Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Isolation, Culture, and Characterization
2.2. Fabrication of Substrates with Various Stiffnesses
2.3. RNA Isolation for Oligonucleotide Microarray and qPCR
2.4. Staining and Histological Analysis
2.5. Statistical Analysis
3. Results
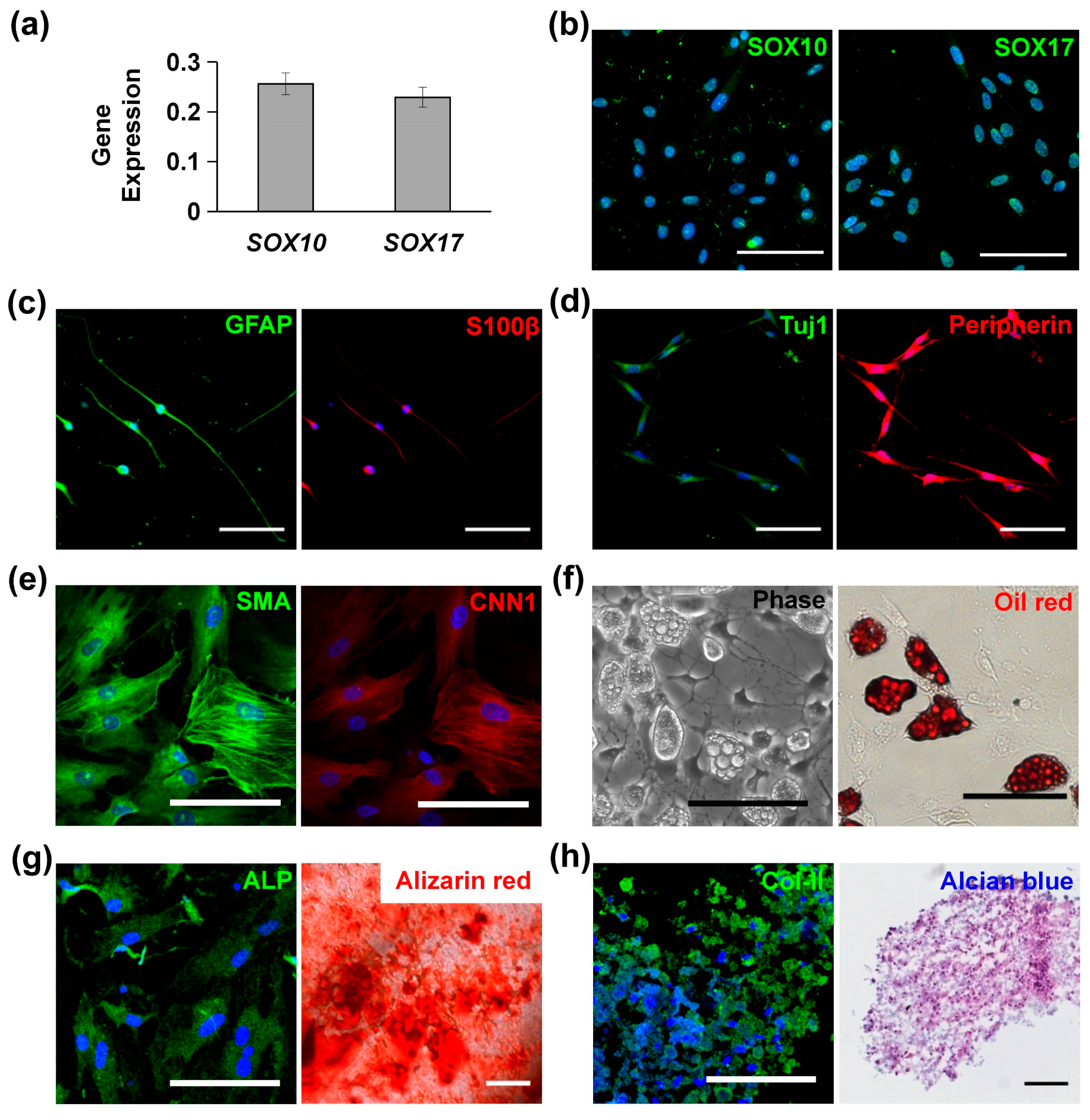
3.1. Characterization and Multipotency of MVSCs
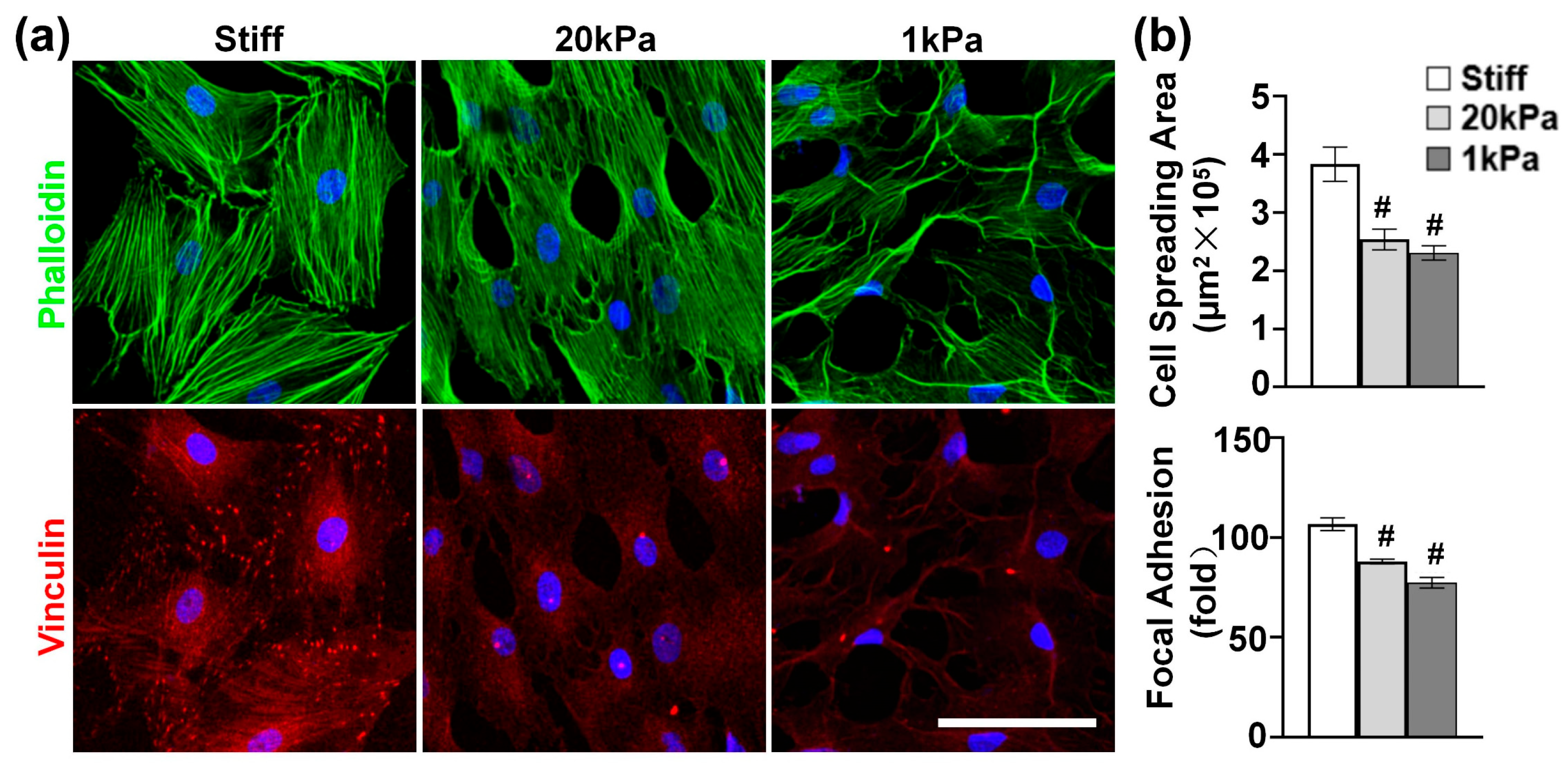
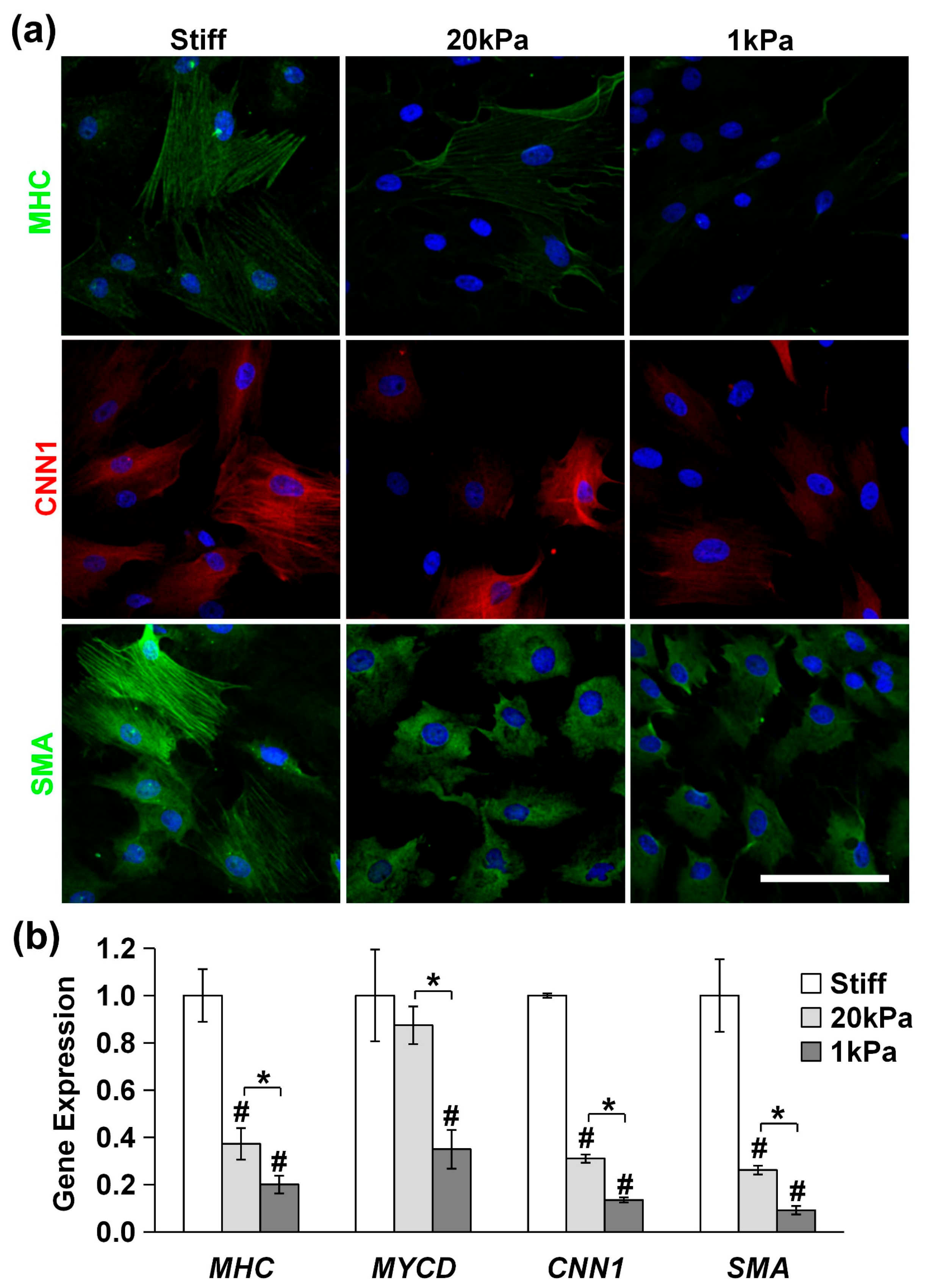
3.2. Varying the Stiffness of PA Gels Modulates Cytoskeleton Organization
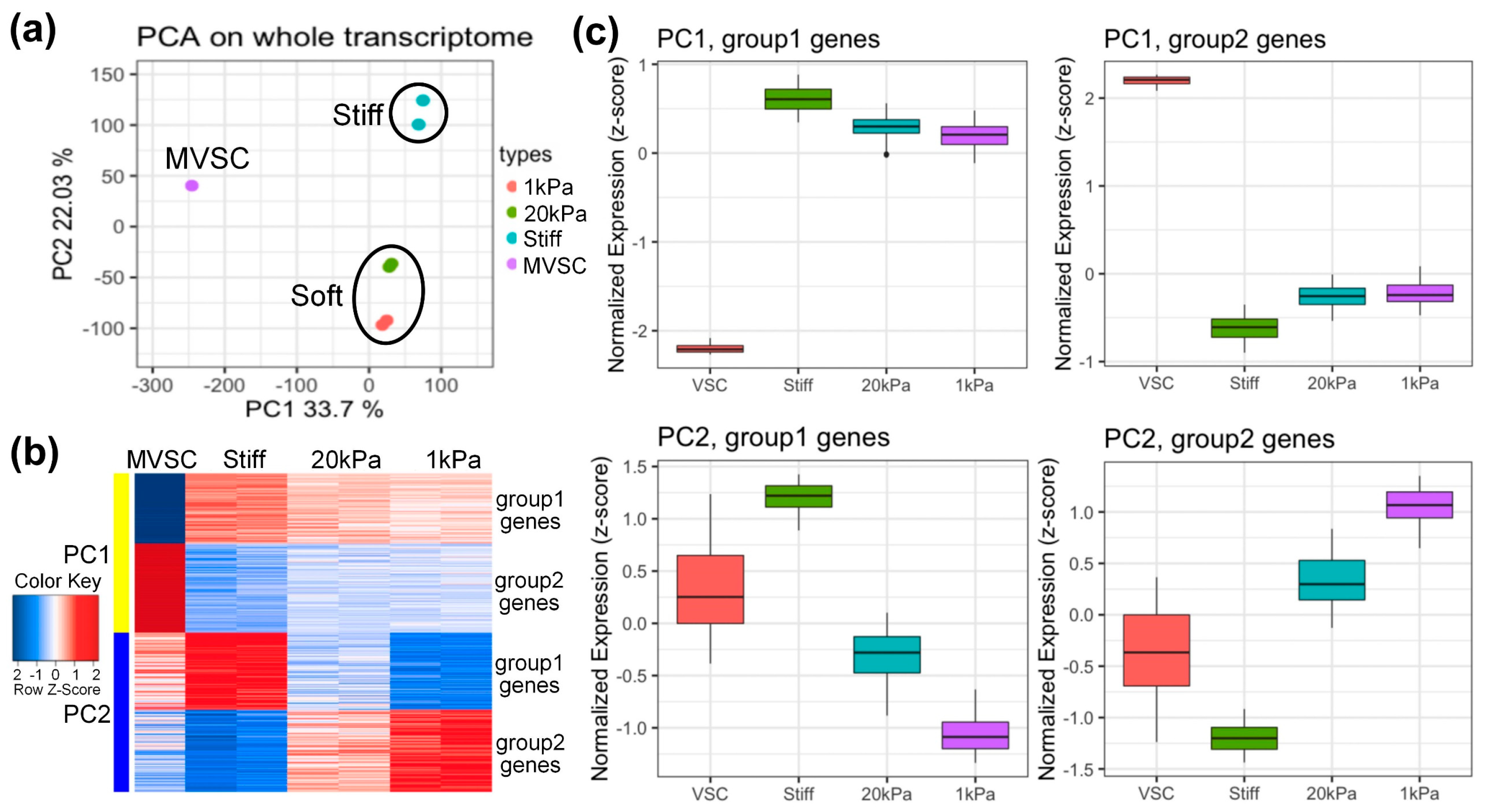
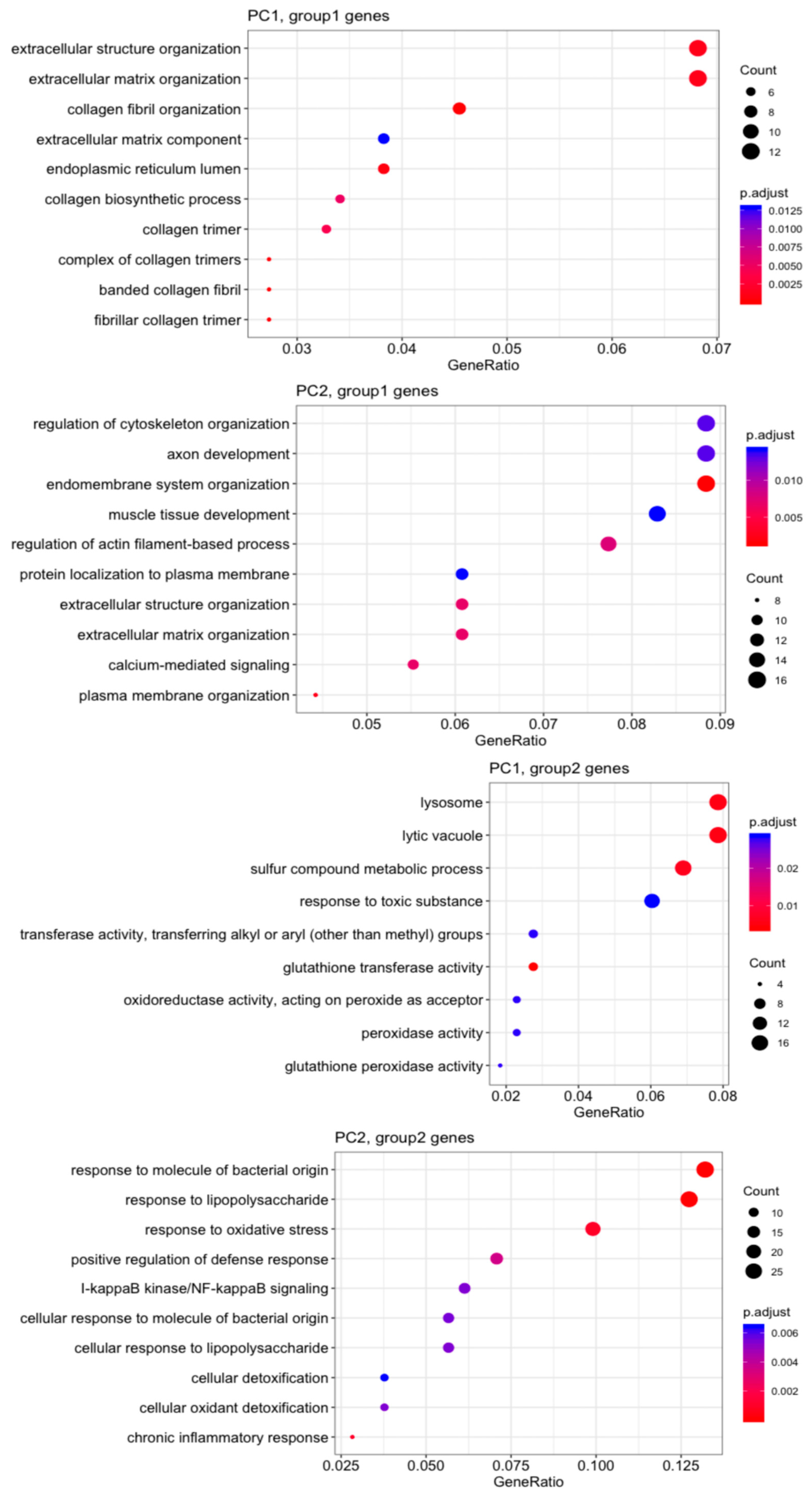
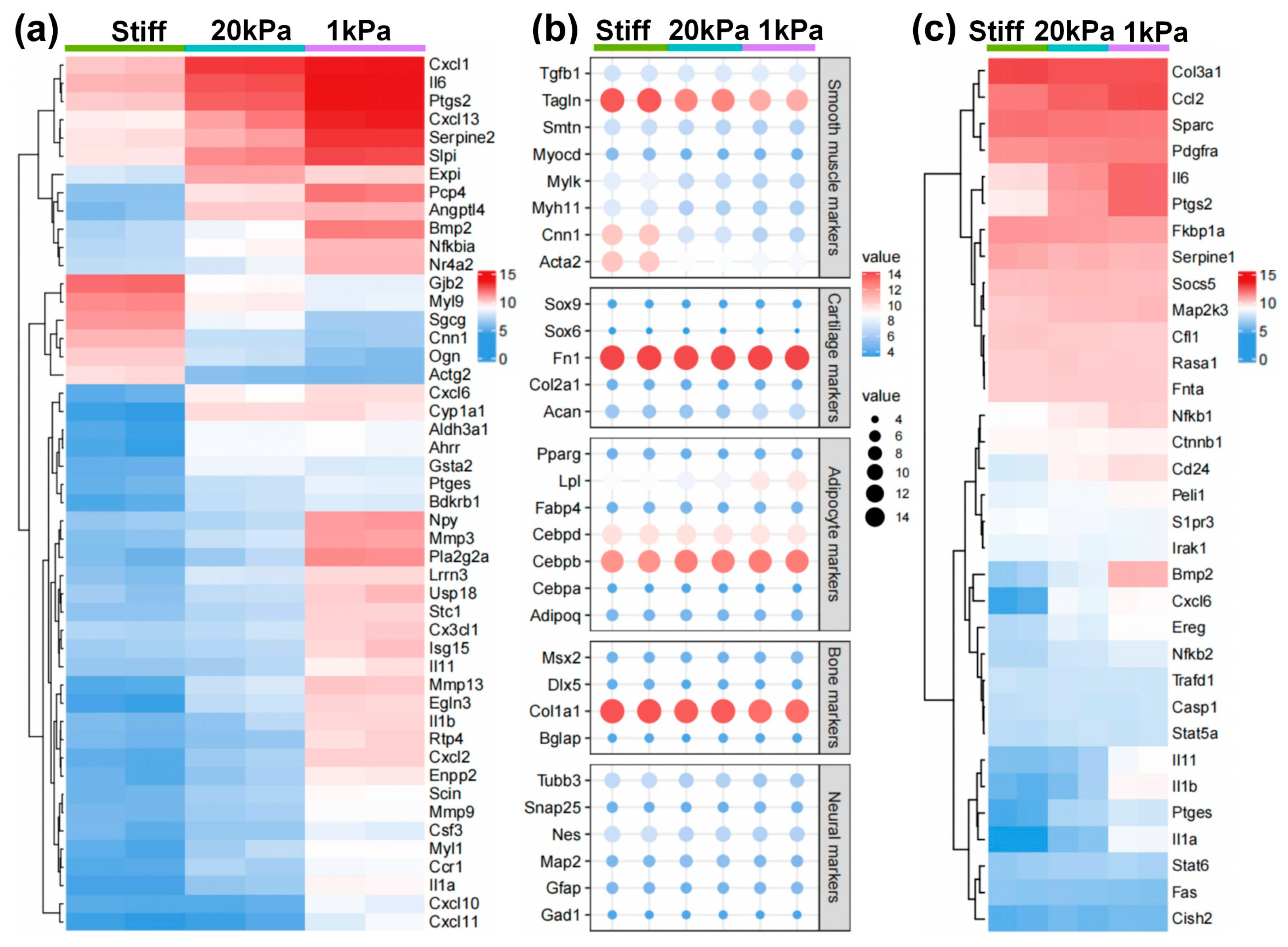
3.3. Microarray Analysis of MVSCs on PA Gels with Varying Stiffnesses
3.4. Stiff Substrates Promote MVSC Differentiation into SMC Lineage
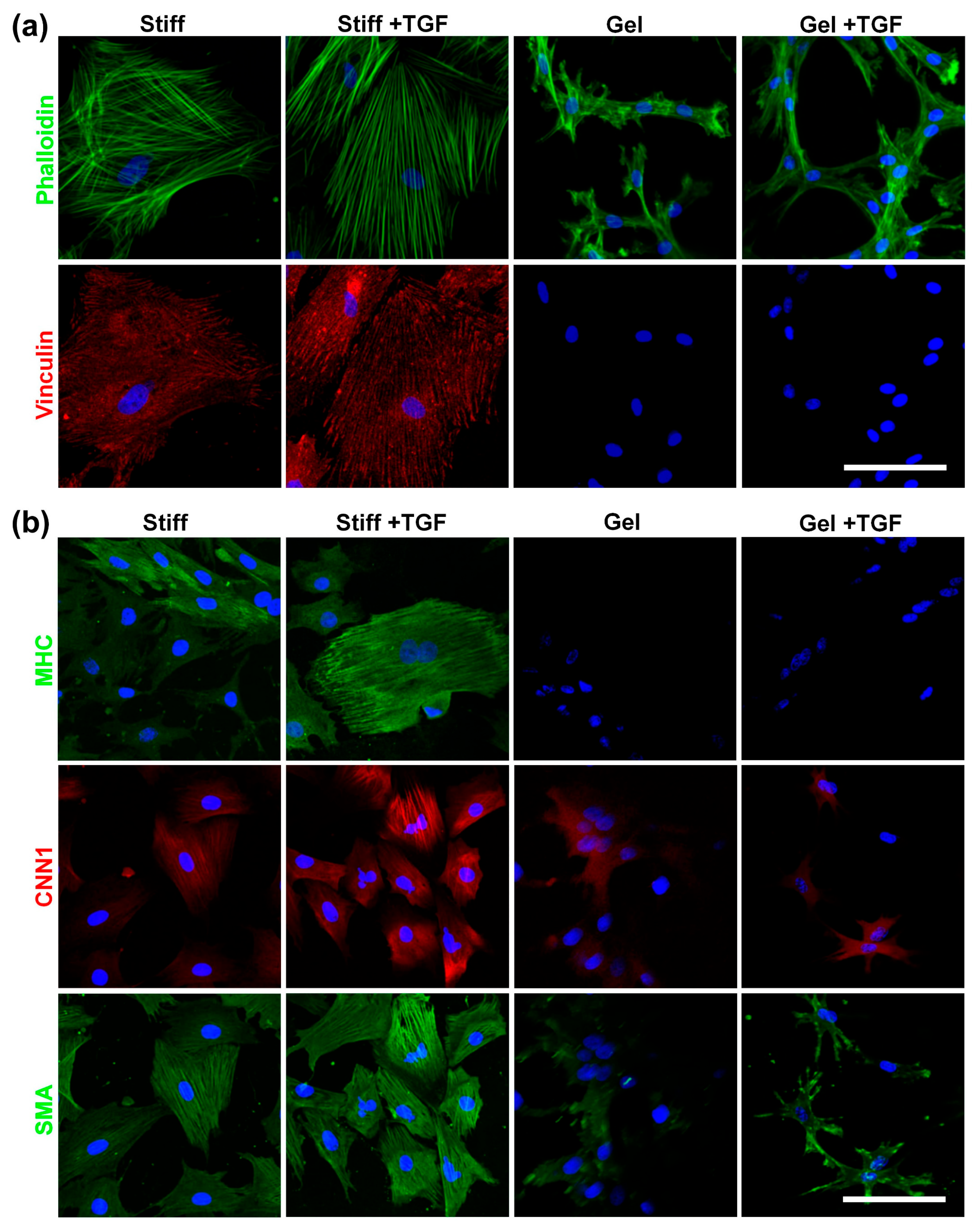
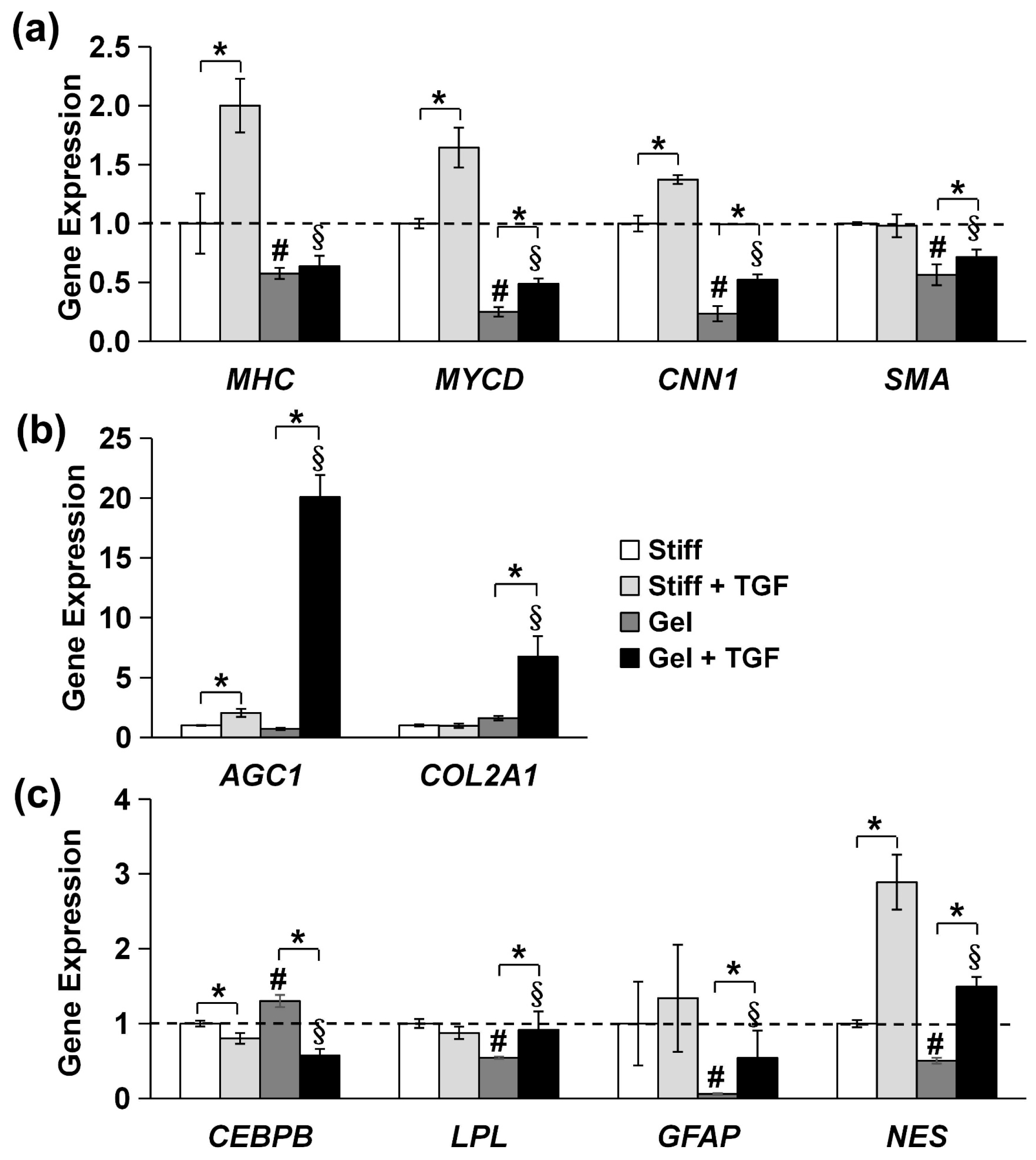
3.5. Validation of Cytoskeleton Organization and Lineage Commitment of MVSCs on Varying Stiffnesses of Collagen Gels and TGF-β1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Atherosclerosis |
| MVSCs | Multipotent vascular stem cells |
| SMCs | Vascular smooth muscle cells |
| MHC | Myosin heavy chain |
| MYCD | Myocardin |
