Berberine Suppression of Human IgE but Not IgG Production via Inhibition of STAT6 Binding Activity at IgE Promoter by BCL6
Abstract
1. Introduction
2. Materials and Methods
2.1. Tonsil Collection
2.2. Tonsil Processing and Single Cell Isolation
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of Berberine
2.4. Cell Stimulation
2.5. Cell Viability
2.6. Antibody Measurement
2.7. Quantitative Real-Time PCR
2.8. The Chromatin Immunoprecipitation (ChIP) Assay
2.9. Statistical Analysis
3. Results
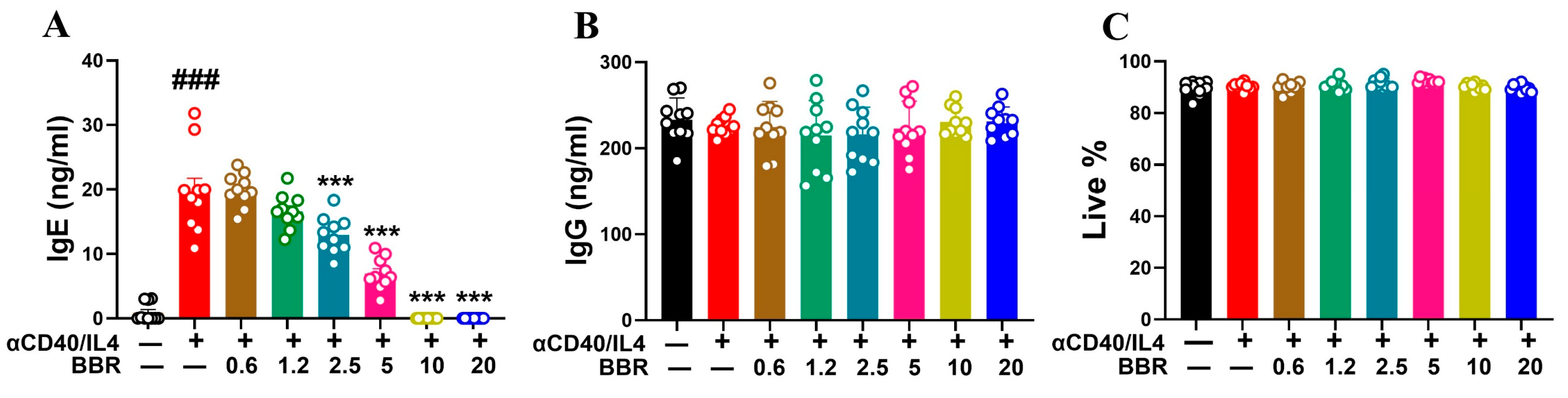
3.1. Berberine Dose Dependently Inhibited IgE Production Following Anti-CD40/IL-4 Stimulation
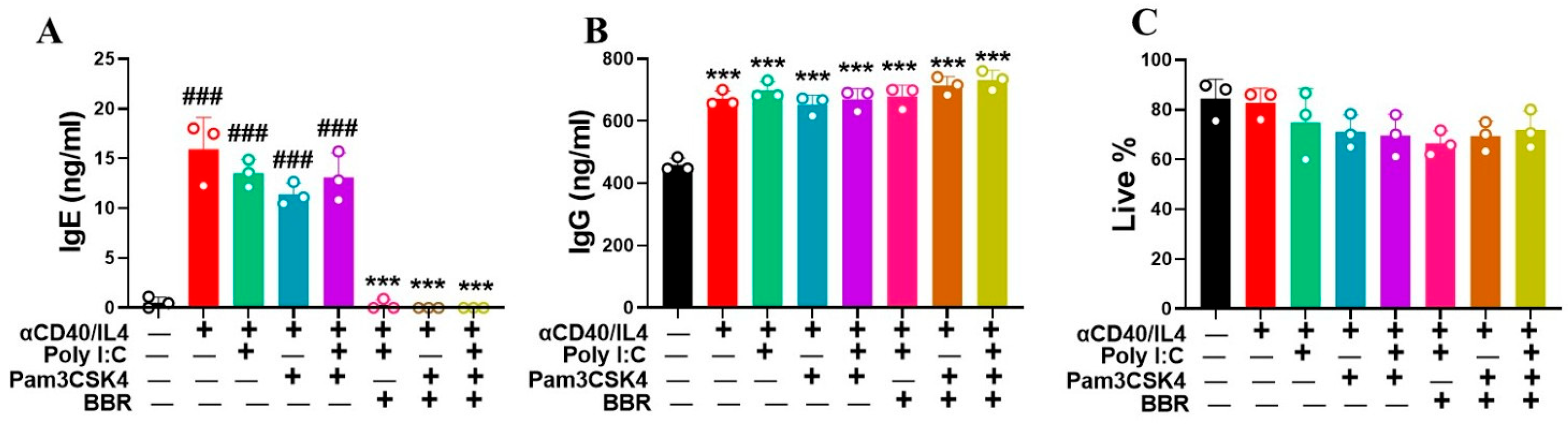
3.2. Berberine Suppressed IgE Production by Tonsil Cells Following T-Cell-Dependent Stimulation with Poly IC and Pam3CSK4
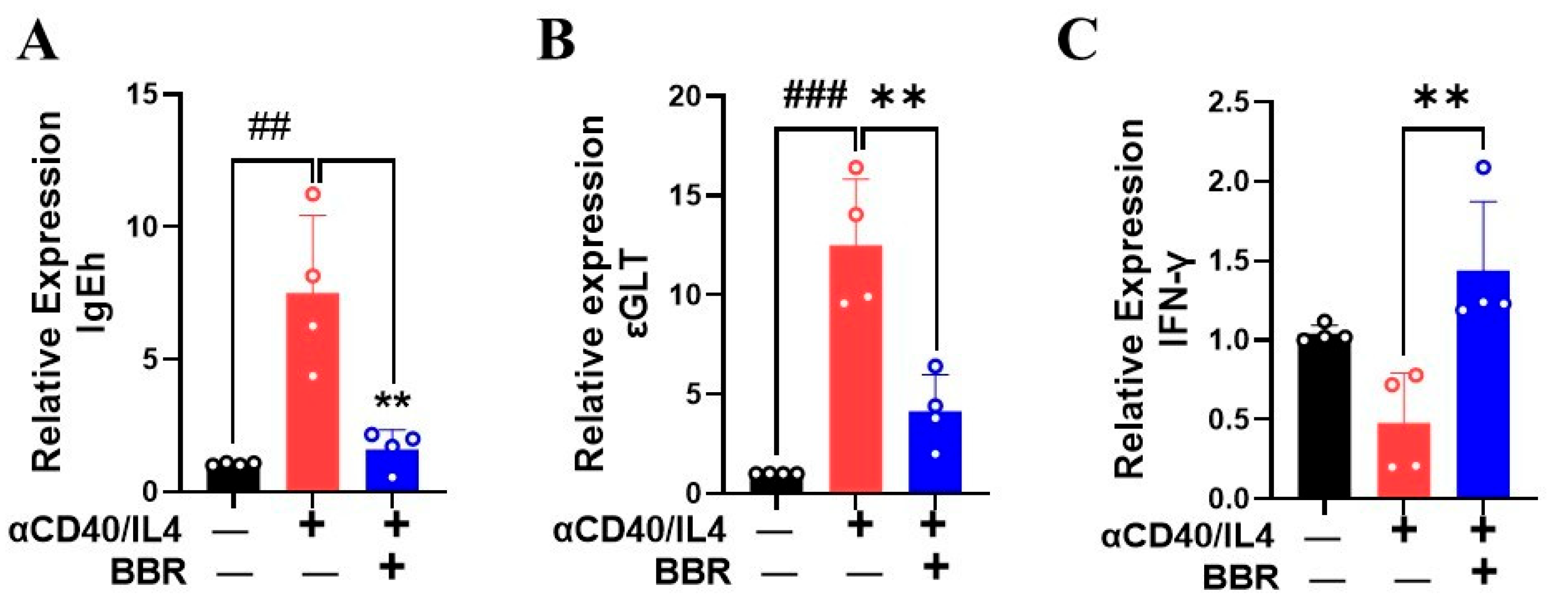
3.3. Berberine Inhibited IgE Heavy Chain and Epsilon GLT mRNA Expression
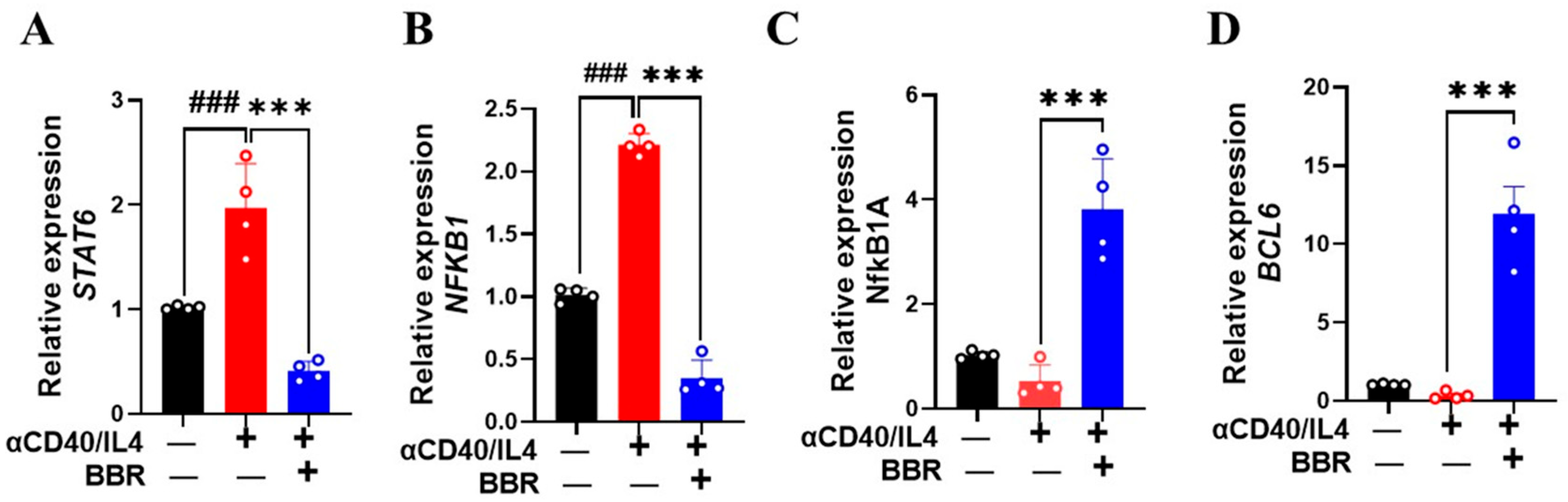
3.4. Berberine Increased BCL6 and Decreased STAT6 Expression
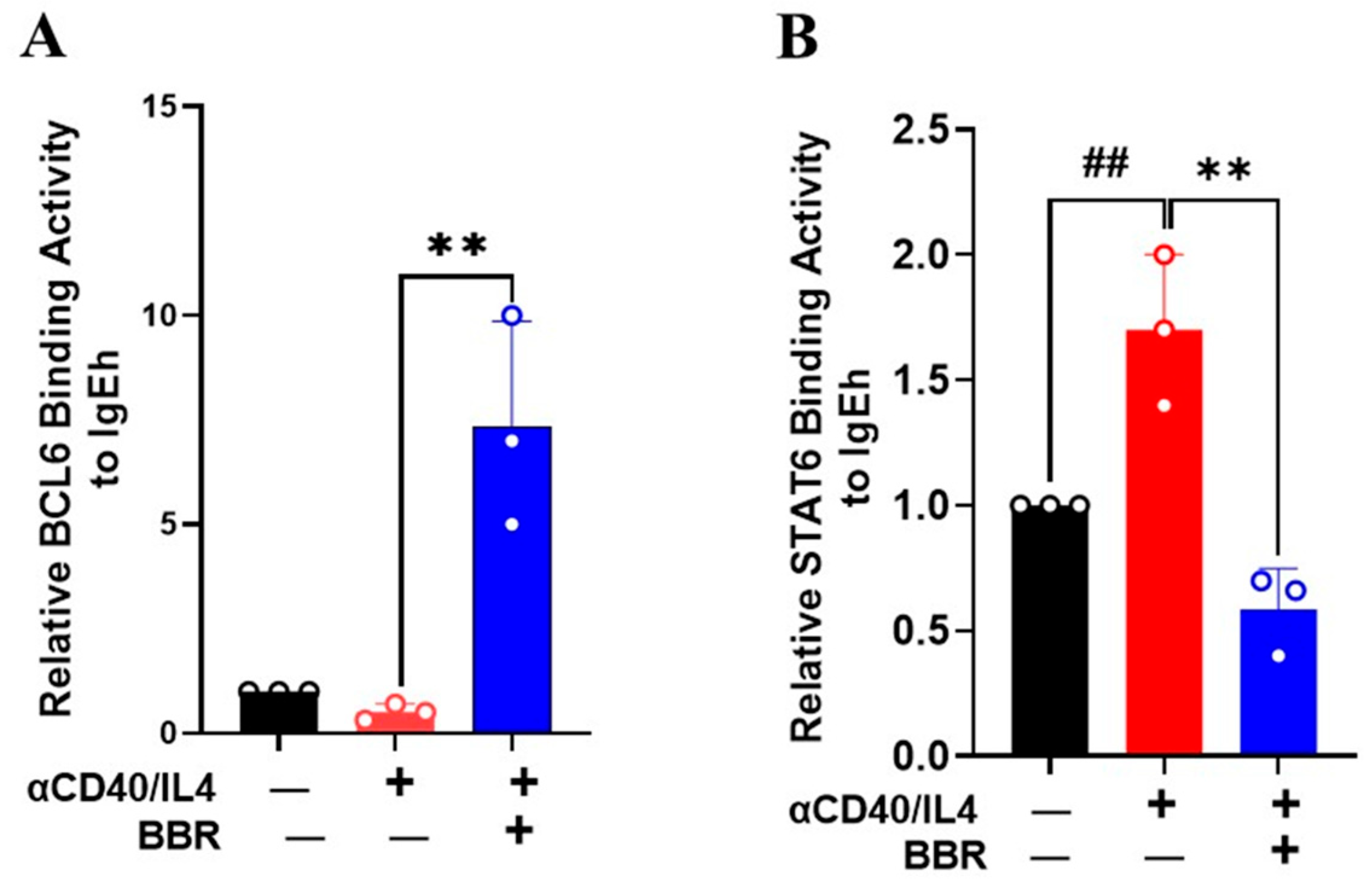
3.5. Berberine Inhibited STAT6 Binding Activity but Increased BCL6 Binding Activity on IgE Promoter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105. [Google Scholar] [CrossRef]
- Abbas, M.; Moussa, M.; Akel, H. Type I Hypersensitivity Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gauchat, J.F.; Aversa, G.; Gascan, H.; de Vries, J.E. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int. Immunol. 1992, 4, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Jumper, M.D.; Meek, K.; Lipsky, P.E. Evidence for a CD40 response element, distinct from the IL-4 response element, in the germline epsilon promoter. Int. Immunol. 1995, 7, 1529–1533. [Google Scholar] [CrossRef]
