and chromatin accessibility data to uncover disease-specific transcriptional and epigenetic signatures [154,155]. Their study demonstrated that COPD-associated AM subsets exhibit differential enhancer activity in genes involved in immune response regulation and tissue repair, linking epigenetic changes to altered AM function [155]. Collectively, this knowledge may open avenues for developing targeted therapies aimed at modulating histone modifications to alleviate inflammation and improve the overall condition of individuals affected by COPD.
5.3. Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH), identified by increased blood pressure in the pulmonary arteries, entails complex interactions with resident macrophages, particularly AMs and IMs of the lungs [156,157]. These macrophages play a crucial role in orchestrating vascular remodeling, particularly in response to injury, inflammation, or hypoxia [1,158]. One of the central mechanisms by which AMs exert this influence is through the secretion of cytokines that not only activate traditional signaling pathways but also induce epigenetic reprogramming in vascular endothelial and smooth muscle cells [159,160,161]. These macrophages play a substantial role in the pathogenesis of PAH by establishing an inflammatory environment vascular remodeling [156,162]. Various key cytokines released by macrophages include TNF-α, IL-6, IL-1β, TGF-β, and VEGF, as well as chemokines like CXCL12 [160,161]. Through the release of pro-inflammatory cytokines like IL-6 and TNF-α, they contribute to vascular dysfunction and remodeling. These molecules engage their respective receptors on vascular cells, activating intracellular cascades such as the NF-κB, STAT3, and SMAD pathways, which in turn modulate the activity of epigenetic enzymes [161]. Furthermore, macrophages influence the proliferation and migration of vascular smooth muscle cells, contributing to pulmonary artery narrowing and increased vascular resistance in PAH through vascular remodeling [161,162]. Additionally, they play a role in endothelial dysfunction, a hallmark of PAH, and their dysregulated immune response sustains inflammation and drives vascular changes [160,161].
5.3.1. DNA Methylation
Although concrete data are currently insufficient to firmly establish an epigenetic role of AM in PAH, recent findings highlight that altered DNA methylation levels at specific gene loci such as superoxide dismutase 2 and granulysin, variations in histone H1 levels, aberrant expression of HDACs and bromodomain-containing protein 4, and disruptions in microRNA networks contribute to the emerging role of epigenetics in PAH [58,163]. Notably, studies indicate that vascular cells, including pulmonary artery endothelial cells, smooth muscle cells, and adventitial fibroblasts isolated from hypertensive lung vessels maintain a hyper-proliferative, apoptosis-resistant phenotype ex vivo, suggesting heritability and contributing to PAH progression [38,163]. The intersection with AMs in this context adds a layer of complexity, as macrophages are known for their involvement in inflammation and the vascular remodeling of key aspects of PAH pathology [164]. Additionally, macrophage-derived cytokines like TGF-β modulate DNA methylation patterns [161,165]. TGF-β activates SMAD-dependent pathways in endothelial and smooth muscle cells, upregulating DNA methyltransferases such as DNMT1 and DNMT3a [166,167]. This results in the hypermethylation of promoters of genes that typically inhibit fibrosis and proliferation, including SMAD7 and RASSF1A, thereby repressing their expression and promoting a vascular remodeling phenotype in PAH [167,168]
5.3.2. Histone Modification
Histone post-translational modifications serve as crucial regulators of chromatin modification processes. Among these modifications, core histones lysine acetylation tightly controlled the opposing actions of HATs and HDACs [41,169]. Among various cytokines released from AMs in PAH, TNF-α and IL-6 activate the NF-κB and STAT3 pathways, respectively [170,171]. NF-κB, once translocated to the nucleus, recruit HATs such as p300/CBP to promoters of pro-angiogenic and matrix-remodeling genes like MMP9 and VEGF [170]. This leads to increased H3K27 acetylation, an epigenetic mark associated with active transcription, thereby enhancing expression of these genes and promoting vascular permeability and extracellular matrix breakdown [172,173]. Concurrently, IL-6-driven STAT3 activation has been shown to recruit EZH2, a histone methyltransferase, to the promoters of anti-angiogenic genes such as THBS1, leading to trimethylation of H3K27 and transcriptional silencing of these inhibitory signals [174,175,176]. This shift in gene expression promotes angiogenesis, endothelial proliferation smooth muscle cell proliferation, and vascular remodeling. Importantly, the dysregulation of HDAC activity has emerged as a significant player in the pathogenesis of PAH, and a study found promising therapeutic outcomes using small-molecule HDAC inhibitors in diverse animal models of PAH [167]. HDAC inhibitors, recognized for their ability to impede cell proliferation and induce apoptosis in transformed cells, have shown therapeutic promise in preclinical studies [177]. These inhibitors effectively dampen inflammation, mitigate fibrosis, and inhibit restenosis, showcasing their efficacy in animal models of left ventricular dysfunction and in the treatment of PAH [178].
5.3.3. Non-Coding RNA
Next, numerous studies have identified distinct expression patterns of miRNA in PAH lung samples and associated cells. In lung PAH tissue, miR-29, miR-17, and miR-223 showed high expression, while miR-1, miR-124, miR-130, miR-138, miR-140, and miR-210 were downregulated [147,179]. In PAH smooth muscles, miR-204 and miR-29 were downregulated, and miR-424 was upregulated [147,180]. Likewise, PAH epithelial cells exhibited upregulation of miR-140, and miR-21, with miR-301 showing lower expression compared with healthy controls [180]. This suggests a potential role of microRNA in PAH. Furthermore, induction of cytokine signaling in PAH can also influence non-coding RNAs. For example, IL-1β and TNF-α upregulate miR-155, which targets negative regulators of inflammation and angiogenesis, further reinforcing the remodeling process [181,182]. Collectively, these epigenetic changes induced by AM-derived cytokines establish a permissive transcriptional landscape in vascular cells, driving structural and functional alterations characteristic of pathological vascular remodeling [183,184,185].
Despite the acknowledged role of AMs and miRNA in PAH, none of the research, to the best of our knowledge, has explored the intricate role of miRNA within AM to PAH. Understanding the epigenetic modulation of AMs could provide crucial insights into the intricate mechanisms driving PAH.
5.4. Lung Cancer
Lung cancer stands as a global health threat, claiming the highest mortality rates among malignancies. The intricate interplay between inflammation and tumor progression is a pivotal factor in the development of lung tumors. In lung cancer, non-small-cell lung carcinoma AMs in particular differentiate into tumor-associated macrophages (TAMs) in the tumor microenvironment, exhibiting diverse effects on the growth, progression, and metastasis of lung tumors [17,91,186]. The dichotomy of TAMs manifests through two distinct phenotypes, the M1 and M2 macrophages, each exerting contrasting influences on neoplasm advancement. TAMs are often immunosuppressive (like M2-polarized macrophages) and facilitate tumor growth, angiogenesis, and metastasis. Their pro-tumoral versus anti-tumoral functions are heavily influenced by epigenetic modifications [104]. Unlike genetic mutations in cancer cells, TAMs undergo mainly epigenetic reprogramming in response to tumor-derived signals [104]. The dynamic functionality of TAMs, shaped by cytokines, chemokines, cell interactions, and signaling pathways, underscores their potential as therapeutic targets in non-small-cell lung cancer [187]. Within the intricate context of lung cancer, AMs, pivotal components of the lung’s immune milieu, contribute to lung cancer epigenetics through various mechanisms. A wealth of studies underscores the pivotal role of M2 in fostering an environment conducive to lung cancer progression. M2 macrophages accomplish this by releasing a spectrum of chemokines and growth factors, including TGF-β, IL-10, and MMP, fueling the growth, invasion, immunosuppression, and metastasis of lung cancer [17,91,187]. In addition, DNA methylation alterations in macrophages may influence the regulation of tumor suppressor genes, potentially affecting their anti-tumor functions and contributing to an immunosuppressive microenvironment that facilitates cancer progression [188,189].
5.4.1. DNA Methylation
Lung cancer exhibits altered methylation in various oncogenes, silencing their expression primarily through hypermethylation of CpG islands in their promoter regions. DNA methylation likely silences certain genes in TAMs that would otherwise attack tumor cells. The hypoxic, cytokine-rich TME can induce DNMTs in TAMs, leading to hypermethylation of promoters for M1-associated genes including IL12B or CXCL9 and other checkpoints that activate T cells. One study noted that tumor-derived lactic acid altered the DNA methylation pattern of oxidative metabolism genes in M1 macrophages, pushing them towards an M2 state [104]. Consistently, inhibiting DNMTs can skew macrophages toward a more inflammatory phenotype. For instance, blocking DNMT activity prevented tumor-induced metabolic reprogramming in macrophages in vitro [104,115,190]. However, direct analyses of TAM-specific DNA methylation remain sparse, and more profiling is needed. It is generally acknowledged that using DNMT inhibitors in cancer therapy can rejuvenate immune responses in the TME, partly by re-activating silenced immune genes in both tumor cells and infiltrating macrophages [104,191,192,193]. In summary, DNA methylation in TAMs contributes to immune evasion by repressing genes for antigen presentation and pro-inflammatory cytokines, but it may be reversible by epigenetic therapy.
EZH2 is a key component of the polycomb repressive complex 2 (PRC2), which plays a crucial role in regulating gene expression by modifying chromatin structure. As a histone methyltransferase, EZH2 plays a pivotal role in regulating cell cycle, autophagy, and apoptosis, particularly in cancer. Stimulation of TAMs showed PRMT1-mediated EZH2 methylation, promoting lung cancer proliferation and metastasis by modulating oncogene methylation through EZH2 [189,194]. Notably, hypermethylation of promoter regions in tumor suppressor genes is a recurring theme, resulting in the silencing of genes that normally act as safeguards against uncontrolled cell growth. Concurrently, hypomethylation in specific genomic regions, including oncogenes, fuels their overexpression, promoting cell proliferation and contributing to the oncogenic milieu [195]. Beyond diagnostics, the understanding of these methylation changes has opened avenues for epigenetic therapies [34,93,196]. Exploration of DNA methyltransferase inhibitors as potential interventions is underway to reverse aberrant DNA methylation and reactivate silenced tumor suppressor genes. The observed epigenetic heterogeneity in lung cancer, characterized by distinct DNA methylation profiles among subtypes, underscores the complexity of the disease and underscores the necessity for tailored therapeutic approaches [197,198].