| CNN1 | Calponin 1 |
| SMA | Smooth muscle α-actin |
| ECM | Extracellular matrix |
| PA | Polyacrylamide |
| MSCs | Bone marrow mesenchymal stem cells |
| NCSCs | Neural crest stem cells |
| DEGs | Differentially expressed genes |
| AGC1 | Aggrecan 1 |
| COL2A1 | Collagen-II |
| CEBPB | CCAAT/enhancer binding protein beta |
| LPL | Lipoprotein lipase |
| PFA | Paraformaldehyde |
| F-actin | Filamentous actin |
| DAPI | 4,6-diamidino-2-phenylindole |
| ALP | Alkaline phosphatase |
| PCA | Principal component analysis |
| PC | Principal component |
| GO | Gene Ontology |
References
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. Inflammation and immunity in diseases of the arterial tree: Players and layers. Circ. Res. 2015, 116, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, A.; Yuan, F.; Yan, Z.; Liu, B.; Chu, J.S.; Helms, J.A.; Li, S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat. Commun. 2012, 3, 875. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Wang, D.; Xu, K.; Wang, J.X.; Zhang, Z.J.; Yang, L.; Yang, G.Y.; Li, S. Contribution of vascular cells to neointimal formation. PLoS ONE 2017, 12, e0168914. [Google Scholar] [CrossRef]
