The Role of A20 in Cancer: Friend or Foe?
Abstract
1. Introduction
2. A20, a Regulator of Inflammation
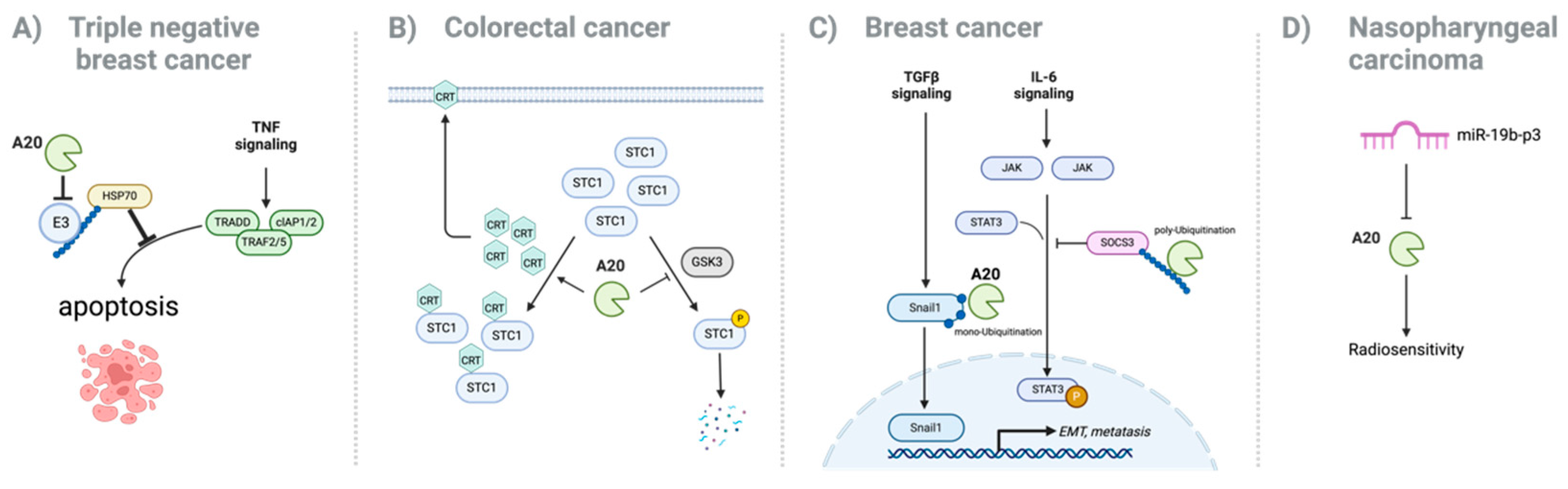
3. Oncogenic Role of A20 in Cancer
4. Tumor-Suppressive Functions of A20 in Cancer
5. Association Between A20 and Autophagy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNFAIP3 | Tumor Necrosis Factor Alpha-Induced Protein 3 |
| Ub | Ubiquitination |
| DUB | Deubiquitination |
| ZnF domains | Zinc Finger domain |
| NF-κB | Nuclear factor kappa B |
| CLL | Chronic lymphocytic leukemia |
| T-ALL | T-cell acute lymphocytic leukemia |
| HCC | Hepatocellular carcinoma |
| NSCLC | Non-small-cell lung cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| ATG | Autophagy-related gene |
| mTOR | Mammalian target of rapamycin |
| MDSC | Myeloid-derived suppressor cell |
References
- Bosanac, I.; Wertz, I.E.; Pan, B.; Yu, C.; Kusam, S.; Lam, C.; Phu, L.; Phung, Q.; Maurer, B.; Arnott, D.; et al. Ubiquitin Binding to A20 Znf4 Is Required for Modulation of Nf-κb Signaling. Mol. Cell 2010, 40, 548–557. [Google Scholar] [PubMed]
- Tokunaga, F.; Nishimasu, H.; Ishitani, R.; Goto, E.; Noguchi, T.; Mio, K.; Kamei, K.; Ma, A.; Iwai, K.; Nureki, O. Specific Recognition of Linear Polyubiquitin by A20 Zinc Finger 7 Is Involved in Nf-κb Regulation. EMBO J. 2012, 31, 3856–3870. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, K.; Carpentier, I.; Kreike, M.; Meloni, L.; Verstrepen, L.; Kensche, T.; Dikic, I.; Beyaert, R. A20 Inhibits Lubac-Mediated Nf-κb Activation by Binding Linear Polyubiquitin Chains Via Its Zinc Finger 7. EMBO J. 2012, 31, 3845–3855. [Google Scholar]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-Ubiquitination and Ubiquitin Ligase Domains of A20 Downregulate Nf-Kappab Signalling. Nature 2004, 430, 694–699. [Google Scholar]
- Dixit, V.M.; Green, S.; Sarma, V.; Holzman, L.B.; Wolf, F.W.; O’Rourke, K.; Ward, P.A.; Prochownik, E.V.; Marks, R.M. Tumor Necrosis Factor-Alpha Induction of Novel Gene Products in Human Endothelial Cells Including a Macrophage-Specific Chemotaxin. J. Biol. Chem. 1990, 265, 2973–2978. [Google Scholar]
- Heyninck, K.; De Valck, D.; Berghe, W.V.; Van Criekinge, W.; Contreras, R.; Fiers, W.; Haegeman, G.; Beyaert, R. The Zinc Finger Protein A20 Inhibits Tnf-Induced Nf-Kappab-Dependent Gene Expression by Interfering with an Rip- or Traf2-Mediated Transactivation Signal and Directly Binds to a Novel Nf-Kappab-Inhibiting Protein Abin. J. Cell Biol. 1999, 145, 1471–1482. [Google Scholar] [PubMed]