5.4.2. Histone Modification
Moreover, histone modifications within macrophages play a crucial role in shaping their immune response [43,45]. Changes in histone acetylation and methylation patterns can influence the pro-inflammatory or anti-inflammatory phenotype of macrophages, impacting their ability to recognize and eliminate cancer cells [189]. Unraveling the specific contributions of macrophage-associated epigenetic changes in lung cancer progression holds promise for developing targeted therapeutic strategies that leverage the interplay between the immune system and the epigenetic landscape of cancer cells [189,199]. Furthermore, TAMs exhibit characteristic histone modification patterns that promote an anti-inflammatory, pro-tumor phenotype. IL-4 and IL-10 in the TME upregulate JMJD3 in TAMs, as mentioned earlier, which removes the H3K27me3 repressive marks from M2 genes (e.g., ARG1, CHI3L1) and from genes that inhibit inflammation (like IL1RA) [104]. The same mechanism is operative in tumors: high JMJD3 levels in TAMs correlate with enhanced expression of M2 markers and immunosuppressive factors. On the other hand, certain histone methyltransferases can restrain macrophage activation. For example, SUV420H1-mediated H4K20 trimethylation and SMYD2-mediated H3K36 dimethylation set repressive checkpoints on TLR4-inducible genes in macrophages [104]. If tumor signals downregulate these enzymes, TAMs might overproduce inhibitory cytokines like IL-10. Additionally, histone acetylation is widely modulated in TAMs: tumor TAMs often have low expression of class II HDACs (e.g., HDAC9, HDAC10), leading to hyperacetylation and activation of genes that paradoxically encourage macrophages to be trophic rather than toxic to tumors. Notably, HDAC11 stands out—it is a negative regulator of IL-10 in macrophages. Tumor exosomes containing miR-145 have been shown to downregulate HDAC11 in TAMs, unleashing IL-10 production and thereby promoting tumor immune evasion [104]. Neutralizing exosomal miR-145 or inhibiting IL-10 signaling can reduce tumor growth in such models [104]. Moreover, TAMs in many cancers exhibit increased histone lactylation due to abundant tumor cell-derived lactate. Lactylation of histone H3 induces transcription of Arg1 and VEGF in TAMs, reinforcing their tissue repair, pro-angiogenic program. Overall, tumors create an epigenetic milieu (via cytokines, exo-metabolites, and exosomes) that re-wires histone marks in AMs/TAMs to favor tumor progression.
Recent clinical trials have explored the effects of HDAC and DNMT inhibitors on AM function, particularly in the context of lung cancer. A Phase II trial combined the DNMT inhibitor 5-azacitidine with the HDAC inhibitor entinostat in patients with advanced non-small-cell lung cancer. This combination aimed to reverse the epigenetic silencing of tumor suppressor genes [200,201]. Another Phase I clinical trial investigated the combination of panobinostat, a pan-HDAC inhibitor, with PDR001, an immune checkpoint inhibitor, in patients with advanced non-small-cell lung adenocarcinoma. This study explored the potential of combining epigenetic modulation with immune checkpoint blockade to enhance anti-tumor immunity [202,203,204]. Beyond clinical trials, preclinical research has demonstrated that combining DNMT and HDAC inhibitors can modulate macrophage responses. A study combining 5-azacitidine and trichostatin A in a mouse model of acute lung injury showed reduced inflammation and apoptosis, suggesting a potential therapeutic approach for conditions involving macrophage-mediated lung injury [205]. Furthermore, dual inhibitors targeting both DNMT and HDAC pathways have been developed for cancer therapy. Compound (R)-23a, a dual DNMT1/HDAC inhibitor, has shown potent inhibition of both targets, effectively reactivating tumor suppressor genes and suppressing tumor growth in xenograft models [206]. Another compound, C02S, demonstrated strong inhibitory activity against DNMT1, DNMT3A, DNMT3B, and HDAC1, leading to apoptosis, cell cycle arrest, reduced angiogenesis, and decreased tumor proliferation in vitro and in vivo [207]. These findings underscore the potential of dual epigenetic inhibitors in reshaping macrophage function and improving therapeutic outcomes in lung cancers and inflammatory lung conditions.
5.4.3. Non-Coding RNA
Next, TAMs are heavily influenced by ncRNA-mediated regulation. Tumors secrete exosomes packed with microRNAs that enter macrophages and modulate their epigenetic and signaling pathways. We saw the example of miR-145 downregulating HDAC11 [104,208]. Another example is presented here: miR-138-5p delivered by tumor exosomes was found to target KDM6B (JMJD3) in macrophages, thereby upregulating H3K27me3 and blunting an M2-to-M1 reprogramming attempt [209]. Indeed, epigenetic therapies aimed at TAMs—such as combined DNA demethylating agents and HDAC inhibitors—have shown success in mouse models by disrupting immunosuppressive pre-metastatic niches and improving survival [210].
6. Chromatin Remodeler and Transcription Factor in the Epigenetic Regulation of AMs
To fully understand how epigenetic regulation shapes AM behavior, particularly in disease contexts, it is essential to consider the role of transcription factor (TF)-based epigenetic regulators, which modulate gene expression by altering chromatin structure without changing the underlying DNA sequence. These factors help control various processes such as development, differentiation, immune responses, and disease progression, including in lung diseases. Among various key epigenetic chromatin remodelers in regulating AMs function, particularly in the context of various lung diseases, bromodomain and extra-terminal (BET) proteins, including BRD2, BRD3, and BRD4, are critical for regulating the transcription of inflammatory genes [211,212]. These proteins recognize acetylated lysine residues on histones and non-histone proteins, facilitating the recruitment of transcriptional machinery to chromatin [213]. In diseases like COPD and lung disease conditions, BET proteins influence the expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β [214,215]. Inhibition of BET proteins with small-molecule inhibitors, like JQ1, has shown promise in reducing inflammation and modulating macrophage polarization, suggesting potential therapeutic applications in pulmonary diseases [215]. Additionally, the BET inhibitor apabetalone was tested in the BETonMACE trial and showed cardiovascular and anti-inflammatory benefits [216,217]. Similarly, vorinostat, a histone deacetylase inhibitor (HDACi), has been investigated in early-phase trials for its capacity to suppress pro-inflammatory cytokine expression in pulmonary and fibrotic contexts [218]. While these agents show promising immune modulatory effects, several challenges limit their translational application to lung diseases. Among them is the issue of off-target effects, as most current epigenetic drugs act systemically and lack cell-type specificity, raising concerns about toxicity and global immune suppression. Additionally, effective delivery to AMs remains a significant barrier due to the anatomical complexity of the lung and the inaccessibility of these cells via traditional systemic routes [219]. Additionally, histone modifiers, such as HATs and HMTs, play pivotal roles in shaping macrophage gene expression. For instance, p300/CBP, major HATs, are involved in activating transcription by acetylating histones at the promoters of inflammatory genes [220]. G9a and SUV39H1, histone methyltransferases, modulate histone methylation, which in turn influences macrophage activation and polarization [221]. In diseases such as pulmonary fibrosis, aberrant histone modifications can drive chronic inflammation and fibrosis, underscoring the need for targeted therapies that modulate these chromatin modifiers [108]. In diseases like COPD, altered DNA methylation by DNMTs can affect immune gene expression, potentially impairing immune responses or promoting excessive inflammation [219].
Chromatin remodelers are protein complexes that regulate the accessibility of chromatin to transcriptional machinery, thereby influencing gene expression. In lung diseases, transcription factors like NF-κB, AP-1, and IRF3 also play vital roles in regulating key genes in AMs. NF-κB is a central regulator of inflammation and is modulated by epigenetic changes such as histone acetylation [20,109]. NF-κB signaling contributes to the production of pro-inflammatory cytokines, while AP-1 and IRF3 regulate macrophage activation and immune responses to infection [77,170]. These TFs often interact with chromatin modifiers, affecting macrophage responses to environmental stimuli. The impact of these epigenetic factors varies across different conditions. In lung injury and chronic lung disease, the combined actions of transcription factors like NF-κB and AP-1, along with chromatin modifiers, regulate immune gene expression, leading to excessive inflammation [222]. Epigenetic therapies targeting these pathways could potentially reduce lung injury and promote tissue repair. In lung cancer, aberrant activation of BET proteins and altered DNA methylation can reshape the tumor microenvironment, with TAMs being reprogrammed to support tumor growth [212,214]. Targeting these pathways may reprogram TAMs from a pro-tumor to an anti-tumor phenotype, enhancing anti-cancer immunity. In pulmonary fibrosis, altered histone modification and DNA methylation contribute to fibroblast activation and excessive matrix deposition, and understanding how chromatin modifiers influence these processes in AMs may lead to strategies for reversing fibrosis. Furthermore, the therapeutic potential of epigenetic modifiers in treating chronic lung diseases such as COPD, pulmonary fibrosis, and PAH is increasingly recognized due to their capacity to reprogram macrophage phenotypes and attenuate pathogenic gene expression.
Technological advances, including CUT&RUN and single-cell RNA sequencing, have enabled precise profiling of transcription factors and histone modifications, providing new insights into how chromatin accessibility and metabolic states shape cellular identity and function within AMs [223]. Integration of multi-omics platforms offers a more comprehensive view of how environmental cues influence epigenetic regulation in lung health and disease [224]. Moreover, computational approaches—including machine learning—are increasingly being used to analyze these large datasets, identify novel AM subtypes, and predict disease-specific cellular behaviors. These technologies not only help uncover disease-associated macrophage subtypes but also facilitate the discovery of novel epigenetic biomarkers and therapeutic targets, offering a more detailed understanding of how the AM epigenome is reprogrammed during disease progression.
7. Microbial and Cellular Metabolites in the Epigenetic Regulation of AMs
Emerging evidence reveals that the gut microbiome profoundly influences the epigenetic landscape of AMs, offering new insights into the gut–lung axis [225,226]. Gut-derived short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate, act as signaling molecules that extend far beyond the intestinal environment [227]. Their capacity to inhibit HDACs is particularly noteworthy as HDACs are enzymes that remove acetyl groups from histone proteins, leading to chromatin condensation and gene silencing. By inhibiting HDACs, SCFAs promote histone acetylation, generally resulting in a more open chromatin structure and enhanced gene transcription [228]. In the context of AMs, the constant exposure to systemic SCFAs originating from the gut likely plays a role in shaping their baseline epigenetic profile during homeostasis [229]. This could influence the expression of genes critical for their sentinel functions, including efferocytosis, a process vital for maintaining alveolar integrity and preventing the release of pro-inflammatory contents. For instance, butyrate-mediated HDAC inhibition might upregulate genes involved in the recognition and engulfment of dead cells, contributing to tissue remodeling and resolution of minor injury in the lungs [230]. However, when the delicate balance of the gut microbiome is disrupted, the production of SCFAs can be altered, both in quantity and composition. This shift in the systemic availability of HDAC inhibitors can have profound consequences for the epigenetic programming of AMs. In diseases such as COPD [231], where altered gut microbiota profiles are commonly observed, reduced levels of SCFAs may lead to decreased histone acetylation in AMs. This epigenetic shift could promote a pro-inflammatory phenotype, increasing their responsiveness to allergens and contributing to airway hyperresponsiveness and inflammation. In COPD specifically, where impaired clearance of cellular debris and chronic inflammation are key features, dysbiosis-related changes in SCFA levels may further impair the efferocytosis capacity of AMs, exacerbating tissue damage and sustaining inflammation [227,228,232]. Understanding these intricate epigenetic mechanisms driven by gut microbial metabolites offers promising avenues for therapeutic interventions aimed at modulating the gut microbiome or directly targeting epigenetic pathways in AMs to restore lung health.