- Ciavarella, C.; Valente, S.; Pasquinelli, G. The The characteristics and survival potential under sub-lethal stress of mesenchymal stromal/stem cells isolated from the human vascular wall. Stem Cells 2022, 40, 1071–1077. [Google Scholar] [CrossRef]
- Putra, V.D.L.; Kilian, K.A.; Knothe Tate, M.L. Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment. Commun. Biol. 2023, 6, 75. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of electrospun scaffolds and biological polymers for enhancing the delivery and efficacy of mesen-chymal stem/stromal cell therapies. Front. Biosci. 2024, 29, 228. [Google Scholar] [CrossRef]
- Kureel, S.K.; Maroto, R.; Davis, K.; Sheetz, M. Cellular mechanical memory: A potential tool for mesenchymal stem cell-based therapy. Stem Cell Res. Ther. 2025, 16, 159. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3d. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Lyle, A.N.; Raaz, U. Killing me unsoftly: Causes and mechanisms of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 4. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Califano, J.P.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011, 3, 3002761. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Soto, J.; Wong, S.Y.; Wu, Y.; Hoffman, T.; Akhtar, N.; Norris, S.; Chu, J.; Park, H.; Kelkhoff, D.O.; et al. Biphasic regulation of epigenetic state by matrix stiffness during cell reprogramming. Sci. Adv. 2024, 10, eadk0639. [Google Scholar] [CrossRef]
- Matsumoto, T.; Abe, H.; Ohashi, T.; Kato, Y.; Sato, M. Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol. Meas. 2002, 23, 635–648. [Google Scholar] [CrossRef]
- Hansen, L.; Taylor, W.R. Is increased arterial stiffness a cause or consequence of atherosclerosis? Atherosclerosis 2016, 249, 226–227. [Google Scholar] [CrossRef]
- Raaz, U.; Zollner, A.M.; Schellinger, I.N.; Toh, R.; Nakagami, F.; Brandt, M.; Emrich, F.C.; Kayama, Y.; Eken, S.; Adam, M.; et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015, 131, 1783–1795. [Google Scholar] [CrossRef]
- Sainz, J.; Al Haj Zen, A.; Caligiuri, G.; Demerens, C.; Urbain, D.; Lemitre, M.; Lafont, A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 281–286. [Google Scholar] [CrossRef]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024, 30, 706–750. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to tgf-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, A.; Wu, F.; Qiu, X.; Li, Y.; Chu, J.; Huang, W.C.; Xu, K.; Gong, X.; Li, S. Sox10+ adult stem cells contribute to biomaterial encapsulation and microvascularization. Sci. Rep. 2017, 7, 40295. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lao, J.; Chen, B.P.; Li, Y.S.; Zhao, Y.; Chu, J.; Chen, K.D.; Tsou, T.C.; Peck, K.; Chien, S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003, 17, 97–99. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile mechanical properties of three-dimensional type i collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef]
- Pan, M.; Chew, T.W.; Wong, D.C.P.; Xiao, J.; Ong, H.T.; Chin, J.F.L.; Low, B.C. Bnip-2 retards breast cancer cell migration by coupling microtubule-mediated gef-h1 and RhoA activation. Sci. Adv. 2020, 6, eaaz1534. [Google Scholar] [CrossRef]
- Sun, A.R.; Ramli, M.F.H.; Shen, X.; Chen, D.; Foo, R.S.; Zhu, J.; Ackers-Johnson, M.; Young, J.L. Hybrid hydrogel-extracellular matrix scaffolds identify distinct ligand and mechanical signatures in cardiac aging. bioRxiv 2023. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Majesky, M.W.; Dong, X.R.; Regan, J.N.; Hoglund, V.J. Vascular smooth muscle progenitor cells: Building and repairing blood vessels. Circ. Res. 2011, 108, 365–377. [Google Scholar] [CrossRef]
