LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation
Abstract
1. Introduction
2. Methods and Materials
2.1. Animals
2.2. LRG1 Protein
2.3. LRG1 Overexpression Vector
2.4. Oxygen-Induced Retinopathy (OIR)
2.5. Vascular Leakage
2.6. Immunohistochemistry
2.7. Single-Cell RNA Sequencing Analysis
2.8. Transmission Electron Microscopy
2.9. Analysis and Statistics
3. Results
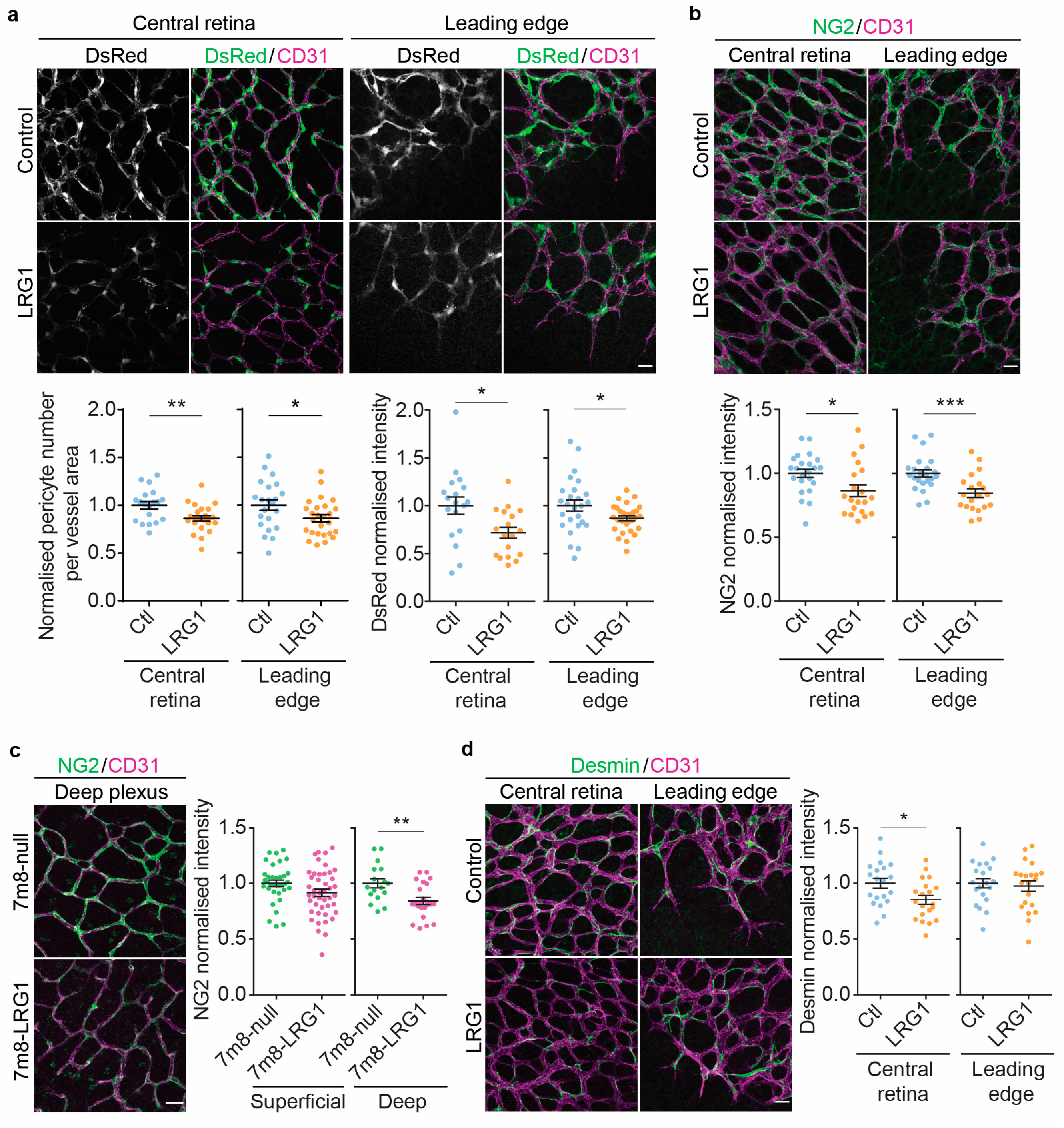
3.1. LRG1 Reduces Pericyte Coverage and Pericytic NG2 Expression in the Retina
3.2. LRG1 Reduces Vessel Collagen IV Coverage
3.3. LRG1 Increases Vascular Leakage Without Altering the Expression of Tight Junction Proteins
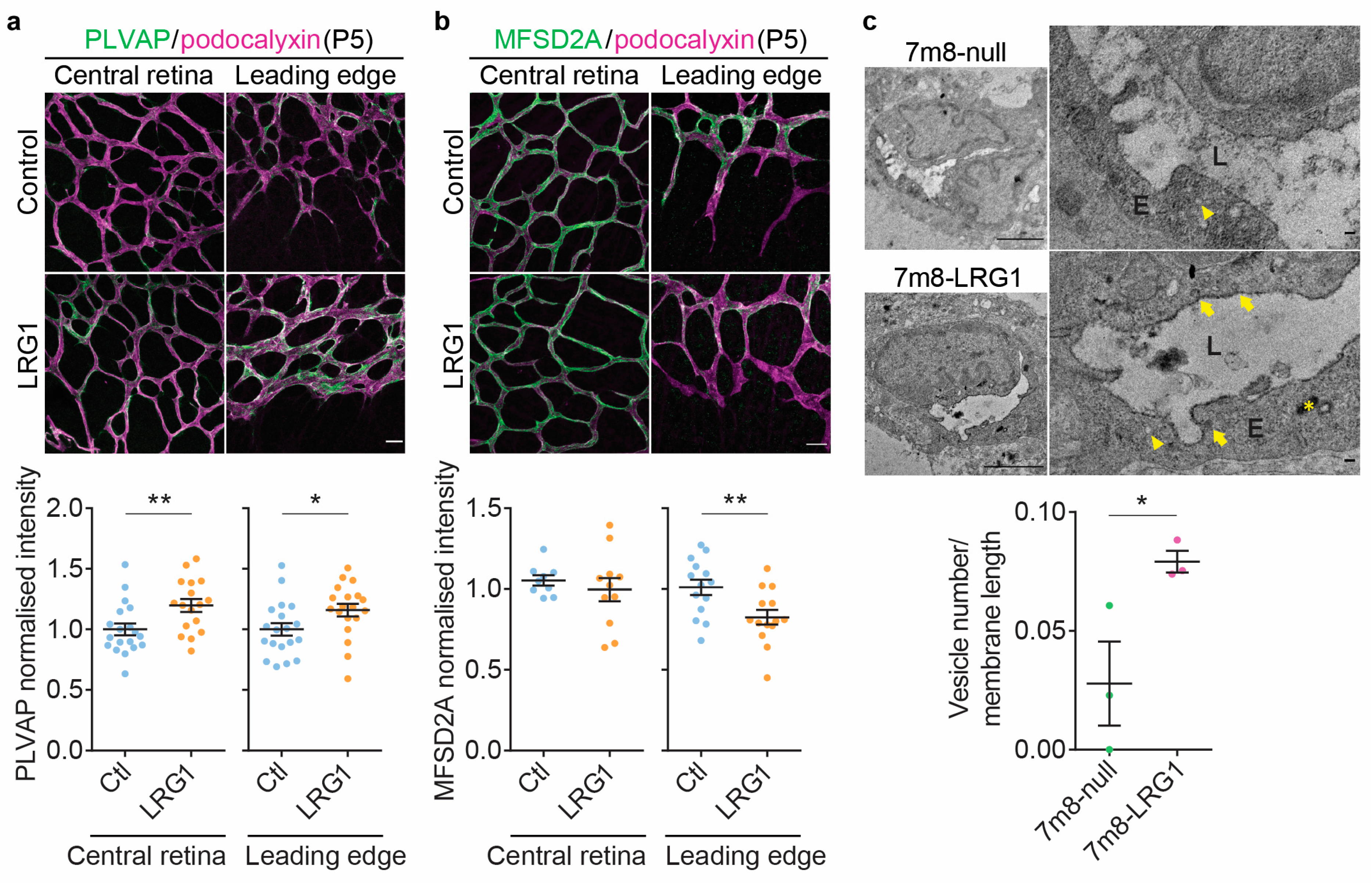
3.4. LRG1 Alters the Expression of Genes Associated with Transcellular Transport and Increases Endothelial Vesicular Trafficking
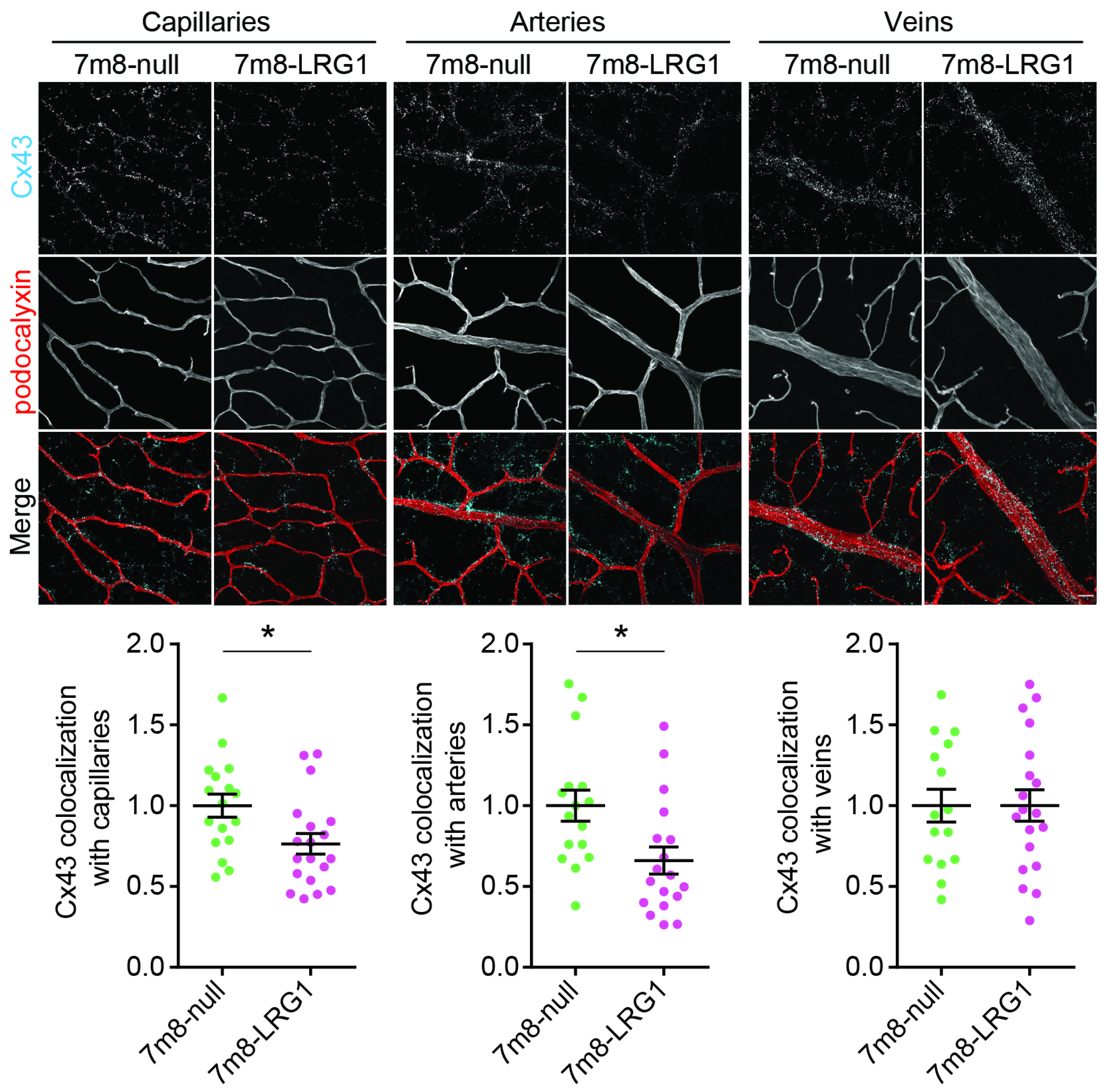
3.5. LRG1 Gene Delivery Reduces the Expression of Connexin43
3.6. LRG1 Reduces Microvascular Density and Vessel Size of Deep Capillaries and Veins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333.e1323. [Google Scholar] [CrossRef]
- Stratman, A.N.; Davis, G.E. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: Influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 2012, 18, 68–80. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Camilli, C.; Hoeh, A.E.; De Rossi, G.; Moss, S.E.; Greenwood, J. LRG1: An emerging player in disease pathogenesis. J. Biomed. Sci. 2022, 29, 6. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Wang, X.; Abraham, S.; McKenzie, J.A.G.; Jeffs, N.; Swire, M.; Tripathi, V.B.; Luhmann, U.F.O.; Lange, C.A.K.; Zhai, Z.; Arthur, H.M.; et al. LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature 2013, 499, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Dritsoula, A.; Camilli, C.; Moss, S.E.; Greenwood, J. The disruptive role of LRG1 on the vasculature and perivascular microenvironment. Front. Cardiovasc. Med. 2024, 11, 1386177. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheng, J.; Yu, B.J.; Zhou, L.; Xu, H.F.; Yang, L.L. LRG1 promotes corneal angiogenesis and lymphangiogenesis in a corneal alkali burn mouse model. Int. J. Ophthalmol. 2020, 13, 365–373. [Google Scholar] [CrossRef]