- Shembade, N.; Harhaj, E.W. Regulation of Nf-κb Signaling by the A20 Deubiquitinase. Cell Mol. Immunol. 2012, 9, 123–130. [Google Scholar]
- Mooney, E.C.; Sahingur, S.E. The Ubiquitin System and A20: Implications in Health and Disease. J. Dent. Res. 2021, 100, 10–20. [Google Scholar]
- Shi, Y.; Wang, X.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L. The Dual Roles of A20 in Cancer. Cancer Lett. 2021, 511, 26–35. [Google Scholar]
- Guo, W.; Ma, J.; Guo, S.; Wang, H.; Wang, S.; Shi, Q.; Liu, L.; Zhao, T.; Yang, F.; Chen, S.; et al. A20 Regulates the Therapeutic Effect of Anti-Pd-1 Immunotherapy in Melanoma. J. Imunother. Cancer 2020, 8, e001866. [Google Scholar]
- Zou, J.; Xia, H.; Zhang, C.; Xu, H.; Tang, Q.; Zhu, G.; Li, J.; Bi, F. Casp8 Acts through A20 to Inhibit Pd-L1 Expression: The Mechanism and Its Implication in Immunotherapy. Cancer Sci. 2021, 112, 2664–2678. [Google Scholar] [CrossRef]
- Shembade, N.; Parvatiyar, K.; Harhaj, N.S.; Harhaj, E.W. The Ubiquitin-Editing Enzyme A20 Requires Rnf11 to Downregulate Nf-Kappab Signalling. EMBO J. 2009, 28, 513–522. [Google Scholar]
- Wertz, I.E.; Dixit, V.M. Regulation of Death Receptor Signaling by the Ubiquitin System. Cell Death Differ. 2010, 17, 14–24. [Google Scholar] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in Inflammation and Autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [PubMed]
- Boone, D.L.; Turer, E.E.; Lee, E.G.; Ahmad, R.C.; Wheeler, M.T.; Tsui, C.; Hurley, P.; Chien, M.; Chai, S.; Hitotsumatsu, O.; et al. The Ubiquitin-Modifying Enzyme A20 Is Required for Termination of Toll-Like Receptor Responses. Nat. Immunol. 2004, 5, 1052–1060. [Google Scholar] [PubMed]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to Regulate Tnf-Induced Nf-Kappab and Cell Death Responses in A20-Deficient Mice. Science 2000, 289, 2350–2354. [Google Scholar]
- Kool, M.; van Loo, G.; Waelput, W.; De Prijck, S.; Muskens, F.; Sze, M.; van Praet, J.; Branco-Madeira, F.; Janssens, S.; Reizis, B.; et al. The Ubiquitin-Editing Protein A20 Prevents Dendritic Cell Activation, Recognition of Apoptotic Cells, and Systemic Autoimmunity. Immunity 2011, 35, 82–96. [Google Scholar]
- Tavares, R.M.; Turer, E.E.; Liu, C.L.; Advincula, R.; Scapini, P.; Rhee, L.; Barrera, J.; Lowell, C.A.; Utz, P.J.; Malynn, B.A.; et al. The Ubiquitin Modifying Enzyme A20 Restricts B Cell Survival and Prevents Autoimmunity. Immunity 2010, 33, 181–191. [Google Scholar]