- Vitte, J.; Vibhushan, S.; Bratti, M.; Montero-Hernandez, J.E.; Blank, U. Allergy, Anaphylaxis, and Nonallergic Hypersensitivity: IgE, Mast Cells, and Beyond. Med. Princ. Pract. 2022, 31, 501–515. [Google Scholar] [CrossRef]
- Oettgen, H.C. Regulation of the IgE isotype switch: New insights on cytokine signals and the functions of epsilon germline transcripts. Curr. Opin. Immunol. 2000, 12, 618–623. [Google Scholar] [CrossRef]
- Liongue, C.; Almohaisen, F.L.J.; Ward, A.C. B Cell Lymphoma 6 (BCL6): A Conserved Regulator of Immunity and Beyond. Int. J. Mol. Sci. 2024, 25, 10968. [Google Scholar] [CrossRef]
- Balbino, B.; Herviou, P.; Godon, O.; Stackowicz, J.; Goff, O.R.; Iannascoli, B.; Sterlin, D.; Brûlé, S.; Millot, G.A.; Harris, F.M.; et al. The anti-IgE mAb omalizumab induces adverse reactions by engaging Fcγ receptors. J. Clin. Investig. 2020, 130, 1330–1335. [Google Scholar] [CrossRef]
- Wang, J.; Jones, S.M.; Pongracic, J.A.; Song, Y.; Yang, N.; Sicherer, S.H.; Makhija, M.M.; Robison, R.G.; Moshier, E.; Godbold, J.; et al. Safety, clinical, and immunologic efficacy of a Chinese herbal medicine (Food Allergy Herbal Formula-2) for food allergy. J. Allergy Clin. Immunol. 2015, 136, 962–970.e1. [Google Scholar] [CrossRef]
- Srivastava, K.D.; Qu, C.; Zhang, T.; Goldfarb, J.; Sampson, H.A.; Li, X.M. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J. Allergy Clin. Immunol. 2009, 123, 443–451. [Google Scholar] [CrossRef]
- Srivastava, K.; Yang, N.; Chen, Y.; Lopez-Exposito, I.; Song, Y.; Goldfarb, J.; Zhan, J.; Sampson, H.; Li, X.-M. Efficacy, safety and immunological actions of butanol-extracted Food Allergy Herbal Formula-2 on peanut anaphylaxis. Clin. Exp. Allergy 2011, 41, 582–591. [Google Scholar] [CrossRef]