- Schlecht, A.; Thien, A.; Wolf, J.; Prinz, G.; Agostini, H.; Schlunck, G.; Wieghofer, P.; Boneva, S.; Lange, C. Immunosenescence in Choroidal Neovascularization (CNV)-Transcriptional Profiling of Naive and CNV-Associated Retinal Myeloid Cells during Aging. Int. J. Mol. Sci. 2021, 22, 13318. [Google Scholar] [CrossRef]
- Kallenberg, D.; Tripathi, V.; Javaid, F.; Pilotti, C.; George, J.; Davis, S.; Blackburn, J.W.D.; O’Connor, M.; Dowsett, L.; Bowers, C.E.; et al. A Humanized Antibody against LRG1 that Inhibits Angiogenesis and Reduces Retinal Vascular Leakage. bioRxiv 2021. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; Zhang, W.; Schlondorff, D.; Wu, J.; Cai, M.; He, W.; Baron, M.H.; Chuang, P.Y.; Liu, Z.; et al. Gene expression profiles of glomerular endothelial cells support their role in the glomerulopathy of diabetic mice. Kidney Int. 2018, 94, 326–345. [Google Scholar] [CrossRef]
- Haku, S.; Wakui, H.; Azushima, K.; Haruhara, K.; Kinguchi, S.; Ohki, K.; Uneda, K.; Kobayashi, R.; Matsuda, M.; Yamaji, T.; et al. Early Enhanced Leucine-Rich alpha-2-Glycoprotein-1 Expression in Glomerular Endothelial Cells of Type 2 Diabetic Nephropathy Model Mice. Biomed. Res. Int. 2018, 2018, 2817045. [Google Scholar] [CrossRef]
- Hong, Q.; Zhang, L.; Fu, J.; Verghese, D.A.; Chauhan, K.; Nadkarni, G.N.; Li, Z.; Ju, W.; Kretzler, M.; Cai, G.Y.; et al. LRG1 Promotes Diabetic Kidney Disease Progression by Enhancing TGF-beta-Induced Angiogenesis. J. Am. Soc. Nephrol. 2019, 30, 546–562. [Google Scholar] [CrossRef]
- Hisata, S.; Racanelli, A.C.; Kermani, P.; Schreiner, R.; Houghton, S.; Palikuqi, B.; Kunar, B.; Zhou, A.; McConn, K.; Capili, A.; et al. Reversal of emphysema by restoration of pulmonary endothelial cells. J. Exp. Med. 2021, 218, e20200938. [Google Scholar] [CrossRef]
- Nitkin, C.R.; Xia, S.; Menden, H.; Yu, W.; Xiong, M.; Heruth, D.P.; Ye, S.Q.; Sampath, V. FOSL1 is a novel mediator of endotoxin/lipopolysaccharide-induced pulmonary angiogenic signaling. Sci. Rep. 2020, 10, 13143. [Google Scholar] [CrossRef]