The epigenetic regulation of AMs is inherently dynamic and closely intertwined with cellular metabolism [67]. Beyond the influence of microbial-derived SCFAs, a wide range of endogenous metabolites act as direct regulators of the enzymes responsible for establishing and maintaining epigenetic marks [70,227]. These metabolites effectively connect the metabolic state of AMs to their gene expression patterns and functional outcomes. A central player in this metabolic–epigenetic interface is the one-carbon metabolism pathway, which includes folate and methionine cycles [233]. Methionine is converted into S-adenosylmethionine (SAM), the primary methyl donor used by DNMTs to add methyl groups to cytosine residues in DNA—a modification typically associated with gene repression. The availability of methionine, which is influenced by both diet and cellular metabolic activity, directly impacts SAM levels and thereby modulates DNA methylation patterns across the AM genome [234,235]. These changes can significantly affect the expression of genes involved in inflammation, phagocytosis, and tissue repair. Similarly, the tricarboxylic acid (TCA) cycle, a key source of cellular energy metabolism, produces various intermediates that serve as cofactors or inhibitors for histone-modifying enzymes [236]. For example, α-ketoglutarate is an essential co-substrate for Jumonji C domain-containing histone demethylases (KDMs), which remove methyl groups from lysine residues on histones [237]. This activity typically leads to a more relaxed chromatin structure and promotes gene transcription. Alterations in glucose and glutamine metabolism can influence intracellular α-KG levels, thereby modulating KDM activity and shaping chromatin accessibility in AMs in response to various stimuli [65]. Conversely, TCA cycle intermediates like succinate and fumarate can act as inhibitors of specific histone demethylases and methyltransferases [238]. Under inflammatory conditions, succinate can accumulate and inhibit KDMs, resulting in increased histone methylation and a transcriptional profile skewed toward pro-inflammatory responses [239]. Meanwhile, fumarates modify cysteine residues on proteins including epigenetic enzymes to alter their function [238]. Another key metabolite, acetyl-CoA, produced from glucose and fatty acid metabolism, serves as the primary acetyl donor for HATs [72]. The availability of acetyl-CoA directly influences global levels of histone acetylation, a modification generally linked to transcriptional activation [65,240]. Shifts in fuel utilization by the cell can rapidly affect histone acetylation patterns, enabling AMs to swiftly adjust gene expression in response to environmental cues.
In essence, cellular metabolism acts as a finely tuned regulatory layer over the alveolar macrophage epigenome. By sensing and integrating metabolic signals, AMs can adapt their transcriptional programs to meet the diverse demands of the lung microenvironment, whether it is clearing apoptotic cells during homeostasis or mounting robust immune responses during infection or inflammation [240,241]. Unraveling these metabolic–epigenetic interactions hold great promise for developing therapies that target macrophage function by modulating metabolic pathways or epigenetic enzymes.
8. Targeting Macrophages as a Therapeutic Approach
The therapeutic targeting of macrophages in lung diseases has gained substantial attention, given their crucial role in both lung health and disease. Whether they are addressing infectious lung diseases like COPD [120], pulmonary fibrosis [86], PAH [167], or lung cancer [189], macrophages intricately contribute to lung immunity, inflammation, and tissue repair. Strategies for infectious diseases may involve enhancing macrophage function for effective pathogen combat or modulating responses to mitigate excessive inflammation. In pulmonary fibrosis, directly targeting pro-inflammatory monocyte-derived AMs show promise in preventing fibrosis progression [91]. Balancing pro-inflammatory and anti-inflammatory macrophages is explored in COPD and PAH. Targeting macrophages in lung cancer involves reprogramming tumor-associated macrophages for anti-tumor effects and enhancing treatment efficacy [1,86,91,242]. Immunomodulation to transition macrophages to anti-inflammatory or tissue-repairing states is also considered.
Although HDAC and DNMT inhibitors are primarily studied for their roles in cancer and other conditions [243,244,245], but their potential impact on AM function presents a compelling area for further exploration. HDAC inhibitors work by preventing the deacetylation of histones and increased gene expression. In AMs, this may enhance inflammatory responses by promoting the expression of pro-inflammatory cytokines and chemokines, thus increasing macrophage activation [43,246]. Additionally, HDAC inhibitors may influence macrophage polarization, potentially skewing them towards a more pro-inflammatory or anti-inflammatory state depending on the specific context [246,247]. In lung diseases like COPD, these inhibitors might help resolve inflammation by promoting the production of anti-inflammatory mediators. In the case of lung fibrosis, HDAC inhibitors could reduce excessive collagen deposition by modulating the interactions between macrophages and fibroblasts [108,248]. DNMT inhibitors, on the other hand, prevent DNA methylation, which can reactivate previously silenced genes [249]. In AMs, this could lead to the reactivation of tumor suppressor genes or genes that regulate immune responses, potentially altering macrophage-mediated phagocytosis, cytokine production, or antigen presentation. In chronic inflammatory diseases like COPD or interstitial lung disease, DNMT inhibitors might modulate the expression of genes involved in inflammation [250,251].
Above all, macrophage-directed therapy, utilizing diverse approaches such as targeted drug delivery, immunotherapies, gene therapies, and combination strategies, presents a promising avenue for revolutionizing the management of complex lung diseases. However, a comprehensive understanding of their mechanisms and clinical efficacy requires further investigation. In the broader context, while the therapeutic potential of specific inhibitors within AMs is still under scrutiny, their combination with other immunotherapies holds the potential to unlock novel treatment strategies for conditions beyond lung disease, including lung cancer, infections, and autoimmune disorders.
9. Conclusions
In conclusion, AMs emerge as pivotal contributors in lung diseases, thus positioning them as promising targets for therapeutic interventions. Strategies range from enhancing their functional capabilities to modulating their responses, with tailored approaches being designed for specific lung conditions. These strategies harness the potential of AMs for the effective management of respiratory disorders. Importantly, epigenetics plays a central role as a key determinant in shaping these therapeutic avenues, further highlighting the intricate molecular mechanisms governing AM functions in the context of lung health and disease. The intricate interplay among methylation, histone modifications, microRNA, and histone variants, individually or through crosstalk, establishes a critical epigenetic relationship.
Author Contributions
Conceptualization: Q.-S.M. and L.Z.; resources, validation: L.Z. and Q.-S.M.; visualization: L.Z. and Q.-S.M.; writing—original draft preparation: N.P., K.S., X.K.S., A.J., L.Z. and Q.-S.M.; writing—review and editing, N.P., K.S., X.K.S., A.J., L.Z. and Q.-S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study is partially supported by the National Institutes of Health grants R01AR072046 (L.Z.), R01AR069681, and R01AI119041 (Q.M.) and the Henry Ford Immunology Program grants T71017 (L.Z.) and T71016 (Q.M.).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank all laboratory members for their help and encouragement.
Conflicts of Interest
The authors declare no competing interests.