- Han, Y.; Yan, L.; Xia, L.; Li, S.; Zhang, Q.; Jin, C. Global trends and frontier topics about vascular smooth muscle cells phenotype switch: A bibliometric analysis from 1999 to 2021. Front. Pharmacol. 2022, 13, 1004525. [Google Scholar] [CrossRef]
- Du, L.; Sun, X.; Gong, H.; Wang, T.; Jiang, L.; Huang, C.; Xu, X.; Li, Z.; Xu, H.; Ma, L.; et al. Single cell and lineage tracing studies reveal the impact of CD34+ cells on myocardial fibrosis during heart failure. Stem Cell Res. Ther. 2023, 14, 33. [Google Scholar] [CrossRef]
- Lyu, L.; Li, Z.; Wen, Z.; He, Y.; Wang, X.; Jiang, L.; Zhou, X.; Huang, C.; Wu, Y.; Chen, T.; et al. Fate mapping rna-sequencing reveal malat1 regulates sca1+ progenitor cells to vascular smooth muscle cells transition in vascular remodeling. Cell Mol. Life Sci. 2023, 80, 118. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, M.; Fitzpatrick, E.; Burtenshaw, D.; Liu, W.; Helt, J.-C.; Hakimjavadi, R.; Corcoran, E.; Gusti, Y.; Sheridan, D.; Harman, S.; et al. The calcium binding protein s100β marks hedgehog-responsive resident vascular stem cells within vascular lesions. Npj Regen. Med. 