- Meng, H.; Song, Y.; Zhu, J.; Liu, Q.; Lu, P.; Ye, N.; Zhang, Z.; Pang, Y.; Qi, J.; Wu, H. LRG1 promotes angiogenesis through upregulating the TGFbeta1 pathway in ischemic rat brain. Mol. Med. Rep. 2016, 14, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, C.; Nong, Q.; Long, F.; Liu, J.; Mu, Z.; Chen, B.; Wu, D.; Wu, H. Exosomal Leucine-Rich-Alpha2-Glycoprotein 1 Derived from Non-Small-Cell Lung Cancer Cells Promotes Angiogenesis via TGF-beta Signal Pathway. Mol. Ther. Oncolytics 2019, 14, 313–322. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Feng, A.; Guo, H.; Huang, H.; Deng, Q.; Zhao, E.; Yang, M. LRG1 mediated by ATF3 promotes growth and angiogenesis of gastric cancer by regulating the SRC/STAT3/VEGFA pathway. Gastric Cancer 2022, 25, 527–541. [Google Scholar] [CrossRef]
- O’Connor, M.N.; Kallenberg, D.M.; Camilli, C.; Pilotti, C.; Dritsoula, A.; Jackstadt, R.; Bowers, C.E.; Watson, H.A.; Alatsatianos, M.; Ohme, J.; et al. LRG1 destabilizes tumor vessels and restricts immunotherapeutic potency. Med 2021, 2, 1231–1252.e1210. [Google Scholar] [CrossRef]
- De Rossi, G.; Da Vitoria Lobo, M.E.; Greenwood, J.; Moss, S.E. LRG1 as a novel therapeutic target in eye disease. Eye 2022, 36, 328–340. [Google Scholar] [CrossRef]
- Javaid, F.; Pilotti, C.; Camilli, C.; Kallenberg, D.; Bahou, C.; Blackburn, J.; Baker, J.R.; Greenwood, J.; Moss, S.E.; Chudasama, V. Leucine-rich alpha-2-glycoprotein 1 (LRG1) as a novel ADC target. RSC Chem. Biol. 2021, 2, 1206–1220. [Google Scholar] [CrossRef]
- Mundo, L.; Tosi, G.M.; Lazzi, S.; Pertile, G.; Parolini, B.; Neri, G.; Posarelli, M.; De Benedetto, E.; Bacci, T.; Silvestri, E.; et al. LRG1 Expression Is Elevated in the Eyes of Patients with Neovascular Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 8879. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Huang, H.; Han, C.; Qu, Y.; Jin, H.; Niu, T.; Zhang, Y.; Liu, K.; Xu, X. Elevated plasma and vitreous levels of leucine-rich-alpha2-glycoprotein are associated with diabetic retinopathy progression. Acta Ophthalmol. 2019, 97, 260–264. [Google Scholar] [CrossRef]