References
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.L.; Harrison, R.E. Microbial Phagocytic Receptors and Their Potential Involvement in Cytokine Induction in Macrophages. Front. Immunol. 2021, 12, 662063. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Xiao, K.; Tang, L.; Xie, L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front. Immunol. 2021, 12, 753940. [Google Scholar] [CrossRef] [PubMed]
- Ardain, A.; Marakalala, M.J.; Leslie, A. Tissue-resident innate immunity in the lung. Immunology 2020, 159, 245–256. [Google Scholar] [CrossRef]
- Malainou, C.; Abdin, S.M.; Lachmann, N.; Matt, U.; Herold, S. Alveolar macrophages in tissue homeostasis, inflammation, and infection: Evolving concepts of therapeutic targeting. J. Clin. Investig. 2023, 133, e170501. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, L.; Gillet, L.; Machiels, B. Shaping of the alveolar landscape by respiratory infections and long-term consequences for lung immunity. Front. Immunol. 2023, 14, 1149015. [Google Scholar] [CrossRef]
- Hawley, C.A.; Rojo, R.; Raper, A.; Sauter, K.A.; Lisowski, Z.M.; Grabert, K.; Bain, C.C.; Davis, G.M.; Louwe, P.A.; Ostrowski, M.C.; et al. Csf1r-mApple Transgene Expression and Ligand Binding In Vivo Reveal Dynamics of CSF1R Expression within the Mononuclear Phagocyte System. J. Immunol. 2018, 200, 2209–2223. [Google Scholar] [CrossRef]
- Strickland, A.B.; Chen, Y.; Sun, D.; Shi, M. Alternatively activated lung alveolar and interstitial macrophages promote fungal growth. iScience 2023, 26, 106717. [Google Scholar] [CrossRef]
- Meziani, L.; Mondini, M.; Petit, B.; Boissonnas, A.; de Montpreville, V.T.; Mercier, O.; Vozenin, M.C.; Deutsch, E. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 2018, 51, 1702120. [Google Scholar] [CrossRef]
- Tan, S.Y.; Krasnow, M.A. Developmental origin of lung macrophage diversity. Development 2016, 143, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Ringqvist, E.; Willinger, T. Origin and ontogeny of lung macrophages: From mice to humans. Immunology 2020, 160, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.D.; Jeong, D.; Chung, D.H. Development and Functions of Alveolar Macrophages. Mol. Cells 2021, 44, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Okuma, T.; Terasaki, Y.; Kaikita, K.; Kobayashi, H.; Kuziel, W.A.; Kawasuji, M.; Takeya, M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J. Pathol. 2004, 204, 594–604. [Google Scholar] [CrossRef]
- Oliveira, V.L.S.; Pollenus, E.; Berghmans, N.; Queiroz-Junior, C.M.; Blanter, M.; Mattos, M.S.; Teixeira, M.M.; Proost, P.; Van den Steen, P.E.; Amaral, F.A.; et al. Absence of CCR2 Promotes Proliferation of Alveolar Macrophages That Control Lung Inflammation in Acute Respiratory Distress Syndrome in Mice. Int. J. Mol. Sci. 2022, 23, 12920. [Google Scholar] [CrossRef]
- Gibbings, S.L.; Thomas, S.M.; Atif, S.M.; McCubbrey, A.L.; Desch, A.N.; Danhorn, T.; Leach, S.M.; Bratton, D.L.; Henson, P.M.; Janssen, W.J.; et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. Am. J. Respir. Cell Mol. Biol. 2017, 57, 66–76. [Google Scholar] [CrossRef]
- Schyns, J.; Bureau, F.; Marichal, T. Lung Interstitial Macrophages: Past, Present, and Future. J. Immunol. Res. 2018, 2018, 5160794. [Google Scholar] [CrossRef]
- Nayak, D.K.; Zhou, F.; Xu, M.; Huang, J.; Tsuji, M.; Hachem, R.; Mohanakumar, T. Long-Term Persistence of Donor Alveolar Macrophages in Human Lung Transplant Recipients That Influences Donor-Specific Immune Responses. Am. J. Transpl. 2016, 16, 2300–2311. [Google Scholar] [CrossRef]
- Subramanian, S.; Busch, C.J.; Molawi, K.; Geirsdottir, L.; Maurizio, J.; Vargas Aguilar, S.; Belahbib, H.; Gimenez, G.; Yuda, R.A.A.; Burkon, M.; et al. Long-term culture-expanded alveolar macrophages restore their full epigenetic identity after transfer in vivo. Nat. Immunol. 2022, 23, 458–468. [Google Scholar] [CrossRef]
- Hey, J.; Paulsen, M.; Toth, R.; Weichenhan, D.; Butz, S.; Schatterny, J.; Liebers, R.; Lutsik, P.; Plass, C.; Mall, M.A. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat. Commun. 2021, 12, 6520. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Morival, J.; Hazelwood, A.; Lammerding, J. Feeling the force from within-new tools and insights into nuclear mechanotransduction. J. Cell Sci. 2025, 138, JCS263615. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, W.; Yuan, Z.; Chen, H.; Mao, W. Bridging epigenomics and tumor immunometabolism: Molecular mechanisms and therapeutic implications. Mol. Cancer 2025, 24, 71. [Google Scholar] [CrossRef]
- Ji, Y.; Xiao, C.; Fan, T.; Deng, Z.; Wang, D.; Cai, W.; Li, J.; Liao, T.; Li, C.; He, J. The epigenetic hallmarks of immune cells in cancer. Mol. Cancer 2025, 24, 66. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- Philibert, R.A.; Sears, R.A.; Powers, L.S.; Nash, E.; Bair, T.; Gerke, A.K.; Hassan, I.; Thomas, C.P.; Gross, T.J.; Monick, M.M. Coordinated DNA methylation and gene expression changes in smoker alveolar macrophages: Specific effects on VEGF receptor 1 expression. J. Leukoc. Biol. 2012, 92, 621–631. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Chen, Y.; Dessaint, J.A.; Aridgides, D.S.; Channon, J.Y.; Mellinger, D.L.; Christensen, B.C.; Ashare, A. DNA Methylation Changes in Regional Lung Macrophages Are Associated with Metabolic Differences. Immunohorizons 2019, 3, 274–281. [Google Scholar] [CrossRef]
- McErlean, P.; Bell, C.G.; Hewitt, R.J.; Busharat, Z.; Ogger, P.P.; Ghai, P.; Albers, G.J.; Calamita, E.; Kingston, S.; Molyneaux, P.L.; et al. DNA Methylome Alterations Are Associated with Airway Macrophage Differentiation and Phenotype during Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 204, 954–966. [Google Scholar] [CrossRef]
- Kim, M.; Costello, J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, M.N.; Mora, A.L. The Epigenomic Landscape: A Cornerstone of Macrophage Phenotype Regulation in the Fibrotic Lung. Am. J. Respir. Crit. Care Med. 2021, 204, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes. Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Dhar, G.A.; Saha, S.; Mitra, P.; Nag Chaudhuri, R. DNA methylation and regulation of gene expression: Guardian of our health. Nucleus 2021, 64, 259–270. [Google Scholar] [CrossRef]
- Calle-Fabregat, C.; Morante-Palacios, O.; Ballestar, E. Understanding the Relevance of DNA Methylation Changes in Immune Differentiation and Disease. Genes 2020, 11, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Armstrong, D.A.; Salas, L.A.; Hazlett, H.F.; Nymon, A.B.; Dessaint, J.A.; Aridgides, D.S.; Mellinger, D.L.; Liu, X.; Christensen, B.C.; et al. Genome-wide DNA methylation profiling shows a distinct epigenetic signature associated with lung macrophages in cystic fibrosis. Clin. Epigenetics 2018, 10, 152. [Google Scholar] [CrossRef]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Avci, E.; Sarvari, P.; Savai, R.; Seeger, W.; Pullamsetti, S.S. Epigenetic Mechanisms in Parenchymal Lung Diseases: Bystanders or Therapeutic Targets? Int. J. Mol. Sci. 2022, 23, 546. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dasgupta, S.; Mishra, P.K.; Chaudhury, K. Air pollution-induced epigenetic changes: Disease development and a possible link with hypersensitivity pneumonitis. Environ. Sci. Pollut. Res. Int. 2021, 28, 55981–56002. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Alaskhar Alhamwe, B.; Khalaila, R.; Wolf, J.; von Bulow, V.; Harb, H.; Alhamdan, F.; Hii, C.S.; Prescott, S.L.; Ferrante, A.; Renz, H.; et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. Immunol. 2018, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Allahverdi, A.; Zahiri, J. Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders. J. Biosci. 2020, 45, 135. [Google Scholar] [CrossRef]
- Barnes, P.J.; Adcock, I.M.; Ito, K. Histone acetylation and deacetylation: Importance in inflammatory lung diseases. Eur. Respir. J. 2005, 25, 552–563. [Google Scholar] [CrossRef] [PubMed]
- McQuattie-Pimentel, A.C.; Ren, Z.; Joshi, N.; Watanabe, S.; Stoeger, T.; Chi, M.; Lu, Z.; Sichizya, L.; Aillon, R.P.; Chen, C.I.; et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Investig. 2021, 131, e140299. [Google Scholar] [CrossRef]
- Cosio, B.G.; Mann, B.; Ito, K.; Jazrawi, E.; Barnes, P.J.; Chung, K.F.; Adcock, I.M. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 141–147. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Q.; Adrianto, I.; Wu, X.; Glassbrook, J.; Khalasawi, N.; Yin, C.; Yi, Q.; Dong, Z.; Geissmann, F.; et al. Histone deacetylase 3 controls lung alveolar macrophage development and homeostasis. Nat. Commun. 2020, 11, 3822. [Google Scholar] [CrossRef]
- Li, X.; Mara, A.B.; Musial, S.C.; Kolling, F.W.; Gibbings, S.L.; Gerebtsov, N.; Jakubzick, C.V. Coordinated chemokine expression defines macrophage subsets across tissues. Nat. Immunol. 2024, 25, 1110–1122. [Google Scholar] [CrossRef]
- Sokolova, V.; Sarkar, S.; Tan, D. Histone variants and chromatin structure, update of advances. Comput. Struct. Biotechnol. J. 2023, 21, 299–311. [Google Scholar] [CrossRef]
- Stillman, B. Histone Modifications: Insights into Their Influence on Gene Expression. Cell 2018, 175, 6–9. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Dai, W.; Feng, X.; Ding, C.; Shao, Q.; Xiao, R.; Zhao, N.; Peng, W.; Yang, Y.; Cui, Y.; et al. Suppression of lncRNA NLRP3 inhibits NLRP3-triggered inflammatory responses in early acute lung injury. Cell Death Dis. 2021, 12, 898. [Google Scholar] [CrossRef]
- Miguel, V.; Lamas, S.; Espinosa-Diez, C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox Biol. 2020, 37, 101580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, H.; Lai, M. Non-coding RNAs and their epigenetic regulatory mechanisms. Biol. Cell 2010, 102, 645–655. [Google Scholar] [CrossRef]