- Yang, N.; Wang, J.; Liu, C.; Song, Y.; Zhang, S.; Zi, J.; Zhan, J.; Masilamani, M.; Cox, A.; Nowak-Wegrzyn, A.; et al. Berberine and limonin suppress IgE production by human B cells and peripheral blood mononuclear cells from food-allergic patients. Ann. Allergy Asthma. Immunol. 2014, 113, 556–564.e4. [Google Scholar] [CrossRef]
- Yang, N.; Maskey, A.R.; Srivastava, K.; Kim, M.; Wang, Z.; Musa, I.; Shi, Y.; Gong, Y.; Fidan, O.; Wang, J.; et al. Inhibition of pathologic immunoglobulin E in food allergy by EBF-2 and active compound berberine associated with immunometabolism regulation. Front. Immunol. 2023, 14, 1081121. [Google Scholar] [CrossRef]
- Srivastava, K.; Cao, M.; Fidan, O.; Shi, Y.; Yang, N.; Nowak-Wegrzyn, A.; Miao, M.; Zhan, J.; Sampson, H.A.; Li, X.-M. Berberine-containing natural-medicine with boiled peanut-OIT induces sustained peanut-tolerance associated with distinct microbiota signature. Front. Immunol. 2023, 14, 1174907. [Google Scholar] [CrossRef]
- Assadian, F.; Sandström, K.; Laurell, G.; Svensson, C.; Akusjärvi, G.; Punga, T. Efficient Isolation Protocol for B and T Lymphocytes from Human Palatine Tonsils. J. Vis. Exp. 2015, 105, 53374. [Google Scholar]
- Helm, M.; SABR; Gollner, K.; Gollner, U.; Jérôme, V.; Freitag, R. Isolation of primary human B lymphocytes from tonsils compared to blood as alternative source for ex vivo application. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1179, 122853. [Google Scholar] [CrossRef]
- Maskey, A.; Srivastava, K.; Soffer, G.; Dunkin, D.; Yuan, Q.; Li, X.M. Induction of Severe Eosinophilic Esophagitis and Multi-Organ Inflammation by Airborne Allergens is Associated with IL-4/IL-13 and CCL11 but Not IgE in Genetic Susceptible Mice. J. Inflamm. Res. 2022, 15, 5527–5540. [Google Scholar] [CrossRef]