- Amer, R.; Tiosano, L.; Pe’er, J. Leucine-Rich alpha-2-Glycoprotein-1 (LRG-1) Expression in Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 685–692. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, M.; Liu, L.L.; Zhou, R.Y.; Fu, J.; Zhang, C.Z.; Yun, J.P. LRG1 expression indicates unfavorable clinical outcome in hepatocellular carcinoma. Oncotarget 2015, 6, 42118–42129. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Fang, J.; Ge, Z.; Li, X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1alpha activation. J. Exp. Clin. Cancer Res. 2016, 35, 29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wei, H.; Liu, Y.; Sun, Y.; Ye, J.; Lu, P.; Han, B. Relation between LRG1 and CD4+ T cells, cognitive impairment and neurological function in patients with acute ischemic stroke. Biomark. Med. 2024, 18, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.P.J.; McBride, D.W.; Park, E.; Hinds, S.; Paz, A.; Gusdon, A.; Xuefang, R.; Pan, S.; Ahnstedt, H.; Colpo, G.D.; et al. Leucine-Rich Alpha-2-Glycoprotein 1 is a Systemic Biomarker of Early Brain Injury and Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Neurocrit Care 2023, 38, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Dritsoula, A.; Dowsett, L.; Pilotti, C.; O’Connor, M.N.; Moss, S.E.; Greenwood, J. Angiopathic activity of LRG1 is induced by the IL-6/STAT3 pathway. Sci. Rep. 2022, 12, 4867. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Friedlander, M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog. Retin. Eye Res. 2006, 25, 277–295. [Google Scholar] [CrossRef]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008, 135, 145–157. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra176. [Google Scholar] [CrossRef]
- Basche, M.; Kampik, D.; Kawasaki, S.; Branch, M.J.; Robinson, M.; Larkin, D.F.; Smith, A.J.; Ali, R.R. Sustained and Widespread Gene Delivery to the Corneal Epithelium via In Situ Transduction of Limbal Epithelial Stem Cells, Using Lentiviral and Adeno-Associated Viral Vectors. Hum. Gene Ther. 2018, 29, 1140–1152. [Google Scholar] [CrossRef]
- Aurnhammer, C.; Haase, M.; Muether, N.; Hausl, M.; Rauschhuber, C.; Huber, I.; Nitschko, H.; Busch, U.; Sing, A.; Ehrhardt, A.; et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 2012, 23, 18–28. [Google Scholar] [CrossRef]