- Cui, H.; Banerjee, S.; Guo, S.; Xie, N.; Ge, J.; Jiang, D.; Zornig, M.; Thannickal, V.J.; Liu, G. Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight 2019, 4, e124522. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef]
- Wolowiec, L.; Medlewska, M.; Osiak, J.; Wolowiec, A.; Grzesk, E.; Jasniak, A.; Grzesk, G. MicroRNA and lncRNA as the Future of Pulmonary Arterial Hypertension Treatment. Int. J. Mol. Sci. 2023, 24, 9735. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Saxena, A.; Carninci, P. Long non-coding RNA modifies chromatin: Epigenetic silencing by long non-coding RNAs. BioEssays 2011, 33, 830–839. [Google Scholar] [CrossRef]
- Henikoff, S.; Smith, M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015, 7, a019364. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. Histone variants at a glance. J. Cell Sci. 2021, 134, jcs244749. [Google Scholar] [CrossRef] [PubMed]
- Kurumizaka, H.; Kujirai, T.; Takizawa, Y. Contributions of Histone Variants in Nucleosome Structure and Function. J. Mol. Biol. 2021, 433, 166678. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, J.; Rondinelli, B.; Polo, S.E. Histone Variants: Guardians of Genome Integrity. Cells 2020, 9, 2424. [Google Scholar] [CrossRef]
- Valand, A.; Rajasekar, P.; Wain, L.V.; Clifford, R.L. Interplay between genetics and epigenetics in lung fibrosis. Int. J. Biochem. Cell Biol. 2025, 180, 106739. [Google Scholar] [CrossRef]
- Arif, M.; Sadayappan, S.; Becker, R.C.; Martin, L.J.; Urbina, E.M. Epigenetic modification: A regulatory mechanism in essential hypertension. Hypertens. Res. 2019, 42, 1099–1113. [Google Scholar] [CrossRef]
- Zhang, K.; Jagannath, C. Crosstalk between metabolism and epigenetics during macrophage polarization. Epigenetics Chromatin 2025, 18, 16. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, Y.; Ma, L.; Hu, F.; Xie, L. Macrophage polarization and its impact on idiopathic pulmonary fibrosis. Front. Immunol. 2024, 15, 1444964. [Google Scholar] [CrossRef]
- Hughes, A.L.; Kelley, J.R.; Klose, R.J. Understanding the interplay between CpG island-associated gene promoters and H3K4 methylation. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194567. [Google Scholar] [CrossRef]
- Kondo, Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med. J. 2009, 50, 455–463. [Google Scholar] [CrossRef]
- Kaminski, S.; Lukas, J. [Refractive laser surgery of the cornea]. Wien. Med. Wochenschr. 1997, 147, 302–307. [Google Scholar] [PubMed]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Bartczak, K.; Bialas, A.J.; Kotecki, M.J.; Gorski, P.; Piotrowski, W.J. More than a Genetic Code: Epigenetics of Lung Fibrosis. Mol. Diagn. Ther. 2020, 24, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Helling, B.A.; Yang, I.V. Epigenetics in lung fibrosis: From pathobiology to treatment perspective. Curr. Opin. Pulm. Med. 2015, 21, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Song, K.; Tu, B.; Lin, L.C.; Sun, H.; Zhou, Y.; Sha, J.M.; Yang, J.J.; Zhang, Y.; Zhao, J.Y.; et al. Glycolytic reprogramming in organ fibrosis: New dynamics of the epigenetic landscape. Free Radic. Biol. Med. 2023, 207, 1–10. [Google Scholar] [CrossRef]
- Yan, P.; Liu, J.; Li, Z.; Wang, J.; Zhu, Z.; Wang, L.; Yu, G. Glycolysis Reprogramming in Idiopathic Pulmonary Fibrosis: Unveiling the Mystery of Lactate in the Lung. Int. J. Mol. Sci. 2023, 25, 315. [Google Scholar] [CrossRef]
- Bain, C.C.; MacDonald, A.S. The impact of the lung environment on macrophage development, activation and function: Diversity in the face of adversity. Mucosal Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef]
- Dagvadorj, J.; Shimada, K.; Chen, S.; Jones, H.D.; Tumurkhuu, G.; Zhang, W.; Wawrowsky, K.A.; Crother, T.R.; Arditi, M. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2X7 Receptor Leading to Interleukin-1alpha Release. Immunity 2015, 42, 640–653. [Google Scholar] [CrossRef]
- Ahmad, S.; Nasser, W.; Ahmad, A. Epigenetic mechanisms of alveolar macrophage activation in chemical-induced acute lung injury. Front. Immunol. 2024, 15, 1488913. [Google Scholar] [CrossRef]
- Wei, Q.; Deng, Y.; Yang, Q.; Zhan, A.; Wang, L. The markers to delineate different phenotypes of macrophages related to metabolic disorders. Front. Immunol. 2023, 14, 1084636. [Google Scholar] [CrossRef]
- Luppi, F.; Kalluri, M.; Faverio, P.; Kreuter, M.; Ferrara, G. Idiopathic pulmonary fibrosis beyond the lung: Understanding disease mechanisms to improve diagnosis and management. Respir. Res. 2021, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ahn, C.; Kim, T.H. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: A systematic review and meta-analyses. Sci. Rep. 2021, 11, 4318. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Tonelli, R.; Murray, M.; Samarelli, A.V.; Spagnolo, P. Environmental Causes of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 16481. [Google Scholar] [CrossRef] [PubMed]
- Anathy, V.; Lahue, K.G.; Chapman, D.G.; Chia, S.B.; Casey, D.T.; Aboushousha, R.; van der Velden, J.L.J.; Elko, E.; Hoffman, S.M.; McMillan, D.H.; et al. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018, 24, 1128–1135. [Google Scholar] [CrossRef]
- Pokhreal, D.; Crestani, B.; Helou, D.G. Macrophage Implication in IPF: Updates on Immune, Epigenetic, and Metabolic Pathways. Cells 2023, 12, 2193. [Google Scholar] [CrossRef]
- Ogawa, T.; Shichino, S.; Ueha, S.; Matsushima, K. Macrophages in lung fibrosis. Int. Immunol. 2021, 33, 665–671. [Google Scholar] [CrossRef]
- Gu, Y.; Lawrence, T.; Mohamed, R.; Liang, Y.; Yahaya, B.H. The emerging roles of interstitial macrophages in pulmonary fibrosis: A perspective from scRNA-seq analyses. Front. Immunol. 2022, 13, 923235. [Google Scholar] [CrossRef]
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef]
- Venosa, A. Senescence in Pulmonary Fibrosis: Between Aging and Exposure. Front Med. 2020, 7, 606462. [Google Scholar] [CrossRef]
- Sanders, Y.Y.; Ambalavanan, N.; Halloran, B.; Zhang, X.; Liu, H.; Crossman, D.K.; Bray, M.; Zhang, K.; Thannickal, V.J.; Hagood, J.S. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 525–535. [Google Scholar] [CrossRef]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T. Epigenetics Approaches toward Precision Medicine for Idiopathic Pulmonary Fibrosis: Focus on DNA Methylation. Biomedicines 2023, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhong, B.; Fan, Z.; Zhang, H.; Xu, M.; Zhang, X.; Sanders, Y.Y. DNA methylation in pulmonary fibrosis and lung cancer. Expert Rev. Respir. Med. 2022, 16, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Spek, C.A.; Scicluna, B.P.; van der Poll, T.; Duitman, J. Myeloid DNA methyltransferase3b deficiency aggravates pulmonary fibrosis by enhancing profibrotic macrophage activation. Respir. Res. 2022, 23, 162. [Google Scholar] [CrossRef]
- Kishore, A.; Petrek, M. Roles of Macrophage Polarization and Macrophage-Derived miRNAs in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wu, G.R.; Zhou, Q.; Yue, H.; Rao, L.Z.; Yuan, T.; Mo, B.; Wang, F.X.; Chen, L.M.; et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 2021, 7, eabb6075. [Google Scholar] [CrossRef]
- Dogan, F.; Aljumaily, R.M.K.; Kitchen, M.; Forsyth, N.R. DNMT3B Is an Oxygen-Sensitive De Novo Methylase in Human Mesenchymal Stem Cells. Cells 2021, 10, 1032. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Huang, T.; Wu, G.R.; Zhou, Q.; Wang, F.X.; Chen, L.M.; Sun, F.; Lv, Y.; Xiong, F.; et al. The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation. Eur. Respir. J. 2022, 60, 2003697. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, Y.; Zhang, T.; Yin, W. Epigenetic regulation of macrophage activation in chronic obstructive pulmonary disease. Front. Immunol. 2024, 15, 1445372. [Google Scholar] [CrossRef]
- Yu, H.; Liu, S.; Wang, S.; Gu, X. A narrative review of the role of HDAC6 in idiopathic pulmonary fibrosis. J. Thorac. Dis. 2024, 16, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, M.A.; Mantzourani, C.; Kokotos, G. Histone Deacetylase (HDAC) Inhibitors as a Novel Therapeutic Option Against Fibrotic and Inflammatory Diseases. Biomolecules 2024, 14, 1605. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, Y.; Huang, H.; Fan, H.; Hu, L.; Yin, C.; Li, K.; Fulton, D.J.; Chen, F. Epigenetic Regulation of Interleukin 6 by Histone Acetylation in Macrophages and Its Role in Paraquat-Induced Pulmonary Fibrosis. Front. Immunol. 2016, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, J.; Qiao, Y. Epigenetic Modifications in Tumor-Associated Macrophages: A New Perspective for an Old Foe. Front. Immunol. 2022, 13, 836223. [Google Scholar] [CrossRef]
- Chen, L.; Alam, A.; Pac-Soo, A.; Chen, Q.; Shang, Y.; Zhao, H.; Yao, S.; Ma, D. Pretreatment with valproic acid alleviates pulmonary fibrosis through epithelial-mesenchymal transition inhibition in vitro and in vivo. Lab. Investig. 2021, 101, 1166–1175. [Google Scholar] [CrossRef]
- Ayaub, E.A.; Dubey, A.; Imani, J.; Botelho, F.; Kolb, M.R.J.; Richards, C.D.; Ask, K. Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. Sci. Rep. 2017, 7, 13281. [Google Scholar] [CrossRef]
- Coward, W.R.; Watts, K.; Feghali-Bostwick, C.A.; Knox, A.; Pang, L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol. Cell Biol. 2009, 29, 4325–4339. [Google Scholar] [CrossRef]