- Eastmond, N.C.; Banks, E.M.; Coleman, J.W. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-gamma-induced suppression of exocytosis. J. Immunol. 1997, 159, 1444–1450. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz. 2005, 100 (Suppl. S1), 137–144. [Google Scholar] [CrossRef]
- Link, C.W.M.; Rau, C.N.; Udoye, C.C.; Ragab, M.; Korkmaz, R.; Comdühr, S.; Clauder, A.-K.; Lindemann, T.; Frehse, B.; Hofmann, K.; et al. IL-2-Agonist-Induced IFN-γ Exacerbates Systemic Anaphylaxis in Food Allergen-Sensitized Mice. Front. Immunol. 2020, 11, 596772. [Google Scholar] [CrossRef]
- Kitayama, D.; Sakamoto, A.; Arima, M.; Hatano, M.; Miyazaki, M.; Tokuhisa, T. A role for Bcl6 in sequential class switch recombination to IgE in B cells stimulated with IL-4 and IL-21. Mol. Immunol. 2008, 45, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, Y.; Basaki, Y.; Ikizawa, K.; Kajiwara, K.; Koshio, T.; Akiyama, K. Involvement of nuclear factor-kappa B activation in IgE synthesis in human B cells. J. Allergy Clin. Immunol. 1996, 98 Pt 2, S224–S229. [Google Scholar] [CrossRef]
- Zhang, P.; Ruan, C.; Yang, G.; Guan, Y.; Zhu, Y.; Li, Q.; Dai, X.; An, Y.; Shi, X.; Huang, P.; et al. PGRN Inhibits Early B-cell Activation and IgE Production Through the IFITM3-STAT1 Signaling Pathway in Asthma. Adv. Sci. 2024, 11, e2403939. [Google Scholar] [CrossRef]
- Wohlford, E.M.; Baresel, P.C.; Wilmore, J.R.; Mortelliti, A.J.; Coleman, C.B.; Rochford, R. Changes in Tonsil B Cell Phenotypes and EBV Receptor Expression in Children Under 5-Years-Old. Cytometry B Clin. Cytom. 2018, 94, 291–301. [Google Scholar] [CrossRef]