- Zarkada, G.; Howard, J.P.; Xiao, X.; Park, H.; Bizou, M.; Leclerc, S.; Kunzel, S.E.; Boisseau, B.; Li, J.; Cagnone, G.; et al. Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation. Dev. Cell 2021, 56, 2237–2251.e2236. [Google Scholar] [CrossRef] [PubMed]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Greene, C.; Frudd, K.; Araujo Dos Santos, L.; Futter, C.; Nichols, B.J.; Campbell, M.; Turowski, P. Methamphetamine enhances caveolar transport of therapeutic agents across the rodent blood-brain barrier. Cell Rep. Med. 2022, 3, 100497. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef]
- Diaz-Coranguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Runkle, E.A.; Antonetti, D.A. The blood-retinal barrier: Structure and functional significance. Methods Mol. Biol. 2011, 686, 133–148. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef]
- Bosma, E.K.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 2018, 15, 24. [Google Scholar] [CrossRef]
- Shue, E.H.; Carson-Walter, E.B.; Liu, Y.; Winans, B.N.; Ali, Z.S.; Chen, J.; Walter, K.A. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. 2008, 9, 29. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Burt, J.M.; Hirschi, K.D.; Dai, C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ. Res. 2003, 93, 429–437. [Google Scholar] [CrossRef]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J. Neurosci. 2017, 37, 7580–7594. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Augustin, H.G. Mechanisms of Vessel Pruning and Regression. Dev. Cell 2015, 34, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- van der Wijk, A.E.; Wisniewska-Kruk, J.; Vogels, I.M.C.; van Veen, H.A.; Ip, W.F.; van der Wel, N.N.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. Expression patterns of endothelial permeability pathways in the development of the blood-retinal barrier in mice. FASEB J. 2019, 33, 5320–5333. [Google Scholar] [CrossRef]
- Gibby, K.; You, W.K.; Kadoya, K.; Helgadottir, H.; Young, L.J.; Ellies, L.G.; Chang, Y.; Cardiff, R.D.; Stallcup, W.B. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan. Breast Cancer Res. 2012, 14, R67. [Google Scholar] [CrossRef]
- Yotsumoto, F.; You, W.K.; Cejudo-Martin, P.; Kucharova, K.; Sakimura, K.; Stallcup, W.B. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology 2015, 4, e1001204. [Google Scholar] [CrossRef]