- Korfei, M.; Mahavadi, P.; Guenther, A. Targeting Histone Deacetylases in Idiopathic Pulmonary Fibrosis: A Future Therapeutic Option. Cells 2022, 11, 1626. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Xiao, T.; Lu, Q. Epigenetics in immune-mediated pulmonary diseases. Clin. Rev. Allergy Immunol. 2013, 45, 314–330. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, G.; Eom, G.H. HDAC Inhibitors: Therapeutic Potential in Fibrosis-Associated Human Diseases. Int. J. Mol. Sci. 2019, 20, 1329. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, H.; Chen, S.; Tang, X.X. Regulation of macrophage activation by lactylation in lung disease. Front. Immunol. 2024, 15, 1427739. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Ma, Q.; Ying, X.; Wang, F.; Hou, Y.; Wang, D.; Zhu, L.; Huang, J.; He, C. Histone lactylation in macrophage biology and disease: From plasticity regulation to therapeutic implications. EBioMedicine 2025, 111, 105502. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Lv, Y.; Dai, X. Lactate, histone lactylation and cancer hallmarks. Expert Rev. Mol. Med. 2023, 25, e7. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Matthews, Q.L.; Liu, R.M.; Liu, G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Hu, H.; Zhou, Y.; Lin, Z.; Jing, H.; Sun, B. Multiomic analysis of monocyte-derived alveolar macrophages in idiopathic pulmonary fibrosis. J. Transl. Med. 2024, 22, 598. [Google Scholar] [CrossRef]
- Madden, K.; Liang, Y.C.; Rajabalee, N.; Alvarez, G.G.; Sun, J. Surveying the Epigenetic Landscape of Tuberculosis in Alveolar Macrophages. Infect. Immun. 2022, 90, e0052221. [Google Scholar] [CrossRef]
- Ahangari, F.; Price, N.L.; Malik, S.; Chioccioli, M.; Barnthaler, T.; Adams, T.S.; Kim, J.; Pradeep, S.P.; Ding, S.; Cosmos, C., Jr.; et al. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI Insight 2023, 8, e158100. [Google Scholar] [CrossRef]
- Yu, J.H.; Long, L.; Luo, Z.X.; Li, L.M.; You, J.R. Anti-inflammatory role of microRNA let-7c in LPS treated alveolar macrophages by targeting STAT3. Asian Pac. J. Trop. Med. 2016, 9, 72–75. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Mallia, P.; Johnston, S.L. Mechanisms and experimental models of chronic obstructive pulmonary disease exacerbations. Proc. Am. Thorac. Soc. 2005, 2, 361–366; discussion 371–362. [Google Scholar] [CrossRef]
- Akata, K.; van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 435. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, X.; Xiao, C.; Xiong, G.; Ye, X.; Li, L.; Fang, Y.; Chen, H.; Yang, W.; Du, X. The role of lung macrophages in chronic obstructive pulmonary disease. Respir. Med. 2022, 205, 107035. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.D.; Hersh, C.P. Integrative genomics of chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 2014, 452, 276–286. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Weichand, B.; Weis, N.; Weigert, A.; Grossmann, N.; Levkau, B.; Brune, B. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol. 2013, 43, 3306–3313. [Google Scholar] [CrossRef]
- Barnawi, J.; Jersmann, H.; Haberberger, R.; Hodge, S.; Meech, R. Reduced DNA methylation of sphingosine-1 phosphate receptor 5 in alveolar macrophages in COPD: A potential link to failed efferocytosis. Respirology 2017, 22, 315–321. [Google Scholar] [CrossRef]
- Zhang, L.; Valizadeh, H.; Alipourfard, I.; Bidares, R.; Aghebati-Maleki, L.; Ahmadi, M. Epigenetic Modifications and Therapy in Chronic Obstructive Pulmonary Disease (COPD): An Update Review. COPD 2020, 17, 333–342. [Google Scholar] [CrossRef]
- Peng, H.; Yang, M.; Chen, Z.Y.; Chen, P.; Guan, C.X.; Xiang, X.D.; Cai, S.; Chen, Y.; Fang, X. Expression and methylation of mitochondrial transcription factor a in chronic obstructive pulmonary disease patients with lung cancer. PLoS ONE 2013, 8, e82739. [Google Scholar] [CrossRef]
- Creighton, S.D.; Stefanelli, G.; Reda, A.; Zovkic, I.B. Epigenetic Mechanisms of Learning and Memory: Implications for Aging. Int. J. Mol. Sci. 2020, 21, 6918. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, Y.; Wang, S.; Li, J.; Luan, J. Harnessing noncoding RNA-based macrophage polarization: Emerging therapeutic opportunities for fibrosis. Immun. Inflamm. Dis. 2020, 8, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.V.; Wright, C.M.; Davidson, M.R.; Francis, S.M.; Yang, I.A.; Fong, K.M. Epigenomic targets for the treatment of respiratory disease. Expert Opin. Ther. Targets 2009, 13, 625–640. [Google Scholar] [CrossRef] [PubMed]
- He, L.X.; Tang, Z.H.; Huang, Q.S.; Li, W.H. DNA Methylation: A Potential Biomarker of Chronic Obstructive Pulmonary Disease. Front. Cell Dev. Biol. 2020, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Szulakowski, P.; Crowther, A.J.; Jimenez, L.A.; Donaldson, K.; Mayer, R.; Leonard, T.B.; MacNee, W.; Drost, E.M. The effect of smoking on the transcriptional regulation of lung inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 174, 41–50. [Google Scholar] [CrossRef]
- Mroz, R.M.; Holownia, A.; Chyczewska, E.; Braszko, J.J. Chronic obstructive pulmonary disease: An update on nuclear signaling related to inflammation and anti-inflammatory treatment. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 35–42. [Google Scholar]
- Mroz, R.M.; Noparlik, J.; Chyczewska, E.; Braszko, J.J.; Holownia, A. Molecular basis of chronic inflammation in lung diseases: New therapeutic approach. J. Physiol. Pharmacol. 2007, 58 (Suppl. 5), 453–460. [Google Scholar]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Ho, S.M. Environmental epigenetics of asthma: An update. J. Allergy Clin. Immunol. 2010, 126, 453–465. [Google Scholar] [CrossRef]
- Ito, K.; Lim, S.; Caramori, G.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001, 15, 1110–1112. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yao, H.; Rahman, I. Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD 2009, 6, 291–297. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Edirisinghe, I.; Yao, H.; Adenuga, D.; Rahman, I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: Epigenetics in pathogenesis of COPD. Antioxid. Redox Signal. 2008, 10, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhong, X.; He, Z.; Wen, M.; Li, J.; Peng, X.; Liu, G.; Deng, J.; Zhang, J.; Bai, J. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. Int. Immunopharmacol. 2012, 12, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J. A Structural Perspective on Readout of Epigenetic Histone and DNA Methylation Marks. Cold Spring Harb. Perspect. Biol. 2016, 8, a018754. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, K.; Kim, K.; Yi, S.J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef]
- Gunes Gunsel, G.; Conlon, T.M.; Jeridi, A.; Kim, R.; Ertuz, Z.; Lang, N.J.; Ansari, M.; Novikova, M.; Jiang, D.; Strunz, M.; et al. The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD. Nat. Commun. 2022, 13, 1303. [Google Scholar] [CrossRef]
- Shen, W.; Wang, S.; Wang, R.; Zhang, Y.; Tian, H.; Yang, X.; Wei, W. Analysis of the polarization states of the alveolar macrophages in chronic obstructive pulmonary disease samples based on miRNA-mRNA network signatures. Ann. Transl. Med. 2021, 9, 1333. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hobbs, B.D.; Hersh, C.P.; Kechris, K. Identifying miRNA-mRNA Networks Associated With COPD Phenotypes. Front. Genet. 2021, 12, 748356. [Google Scholar] [CrossRef]
- Hayek, H.; Kosmider, B.; Bahmed, K. The role of miRNAs in alveolar epithelial cells in emphysema. Biomed. Pharmacother. 2021, 143, 112216. [Google Scholar] [CrossRef]
- Nahid, M.A.; Pauley, K.M.; Satoh, M.; Chan, E.K. miR-146a is critical for endotoxin-induced tolerance: IMPLICATION IN INNATE IMMUNITY. J. Biol. Chem. 2009, 284, 34590–34599. [Google Scholar] [CrossRef]
- Li, R.; Fang, L.; Pu, Q.; Bu, H.; Zhu, P.; Chen, Z.; Yu, M.; Li, X.; Weiland, T.; Bansal, A.; et al. MEG3-4 is a miRNA decoy that regulates IL-1beta abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci. Signal. 2018, 11, eaao2387. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S.S.; Zhang, R.; Goplen, N.P.; Gao, X.; Narasimhan, H.; Shi, A.; Chen, Y.; Li, Y.; Zang, C.; et al. Single cell RNA sequencing unravels mechanisms underlying senescence-like phenotypes of alveolar macrophages. iScience 2023, 26, 107197. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shao, X.; Xing, L.; Li, X.; Nonis, G.M.; Koelwyn, G.J.; Zhang, X.; Sin, D.D. Single-Cell Sequencing of Lung Macrophages and Monocytes Reveals Novel Therapeutic Targets in COPD. Cells 2023, 12, 2771. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, M.; Mizikova, I.; Cook, D.P.; Andersson, N.; Cyr-Depauw, C.; Lesage, F.; Helle, E.; Renesme, L.; Jankov, R.P.; Heikinheimo, M.; et al. Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat. Commun. 2021, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Han, Y.; Wang, Y.; Zhou, D.; Wu, F.; Cai, W.; Zheng, S.; Xiao, Q.; Zhang, H.; Li, W. scMoresDB: A comprehensive database of single-cell multi-omics data for human respiratory system. iScience 2024, 27, 109567. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Chi, X.; Ruan, W.; Meng, X.; Deng, J.; Pan, M.; Ma, T.; Zhang, J. Advances on the Role of Lung Macrophages in the Pathogenesis of Chronic Obstructive Pulmonary Disease in the Era of Single-Cell Genomics. Int. J. Med. Sci. 2025, 22, 298–308. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Wang, C.C.; Pang, X.B.; Shi, J.Z.; Li, H.R.; Xie, X.M.; Wang, Z.; Zhang, H.D.; Zhou, Y.F.; Chen, J.W.; et al. Role of macrophages in pulmonary arterial hypertension. Front. Immunol. 2023, 14, 1152881. [Google Scholar] [CrossRef]
- Zawia, A.; Arnold, N.D.; West, L.; Pickworth, J.A.; Turton, H.; Iremonger, J.; Braithwaite, A.T.; Canedo, J.; Johnston, S.A.; Thompson, A.A.R.; et al. Altered Macrophage Polarization Induces Experimental Pulmonary Hypertension and Is Observed in Patients With Pulmonary Arterial Hypertension. Arter. Thromb. Vasc. Biol. 2021, 41, 430–445. [Google Scholar] [CrossRef]
- Hong, H.; Tian, X.Y. The Role of Macrophages in Vascular Repair and Regeneration after Ischemic Injury. Int. J. Mol. Sci. 2020, 21, 6328. [Google Scholar] [CrossRef]