2021, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Molony, C.; King, D.; Di Luca, M.; Kitching, M.; Olayinka, A.; Hakimjavadi, R.; Julius, L.A.N.; Fitzpatrick, E.; Gusti, Y.; Burtenshaw, D.; et al. Disease-relevant single cell photonic signatures identify s100β stem cells and their myogenic progeny in vascu-lar lesions. Stem Cell Rev. Rep. 2021, 17, 1713–1740. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Calderwood, D.A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ecm adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2021, 15, 82–102. [Google Scholar] [CrossRef]
- Lang, Z.; Chen, T.; Zhu, S.; Wu, X.; Wu, Y.; Miao, X.; Wang, Q.; Zhao, L.; Zhu, Z.; Xu, R.X. Construction of vascular grafts based on tissue-engineered scaffolds. Mater. Today Bio 2024, 29, 101336. [Google Scholar] [CrossRef]
- Hyun, J.; Kim, S.J.; Cho, S.D.; Kim, H.W. Mechano-modulation of T cells for cancer immunotherapy. Biomaterials 2023, 297, 1. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| SOX10 | CTGGAGGTTGCTGAACGAGAGT | GTCCGGATGGTCTTTTTTGTG |
| SOX17 | AGAACCCGGATCTGCACAAC | AGGATTTGCCTAGCATCTTGCT |
| CNN1 | AGAACAAGCTGGCCCAGAAA | CACCCCTTCGATCCACTCTCT |
| SMA | TCCTGACCCTGAAGTATCCGATA | GGTGCCAGATCTTTTCCATGTC |