- You, W.K.; Yotsumoto, F.; Sakimura, K.; Adams, R.H.; Stallcup, W.B. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis 2014, 17, 61–76. [Google Scholar] [CrossRef]
- Ozerdem, U. Targeting of pericytes diminishes neovascularization and lymphangiogenesis in prostate cancer. Prostate 2006, 66, 294–304. [Google Scholar] [CrossRef]
- Ozerdem, U. Targeting pericytes diminishes neovascularization in orthotopic uveal melanoma in nerve/glial antigen 2 proteoglycan knockout mouse. Ophthalmic Res. 2006, 38, 251–254. [Google Scholar] [CrossRef]
- Nalbach, L.; Schmitt, B.M.; Becker, V.; Scheller, A.; Laschke, M.W.; Menger, M.D.; Ampofo, E. Nerve/glial antigen 2 is crucially involved in the revascularization of freely transplanted pancreatic islets. Cell Tissue Res. 2019, 378, 195–205. [Google Scholar] [CrossRef]
- Ozerdem, U.; Stallcup, W.B. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis 2004, 7, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, J.; Makagiansar, I.T.; Stallcup, W.B. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell 2004, 15, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Goretzki, L.; Burg, M.A.; Grako, K.A.; Stallcup, W.B. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J. Biol. Chem. 1999, 274, 16831–16837. [Google Scholar] [CrossRef]
- Stallcup, W.B.; Huang, F.J. A role for the NG2 proteoglycan in glioma progression. Cell Adhes. Migr. 2008, 2, 192–201. [Google Scholar] [CrossRef]
- Kuo, H.J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef]
- Liu, G.; Li, H.; Cull, G.; Wilsey, L.; Yang, H.; Reemmer, J.; Shen, H.Y.; Wang, F.; Fortune, B.; Bui, B.V.; et al. Downregulation of Retinal Connexin 43 in GFAP-Expressing Cells Modifies Vasoreactivity Induced by Perfusion Ocular Pressure Changes. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef]
- Bobbie, M.W.; Roy, S.; Trudeau, K.; Munger, S.J.; Simon, A.M.; Roy, S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3758–3763. [Google Scholar] [CrossRef]