- Luna, R.C.P.; de Oliveira, Y.; Lisboa, J.V.C.; Chaves, T.R.; de Araujo, T.A.M.; de Sousa, E.E.; Miranda Neto, M.; Pirola, L.; Braga, V.A.; de Brito Alves, J.L. Insights on the epigenetic mechanisms underlying pulmonary arterial hypertension. Braz. J. Med. Biol. Res. 2018, 51, e7437. [Google Scholar] [CrossRef]
- Li, X.; Tan, J.; Wan, J.; Cheng, B.; Wang, Y.H.; Dai, A. Cell Death in Pulmonary Arterial Hypertension. Int. J. Med. Sci. 2024, 21, 1840–1851. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, T.; Yu, J.; Lu, X.; Xiao, S.; Zhang, T.; Qing, T.; Xiao, Z.; Zeng, L.; Luo, L. A new perspective on targeting pulmonary arterial hypertension: Programmed cell death pathways (Autophagy, Pyroptosis, Ferroptosis). Biomed. Pharmacother. 2024, 181, 117706. [Google Scholar] [CrossRef] [PubMed]
- Florentin, J.; Coppin, E.; Vasamsetti, S.B.; Zhao, J.; Tai, Y.Y.; Tang, Y.; Zhang, Y.; Watson, A.; Sembrat, J.; Rojas, M.; et al. Inflammatory Macrophage Expansion in Pulmonary Hypertension Depends upon Mobilization of Blood-Borne Monocytes. J. Immunol. 2018, 200, 3612–3625. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Epigenetic mechanisms in pulmonary arterial hypertension: The need for global perspectives. Eur. Respir. Rev. 2016, 25, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.V.; Schwartz, D.A. Epigenetic control of gene expression in the lung. Am. J. Respir. Crit. Care Med. 2011, 183, 1295–1301. [Google Scholar] [CrossRef]
- Hautefort, A.; Chesne, J.; Preussner, J.; Pullamsetti, S.S.; Tost, J.; Looso, M.; Antigny, F.; Girerd, B.; Riou, M.; Eddahibi, S.; et al. Pulmonary endothelial cell DNA methylation signature in pulmonary arterial hypertension. Oncotarget 2017, 8, 52995–53016. [Google Scholar] [CrossRef]
- Yorn, C.; Kim, H.; Jeong, K. Influence of DNA Methylation on Vascular Smooth Muscle Cell Phenotypic Switching. Int. J. Mol. Sci. 2024, 25, 3136. [Google Scholar] [CrossRef]
- Dave, J.; Jagana, V.; Janostiak, R.; Bisserier, M. Unraveling the epigenetic landscape of pulmonary arterial hypertension: Implications for personalized medicine development. J. Transl. Med. 2023, 21, 477. [Google Scholar] [CrossRef]
- Emon, I.M.; Al-Qazazi, R.; Rauh, M.J.; Archer, S.L. The Role of Clonal Hematopoiesis of Indeterminant Potential and DNA (Cytosine-5)-Methyltransferase Dysregulation in Pulmonary Arterial Hypertension and Other Cardiovascular Diseases. Cells 2023, 12, 2528. [Google Scholar] [CrossRef]
- Kidd, C.D.; Thompson, P.J.; Barrett, L.; Baltic, S. Histone Modifications and Asthma. The Interface of the Epigenetic and Genetic Landscapes. Am. J. Respir. Cell Mol. Biol. 2016, 54, 3–12. [Google Scholar] [CrossRef]
- Groth, A.; Vrugt, B.; Brock, M.; Speich, R.; Ulrich, S.; Huber, L.C. Inflammatory cytokines in pulmonary hypertension. Respir. Res. 2014, 15, 47. [Google Scholar] [CrossRef]
- Paulin, R.; Meloche, J.; Bonnet, S. STAT3 signaling in pulmonary arterial hypertension. JAKSTAT 2012, 1, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kocken, J.M.M.; da Costa Martins, P.A. Epigenetic Regulation of Pulmonary Arterial Hypertension-Induced Vascular and Right Ventricular Remodeling: New Opportunities? Int. J. Mol. Sci. 2020, 21, 8901. [Google Scholar] [CrossRef] [PubMed]
- Adu-Amankwaah, J.; You, Q.; Liu, X.; Jiang, J.; Yang, D.; Liu, K.; Yuan, J.; Wang, Y.; Hu, Q.; Tan, R. Pulmonary Hypertension: Molecular Mechanisms and Clinical Studies. MedComm 2025, 6, e70134. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.L.; Fang, K.; Li, Z.H.; Ren, D.H.; Zhang, J.Y.; Sun, J. EZH2 Inhibition Ameliorates Transverse Aortic Constriction-Induced Pulmonary Arterial Hypertension in Mice. Can. Respir. J. 2018, 2018, 9174926. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, Y.; Fu, X.; Chen, L.; Pan, Z.; Yi, Q.; Zhao, T.; Fu, Z.; Wang, T. THBS1 mediates hypoxia driven EndMT in pulmonary hypertension. Pulm. Circ. 2024, 14, e70019. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.X.; Leng, D.; Li, J.F.; Liang, Y.; Jiang, T. Effect of EZH2 on pulmonary artery smooth muscle cell migration in pulmonary hypertension. Mol. Med. Rep. 2021, 23, 129. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, C.N.; Hajji, N.; Oliver, E.; Cotroneo, E.; Wharton, J.; Wang, D.; Li, M.; McKinsey, T.A.; Stenmark, K.R.; et al. Histone deacetylation inhibition in pulmonary hypertension: Therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation 2012, 126, 455–467. [Google Scholar] [CrossRef]
- Cavasin, M.A.; Demos-Davies, K.; Horn, T.R.; Walker, L.A.; Lemon, D.D.; Birdsey, N.; Weiser-Evans, M.C.; Harral, J.; Irwin, D.C.; Anwar, A.; et al. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ. Res. 2012, 110, 739–748. [Google Scholar] [CrossRef]
- Dang, X.; Qu, X.; Wang, W.; Liao, C.; Li, Y.; Zhang, X.; Xu, D.; Baglole, C.J.; Shang, D.; Chang, Y. Bioinformatic analysis of microRNA and mRNA Regulation in peripheral blood mononuclear cells of patients with chronic obstructive pulmonary disease. Respir. Res. 2017, 18, 4. [Google Scholar] [CrossRef]
- Errington, N.; Iremonger, J.; Pickworth, J.A.; Kariotis, S.; Rhodes, C.J.; Rothman, A.M.; Condliffe, R.; Elliot, C.A.; Kiely, D.G.; Howard, L.S.; et al. A diagnostic miRNA signature for pulmonary arterial hypertension using a consensus machine learning approach. EBioMedicine 2021, 69, 103444. [Google Scholar] [CrossRef]
- Sanchez-Gloria, J.L.; Carbo, R.; Buelna-Chontal, M.; Osorio-Alonso, H.; Henandez-Diazcouder, A.; de la Fuente-Leon, R.L.; Sandoval, J.; Sanchez, F.; Rubio-Gayosso, I.; Sanchez-Munoz, F. Cold exposure aggravates pulmonary arterial hypertension through increased miR-146a-5p, miR-155-5p and cytokines TNF-alpha, IL-1beta, and IL-6. Life Sci. 2021, 287, 120091. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.S.; White, K.; MacLean, M.R.; Baker, A.H. MicroRNAs in pulmonary arterial remodeling. Cell Mol. Life Sci. 2013, 70, 4479–4494. [Google Scholar] [CrossRef] [PubMed]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in pulmonary arterial hypertension: Pathogenesis, diagnosis and treatment. J. Am. Soc. Hypertens. 2015, 9, 221–234. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, T.; Raj, J.U. MicroRNAs in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2015, 52, 139–151. [Google Scholar] [CrossRef]
- Sedighzadeh, S.S.; Khoshbin, A.P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. A narrative review of tumor-associated macrophages in lung cancer: Regulation of macrophage polarization and therapeutic implications. Transl. Lung Cancer Res. 2021, 10, 1889–1916. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Ramazi, S.; Daddzadi, M.; Sahafnejad, Z.; Allahverdi, A. Epigenetic regulation in lung cancer. MedComm 2023, 4, e401. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, X.; Fujiwara, K.; Jurcak, N.; Muth, S.; Zhou, J.; Xiao, Q.; Li, A.; Che, X.; Li, Z.; et al. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct. Target. Ther. 2021, 6, 366. [Google Scholar] [CrossRef]
- Gonda, T.A.; Fang, J.; Salas, M.; Do, C.; Hsu, E.; Zhukovskaya, A.; Siegel, A.; Takahashi, R.; Lopez-Bujanda, Z.A.; Drake, C.G.; et al. A DNA Hypomethylating Drug Alters the Tumor Microenvironment and Improves the Effectiveness of Immune Checkpoint Inhibitors in a Mouse Model of Pancreatic Cancer. Cancer Res. 2020, 80, 4754–4767. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, D.K.; Bao, L.; Bai, F.; Chencheri, S.C.; Lucas, K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol. 2014, 35, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, V.; Buccione, C.; Ziccheddu, G.; Peschiaroli, F.; Sestili, P.; Puglisi, R.; Mattia, G.; Zanetti, C.; Parolini, I.; Bracci, L.; et al. Combining Type I Interferons and 5-Aza-2′-Deoxycitidine to Improve Anti-Tumor Response against Melanoma. J. Investig. Dermatol. 2017, 137, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Tellez, C.S.; Picchi, M.A.; Juri, D.; Do, K.; Desai, D.H.; Amin, S.G.; Hutt, J.A.; Filipczak, P.T.; Belinsky, S.A. Chromatin remodeling by the histone methyltransferase EZH2 drives lung pre-malignancy and is a target for cancer prevention. Clin. Epigenetics 2021, 13, 44. [Google Scholar] [CrossRef]
- Liyanage, C.; Wathupola, A.; Muraleetharan, S.; Perera, K.; Punyadeera, C.; Udagama, P. Promoter Hypermethylation of Tumor-Suppressor Genes p16 (INK4a), RASSF1A, TIMP3, and PCQAP/MED15 in Salivary DNA as a Quadruple Biomarker Panel for Early Detection of Oral and Oropharyngeal Cancers. Biomolecules 2019, 9, 148. [Google Scholar] [CrossRef]
- Hoang, P.H.; Landi, M.T. DNA Methylation in Lung Cancer: Mechanisms and Associations with Histological Subtypes, Molecular Alterations, and Major Epidemiological Factors. Cancers 2022, 14, 961. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, P.; Capalash, N. DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer. Curr. Cancer Drug Targets 2013, 13, 379–399. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; Song, P.; Zhan, X. DNA and histone modifications as potent diagnostic and therapeutic targets to advance non-small cell lung cancer management from the perspective of 3P medicine. EPMA J. 2022, 13, 649–669. [Google Scholar] [CrossRef]
- Witta, S. Histone deacetylase inhibitors in non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, S404–S406. [Google Scholar] [CrossRef]
- Reed, M.D.; Tellez, C.S.; Grimes, M.J.; Picchi, M.A.; Tessema, M.; Cheng, Y.S.; March, T.H.; Kuehl, P.J.; Belinsky, S.A. Aerosolised 5-azacytidine suppresses tumour growth and reprogrammes the epigenome in an orthotopic lung cancer model. Br. J. Cancer 2013, 109, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gang, X.; Yan, J.; Li, X.; Shi, S.; Xu, L.; Liu, R.; Cai, L.; Li, H.; Zhao, M. Immune checkpoint inhibitors rechallenge in non-small cell lung cancer: Current evidence and future directions. Cancer Lett. 2024, 604, 217241. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Johnson, R.W. Targeting Histone Modifications in Bone and Lung Metastatic Cancers. Curr. Osteoporos. Rep. 2021, 19, 230–246. [Google Scholar] [CrossRef]