| MHC | TTCCGGCAACGCTACGA | TCCATCCATGAAGCCTTTGG |
| MYCD | TCTTACAGTTACGGCTTCAACAGAGA | CCTCGGGTCATGGAACTCA |
| AGC1 | CTTCAAGCTGAACTATGACCACTTTACT | CATGGTCTGGAACTTCTTCTGAGA |
| COL2A1 | CCAGGGCTCCAATGATGTG | GTGTTTCGTGCAGCCATCCT |
| CEBPB | CGCCTTTAGACCCATGGAAGT | AGGCAGTCGGGCTCGTAGTAG |
| LPL | TCTCTTGGGATACAGCCTTGGA | GCCAGTAATTCTGTTGACTTTCTTATTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Wang, Y.; Chu, J.S.; Yang, L.; Li, X.; Li, S. Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells. Cells 2025, 14, 611. https://doi.org/10.3390/cells14080611
Yan Y, Wang Y, Chu JS, Yang L, Li X, Li S. Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells. Cells. 2025; 14(8):611. https://doi.org/10.3390/cells14080611
Chicago/Turabian StyleYan, Yujie, Yuhang Wang, Julia S. Chu, Li Yang, Xian Li, and Song Li. 2025. "Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells" Cells 14, no. 8: 611. https://doi.org/10.3390/cells14080611
APA StyleYan, Y., Wang, Y., Chu, J. S., Yang, L., Li, X., & Li, S. (2025). Substrate Stiffness Modulates TGF-β1-Induced Lineage Specification in Multipotent Vascular Stem Cells. Cells, 14(8), 611. https://doi.org/10.3390/cells14080611