- Buchta, C.M.; Bishop, G.A. Toll-like receptors and B cells: Functions and mechanisms. Immunol. Res. 2014, 59, 12–22. [Google Scholar] [CrossRef]
- Barr, T.A.; Brown, S.; Ryan, G.; Zhao, J.; Gray, D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 2007, 37, 3040–3053. [Google Scholar] [CrossRef]
- Weir, G.M.; Karkada, M.; Hoskin, D.; Stanford, M.M.; MacDonald, L.; Mansour, M.; Liwski, R.S. Combination of poly I:C and Pam3CSK4 enhances activation of B cells in vitro and boosts antibody responses to protein vaccines in vivo. PLoS ONE 2017, 12, e0180073. [Google Scholar] [CrossRef]
- Stavnezer, J.; Guikema, J.E.; Schrader, C.E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008, 26, 261–292. [Google Scholar] [CrossRef]
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Stavnezer, J. Antibody class switching. Adv. Immunol. 1996, 61, 79–146. [Google Scholar]
- Richards, M.L.; Katz, D.H. Analysis of the promoter elements necessary for IL-4 and anti-CD40 antibody induction of murine Fc epsilon RII (CD23): Comparison with the germline epsilon promoter. J. Immunol. 1997, 158, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Stavnezer, J. Interaction of stat6 and NF-kappaB: Direct association and synergistic activation of interleukin-4-induced transcription. Mol. Cell. Biol. 1998, 18, 3395–3404. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.B.; Chang, C.C.; Berton, M.T.; Danial, N.N.; Zhang, J.; Kuehner, D.; Ye, B.H.; Kvatyuk, M.; Pandolfi, P.P.; Cattoretti, G.; et al. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: Specific regulation of iepsilon transcription and immunoglobulin E switching. Mol. Cell. Biol. 1999, 19, 7264–7275. [Google Scholar] [CrossRef]
| ID | Age | Gender | Diagnosis |
|---|---|---|---|
| 1 | 5 | M | Obstructive Sleep Apnea |