- Vereecke, L.; Vieira-Silva, S.; Billiet, T.; van Es, J.H.; Guire, C.M.; Slowicka, K.; Sze, M.; van den Born, M.; De Hertogh, G.; Clevers, H.; et al. A20 Controls Intestinal Homeostasis through Cell-Specific Activities. Nat. Commun. 2014, 5, 5103. [Google Scholar]
- Garcia-Carbonell, R.; Wong, J.; Kim, J.Y.; Close, L.A.; Boland, B.S.; Wong, T.L.; Harris, P.A.; Ho, S.B.; Das, S.; Ernst, P.B.; et al. Elevated A20 Promotes Tnf-Induced and Ripk1-Dependent Intestinal Epithelial Cell Death. Proc. Natl. Acad. Sci. USA 2018, 115, E9192–E9200. [Google Scholar]
- Zhu, L.; Zhou, L.; Wang, L.; Chen, C.; Qiao, J.; Huang, X.; Su, X.; Chen, S.; Li, B.; Wu, X.; et al. A20 Promoter Rs5029924 Concomitant with Rs2230926 and Rs5029937 May Be a Prognostic Predictor for Joint Deformity or Refractory Rheumatoid Arthritis. Int. J. Gen. Med. 2024, 17, 1707–1712. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Wang, X.; Zhou, L.; Liao, Z.; Xu, L.; Wu, H.; Ren, J.; Li, Z.; Yang, L.; et al. Characteristics of A20 Gene Polymorphisms and Clinical Significance in Patients with Rheumatoid Arthritis. J. Transl. Med. 2015, 13, 215. [Google Scholar] [CrossRef]
- Musone, S.L.; Taylor, K.E.; Lu, T.T.; Nititham, J.; Ferreira, R.C.; Ortmann, W.; Shifrin, N.; Petri, M.A.; Kamboh, M.I.; Manzi, S.; et al. Multiple Polymorphisms in the Tnfaip3 Region Are Independently Associated with Systemic Lupus Erythematosus. Nat. Genet. 2008, 40, 1062–1064. [Google Scholar] [CrossRef]
- Philip, R.; Elhani, I.; Gallou, S.; Boysson, H.; Silva, N.M.; Georgin-Lavialle, S.; Deshayes, S.; Aouba, A. A20 Haploinsufficiency Diagnosis Beyond Systemic Lupus Erythematosus: A Systematic Review of the Literature. Autoimmun. Rev. 2025, 24, 103722. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.J.; et al. Psoriasis Collaborative Association Study of. Genome-Wide Scan Reveals Association of Psoriasis with Il-23 and Nf-Kappab Pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zang, W.; Tang, Z.; Ji, Y.; Xu, R.; Yang, Y.; Luo, A.; Hu, B.; Zhang, Z.; Liu, Z.; et al. A20/Tnfaip3 Regulates the DNA Damage Response and Mediates Tumor Cell Resistance to DNA-Damaging Therapy. Cancer Res. 2018, 78, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, S.M.; Yang, K.M.; Bae, E.; Ahn, S.G.; Park, J.S.; Seo, D.; Kim, M.; Ha, J.; Lee, J.; et al. A20 Promotes Metastasis of Aggressive Basal-Like Breast Cancers through Multi-Monoubiquitylation Of snail1. Nat. Cell Biol. 2017, 19, 1260–1273. [Google Scholar] [CrossRef]