- Samanta, S.; Zhou, Z.; Rajasingh, S.; Panda, A.; Sampath, V.; Rajasingh, J. DNMT and HDAC inhibitors together abrogate endotoxemia mediated macrophage death by STAT3-JMJD3 signaling. Int. J. Biochem. Cell Biol. 2018, 102, 117–127. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, H.; Li, X.; Zong, L.; Wei, J.; Li, Z.; Luo, C.; Yang, X.; Fang, H.; Kong, X.; et al. Development of a First-in-Class DNMT1/HDAC Inhibitor with Improved Therapeutic Potential and Potentiated Antitumor Immunity. J. Med. Chem. 2024, 67, 16480–16504. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, S.; Gao, C.; Dai, Q.; Zhang, C.; Sun, Q.; Lin, J.S.; Guo, C.; Chen, Y.; Jiang, Y. Development of a versatile DNMT and HDAC inhibitor C02S modulating multiple cancer hallmarks for breast cancer therapy. Bioorg Chem. 2019, 87, 200–208. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, D. M2 macrophage-derived exosomal miR-145-5p protects against the hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes by inhibiting TLR4 expression. Ann. Transl. Med. 2022, 10, 1376. [Google Scholar] [CrossRef]
- Xun, J.; Du, L.; Gao, R.; Shen, L.; Wang, D.; Kang, L.; Chen, C.; Zhang, Z.; Zhang, Y.; Yue, S.; et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 2021, 11, 6847–6859. [Google Scholar] [CrossRef]
- Chang, C.Y.; Armstrong, D.; Corry, D.B.; Kheradmand, F. Alveolar macrophages in lung cancer: Opportunities challenges. Front. Immunol. 2023, 14, 1268939. [Google Scholar] [CrossRef]
- Wang, N.; Wu, R.; Tang, D.; Kang, R. The BET family in immunity and disease. Signal Transduct. Target. Ther. 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Iten, M.; Gschwend, C.; Ostini, A.; Cameron, D.R.; Goepfert, C.; Berger, D.; Haenggi, M. BET-inhibitor DYB-41 reduces pulmonary inflammation and local and systemic cytokine levels in LPS-induced acute respiratory distress syndrome: An experimental rodent study. Intensive Care Med. Exp. 2024, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Kim, C.; Zhou, M.M. The Functions of BET Proteins in Gene Transcription of Biology and Diseases. Front. Mol. Biosci. 2021, 8, 728777. [Google Scholar] [CrossRef] [PubMed]
- Zakarya, R.; Chan, Y.L.; Rutting, S.; Reddy, K.; Bozier, J.; Woldhuis, R.R.; Xenaki, D.; Van Ly, D.; Chen, H.; Brandsma, C.A.; et al. BET proteins are associated with the induction of small airway fibrosis in COPD. Thorax 2021, 76, 647–655. [Google Scholar] [CrossRef]
- Guo, X.; Olajuyin, A.; Tucker, T.A.; Idell, S.; Qian, G. BRD4 as a Therapeutic Target in Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 13231. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Nicholls, S.J.; Toth, P.P.; Sweeney, M.; Halliday, C.; Johansson, J.O.; Wong, N.C.W.; Kulikowski, E.; Kalantar-Zadeh, K.; Ginsberg, H.N.; et al. Relation of insulin treatment for type 2 diabetes to the risk of major adverse cardiovascular events after acute coronary syndrome: An analysis of the BETonMACE randomized clinical trial. Cardiovasc. Diabetol. 2021, 20, 125. [Google Scholar] [CrossRef]
- Ray, K.K.; Nicholls, S.J.; Ginsberg, H.D.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Toth, P.P.; Wong, N.; Cummings, J.L.; Sweeney, M.; et al. Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: Rationale, design, and baseline characteristics of the BETonMACE trial. Am. Heart J. 2019, 217, 72–83. [Google Scholar] [CrossRef]
- Nasser, M.; Larrieu, S.; Si-Mohamed, S.; Ahmad, K.; Boussel, L.; Brevet, M.; Chalabreysse, L.; Fabre, C.; Marque, S.; Revel, D.; et al. Progressive fibrosing interstitial lung disease: A clinical cohort (the PROGRESS study). Eur. Respir. J. 2021, 57, 2002718. [Google Scholar] [CrossRef]
- Ritzmann, F.; Brand, M.; Bals, R.; Wegmann, M.; Beisswenger, C. Role of Epigenetics in Chronic Lung Disease. Cells 2025, 14, 251. [Google Scholar] [CrossRef]
- Yuan, H.; Reddy, M.A.; Sun, G.; Lanting, L.; Wang, M.; Kato, M.; Natarajan, R. Involvement of p300/CBP and epigenetic histone acetylation in TGF-beta1-mediated gene transcription in mesangial cells. Am. J. Physiol. Ren. Physiol. 2013, 304, F601–F613. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of Histone Post-Translational Modifications in Inflammatory Diseases. Front. Immunol. 2022, 13, 852272. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Malaviya, R.; Laskin, J.D. Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants. Toxicol. Sci. 2019, 168, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, S.; Sun, Y.; Zheng, S.; Zong, L.; Li, P. Cut&tag: A powerful epigenetic tool for chromatin profiling. Epigenetics 2024, 19, 2293411. [Google Scholar] [CrossRef] [PubMed]
- Blutt, S.E.; Coarfa, C.; Neu, J.; Pammi, M. Multiomic Investigations into Lung Health and Disease. Microorganisms 2023, 11, 2116. [Google Scholar] [CrossRef]
- Druszczynska, M.; Sadowska, B.; Kulesza, J.; Gasienica-Gliwa, N.; Kulesza, E.; Fol, M. The Intriguing Connection Between the Gut and Lung Microbiomes. Pathogens 2024, 13, 1005. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Verma, A.; Bhagchandani, T.; Rai, A.; Nikita; Sardarni, U.K.; Bhavesh, N.S.; Gulati, S.; Malik, R.; Tandon, R. Short-Chain Fatty Acid (SCFA) as a Connecting Link between Microbiota and Gut-Lung Axis-A Potential Therapeutic Intervention to Improve Lung Health. ACS Omega 2024, 9, 14648–14671. [Google Scholar] [CrossRef]
- McCubbrey, A.L.; Curtis, J.L. Efferocytosis and lung disease. Chest 2013, 143, 1750–1757. [Google Scholar] [CrossRef]
- Ni, Y.F.; Wang, J.; Yan, X.L.; Tian, F.; Zhao, J.B.; Wang, Y.J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef]
- Otake, S.; Chubachi, S.; Miyamoto, J.; Haneishi, Y.; Arai, T.; Iizuka, H.; Shimada, T.; Sakurai, K.; Okuzumi, S.; Kabata, H.; et al. Impact of smoking on gut microbiota and short-chain fatty acids in human and mice: Implications for COPD. Mucosal Immunol. 2025, 18, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xing, Y.; Song, X.; Qian, Y. The impact of lung microbiota dysbiosis on inflammation. Immunology 2020, 159, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N.Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Helly, F.; Tarling, E.; Keane, M.P.; McCarthy, C. Methionine supplementation: Potential for improving alveolar macrophage function through reverse cholesterol transport? Eur. Respir. J. 2022, 59, 2102594. [Google Scholar] [CrossRef]
- Choi, I.; Son, H.; Baek, J.H. Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses. Life 2021, 11, 69. [Google Scholar] [CrossRef]
- Qu, L.; Yin, T.; Zhao, Y.; Lv, W.; Liu, Z.; Chen, C.; Liu, K.; Shan, S.; Zhou, R.; Li, X.; et al. Histone demethylases in the regulation of immunity and inflammation. Cell Death Discov. 2023, 9, 188. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- Mangalhara, K.C.; Varanasi, S.K.; Johnson, M.A.; Burns, M.J.; Rojas, G.R.; Esparza Molto, P.B.; Sainz, A.G.; Tadepalle, N.; Abbott, K.L.; Mendiratta, G.; et al. Manipulating mitochondrial electron flow enhances tumor immunogenicity. Science 2023, 381, 1316–1323. [Google Scholar] [CrossRef]
- Etchegaray, J.P.; Mostoslavsky, R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol. Cell 2016, 62, 695–711. [Google Scholar] [CrossRef]
- He, W.; Li, Q.; Li, X. Acetyl-CoA regulates lipid metabolism and histone acetylation modification in cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.Y. Macrophages: Friend or foe in idiopathic pulmonary fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Q.; Shi, Z.; Tu, Y.; Li, Q.; Zheng, W.; Yuan, Z.; Li, L.; Zu, X.; Hao, Y.; et al. Dual inhibitors of DNMT and HDAC induce viral mimicry to induce antitumour immunity in breast cancer. Cell Death Discov. 2024, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef]
- Pathania, R.; Ramachandran, S.; Mariappan, G.; Thakur, P.; Shi, H.; Choi, J.H.; Manicassamy, S.; Kolhe, R.; Prasad, P.D.; Sharma, S.; et al. Combined Inhibition of DNMT and HDAC Blocks the Tumorigenicity of Cancer Stem-like Cells and Attenuates Mammary Tumor Growth. Cancer Res. 2016, 76, 3224–3235. [Google Scholar] [CrossRef]
- Bhat, M.F.; Srdanovic, S.; Sundberg, L.R.; Einarsdottir, H.K.; Marjomaki, V.; Dekker, F.J. Impact of HDAC inhibitors on macrophage polarization to enhance innate immunity against infections. Drug Discov. Today 2024, 29, 104193. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sharifi, A.; Pourpaknia, R.; Mohammadian, S.; Sahebkar, A. Manipulating macrophage polarization and function using classical HDAC inhibitors: Implications for autoimmunity and inflammation. Crit. Rev. Oncol. Hematol. 2018, 128, 1–18. [Google Scholar] [CrossRef]
- Cheng, H.P.; Jiang, S.H.; Cai, J.; Luo, Z.Q.; Li, X.H.; Feng, D.D. Histone deacetylases: Potential therapeutic targets for idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1426508. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, Y.; Lei, D.; Xiang, J.; Ouyang, L.; Wang, Y.; Yang, J. Recent progress in DNA methyltransferase inhibitors as anticancer agents. Front. Pharmacol. 2022, 13, 1072651. [Google Scholar] [CrossRef]
- Bi, L.; Wang, X.; Li, J.; Li, W.; Wang, Z. Epigenetic modifications in early stage lung cancer: Pathogenesis, biomarkers, and early diagnosis. MedComm 2025, 6, e70080. [Google Scholar] [CrossRef]
- Traynor, S.; Terp, M.G.; Nielsen, A.Y.; Guldberg, P.; Jakobsen, M.; Pedersen, P.G.; Gammelgaard, O.L.; Pedersen, C.B.; Pedersen, M.T.; Rattenborg, S.; et al. DNA methyltransferase inhibition promotes recruitment of myeloid-derived suppressor cells to the tumor microenvironment through induction of tumor cell-intrinsic interleukin-1. Cancer Lett. 2023, 552, 215982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).