- Tien, T.; Muto, T.; Zhang, J.; Sohn, E.H.; Mullins, R.F.; Roy, S. Association of reduced Connexin 43 expression with retinal vascular lesions in human diabetic retinopathy. Exp. Eye Res. 2016, 146, 103–106. [Google Scholar] [CrossRef]
- Jin, Y.; Kaluza, D.; Jakobsson, L. VEGF, Notch and TGFbeta/BMPs in regulation of sprouting angiogenesis and vascular patterning. Biochem. Soc. Trans. 2014, 42, 1576–1583. [Google Scholar] [CrossRef]
- Chang, H.; Huylebroeck, D.; Verschueren, K.; Guo, Q.; Matzuk, M.M.; Zwijsen, A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development 1999, 126, 1631–1642. [Google Scholar] [CrossRef]
- Rochon, E.R.; Menon, P.G.; Roman, B.L. Alk1 controls arterial endothelial cell migration in lumenized vessels. Development 2016, 143, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Vargesson, N.; Laufer, E. Smad7 misexpression during embryonic angiogenesis causes vascular dilation and malformations independently of vascular smooth muscle cell function. Dev. Biol. 2001, 240, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Allinson, K.R.; Zhai, Z.; Oakenfull, R.; Ghandi, P.; Adams, R.H.; Fruttiger, M.; Arthur, H.M. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 2010, 106, 1425–1433. [Google Scholar] [CrossRef]
- Arnold, T.D.; Ferrero, G.M.; Qiu, H.; Phan, I.T.; Akhurst, R.J.; Huang, E.J.; Reichardt, L.F. Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. J. Neurosci. 2012, 32, 1197–1206. [Google Scholar] [CrossRef]
- Li, H.; Zhu, R.; Zhao, R.; Qian, L.; Jiang, L. Role of TGF-Beta1/SMAD2/3 Pathway in Retinal Outer Deep Vascular Plexus and Photoreceptor Damage in Rat 50/10 Oxygen-Induced Retinopathy. Biomed. Res. Int. 2019, 2019, 4072319. [Google Scholar] [CrossRef]
- Tual-Chalot, S.; Mahmoud, M.; Allinson, K.R.; Redgrave, R.E.; Zhai, Z.; Oh, S.P.; Fruttiger, M.; Arthur, H.M. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PLoS ONE 2014, 9, e98646. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Chen, J.; He, L.; Chen, Y. Role of VEGF-A and LRG1 in Abnormal Angiogenesis Associated with Diabetic Nephropathy. Front. Physiol. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Lai, C.M.; Dunlop, S.A.; May, L.A.; Gorbatov, M.; Brankov, M.; Shen, W.Y.; Binz, N.; Lai, Y.K.; Graham, C.E.; Barry, C.J.; et al. Generation of transgenic mice with mild and severe retinal neovascularisation. Br. J. Ophthalmol. 2005, 89, 911–916. [Google Scholar] [CrossRef][Green Version]