| 2 | 9 | F | Enlarged tonsil |
| 3 | 4 | F | Tonsil hypertrophy |
| 4 | 8 | M | Tonsillitis |
| 5 | 8 | F | Enlarged tonsil |
| 6 | 6 | F | Enlarged tonsil |
| 7 | 5 | F | Obstructive Sleep Apnea |
| 8 | 7 | M | Tonsillitis |
| 9 | 2 | M | Obstructive Sleep Apnea |
| 10 | 5 | M | Enlarged tonsils |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maskey, A.R.; Carnazza, M.; Spears, M.; Hemmindinger, S.; Kopulos, D.; Yang, N.; Islam, H.K.; Moscatello, A.L.; Geliebter, J.; Tiwari, R.K.; et al. Berberine Suppression of Human IgE but Not IgG Production via Inhibition of STAT6 Binding Activity at IgE Promoter by BCL6. Cells 2025, 14, 591. https://doi.org/10.3390/cells14080591
Maskey AR, Carnazza M, Spears M, Hemmindinger S, Kopulos D, Yang N, Islam HK, Moscatello AL, Geliebter J, Tiwari RK, et al. Berberine Suppression of Human IgE but Not IgG Production via Inhibition of STAT6 Binding Activity at IgE Promoter by BCL6. Cells. 2025; 14(8):591. https://doi.org/10.3390/cells14080591
Chicago/Turabian StyleMaskey, Anish R., Michelle Carnazza, Madison Spears, Steven Hemmindinger, Daniel Kopulos, Nan Yang, Humayun K. Islam, Augustine L. Moscatello, Jan Geliebter, Raj K. Tiwari, and et al. 2025. "Berberine Suppression of Human IgE but Not IgG Production via Inhibition of STAT6 Binding Activity at IgE Promoter by BCL6" Cells 14, no. 8: 591. https://doi.org/10.3390/cells14080591
APA StyleMaskey, A. R., Carnazza, M., Spears, M., Hemmindinger, S., Kopulos, D., Yang, N., Islam, H. K., Moscatello, A. L., Geliebter, J., Tiwari, R. K., & Li, X.-M. (2025). Berberine Suppression of Human IgE but Not IgG Production via Inhibition of STAT6 Binding Activity at IgE Promoter by BCL6. Cells, 14(8), 591. https://doi.org/10.3390/cells14080591