- van Eeden, P.E.; Tee, L.B.; Lukehurst, S.; Lai, C.M.; Rakoczy, E.P.; Beazley, L.D.; Dunlop, S.A. Early vascular and neuronal changes in a VEGF transgenic mouse model of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4638–4645. [Google Scholar] [CrossRef]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef]
- Cao, J.; Ehling, M.; Marz, S.; Seebach, J.; Tarbashevich, K.; Sixta, T.; Pitulescu, M.E.; Werner, A.C.; Flach, B.; Montanez, E.; et al. Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat. Commun. 2017, 8, 2210. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity Beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Dierkes, M.; Kuppers, V.; Vockel, M.; Tomm, J.; Zeuschner, D.; Rossaint, J.; Zarbock, A.; Koh, G.Y.; Peters, K.; et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 2015, 212, 2267–2287. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeh, A.E.; Chang, J.-H.; Mueller, R.S.; Basche, M.; Fantin, A.; Sepetis, A.; De Rossi, G.; Dritsoula, A.; Ali, R.R.; Turowski, P.; et al. LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation. Cells 2025, 14, 593. https://doi.org/10.3390/cells14080593
Hoeh AE, Chang J-H, Mueller RS, Basche M, Fantin A, Sepetis A, De Rossi G, Dritsoula A, Ali RR, Turowski P, et al. LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation. Cells. 2025; 14(8):593. https://doi.org/10.3390/cells14080593
Chicago/Turabian StyleHoeh, Alexandra E., Jui-Hsien Chang, Ronja S. Mueller, Mark Basche, Alessandro Fantin, Anastasios Sepetis, Giulia De Rossi, Athina Dritsoula, Robin R. Ali, Patric Turowski, and et al. 2025. "LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation" Cells 14, no. 8: 593. https://doi.org/10.3390/cells14080593
APA StyleHoeh, A. E., Chang, J.-H., Mueller, R. S., Basche, M., Fantin, A., Sepetis, A., De Rossi, G., Dritsoula, A., Ali, R. R., Turowski, P., Moss, S. E., & Greenwood, J. (2025). LRG1 Alters Pericyte Phenotype and Compromises Vascular Maturation. Cells, 14(8), 593. https://doi.org/10.3390/cells14080593