- Lee, E.; Ouzounova, M.; Piranlioglu, R.; Ma, M.T.; Guzel, M.; Marasco, D.; Chadli, A.; Gestwicki, J.E.; Cowell, J.K.; Wicha, M.S.; et al. The Pleiotropic Effects of Tnfalpha in Breast Cancer Subtypes Is Regulated by Tnfaip3/A20. Oncogene 2019, 38, 469–482. [Google Scholar] [CrossRef]
- Song, C.; Kendi, A.T.; Lowe, V.J.; Lee, S. The A20/Tnfaip3-Cdc20-Casp1 Axis Promotes Inflammation-Mediated Metastatic Disease in Triple-Negative Breast Cancer. Anticancer Res. 2022, 42, 681–695. [Google Scholar] [CrossRef]
- Yoon, C.I.; Ahn, S.G.; Bae, S.J.; Shin, Y.J.; Cha, C.; Park, S.E.; Lee, J.H.; Ooshima, A.; Lee, H.S.; Yang, K.M.; et al. High A20 Expression Negatively Impacts Survival in Patients with Breast Cancer. PLoS ONE 2019, 14, e0221721. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; Guo, S.; Yi, X.; Zhao, T.; Liu, Y.; Shi, Q.; Gao, T.; Li, C.; Guo, W. A20 Promotes Melanoma Progression Via the Activation of Akt Pathway. Cell Death Dis. 2020, 11, 794. [Google Scholar] [PubMed]
- Du, B.; Liu, M.; Li, C.; Geng, X.; Zhang, X.; Ning, D.; Liu, M. The Potential Role of Tnfaip3 in Malignant Transformation of Gastric Carcinoma. Pathol. Res. Pract. 2019, 215, 152471. [Google Scholar]
- Wisnieski, F.; Santos, L.C.; Calcagno, D.Q.; Geraldis, J.C.; Gigek, C.O.; Anauate, A.C.; Chen, E.S.; Rasmussen, L.T.; Payao, S.L.M.; Artigiani, R.; et al. The Impact of DNA Demethylation on the Upregulation of the Nrn1 and Tnfaip3 Genes Associated with Advanced Gastric Cancer. J. Mol. Med. 2020, 98, 707–717. [Google Scholar] [PubMed]
- Lim, M.C.C.; Maubach, G.; Birkl-Toeglhofer, A.M.; Haybaeck, J.; Vieth, M.; Naumann, M. A20 Undermines Alternative Nf-Kappab Activity and Expression of Anti-Apoptotic Genes in Helicobacter Pylori Infection. Cell Mol. Life Sci. 2022, 79, 102. [Google Scholar] [CrossRef]
- Bavi, P.; Abubaker, J.; Al-Sanea, N.; Abduljabbar, A.; Ashari, L.H.; Alhomoud, S.; Al-Dayel, F.; Uddin, S.; Siraj, A.K.; Al-Kuraya, K.S. Clinico-Pathological Significance of Tnf Alpha-Induced Protein3 (Tnfaip3) in Middle Eastern Colorectal Carcinoma. Clin. Epigenetics 2011, 2, 417–418. [Google Scholar]
- Ungerback, J.; Belenki, D.; Jawad ul-Hassan, A.; Fredrikson, M.; Fransen, K.; Elander, N.; Verma, D.; Soderkvist, P. Genetic Variation and Alterations of Genes Involved in Nfkappab/Tnfaip3- and Nlrp3-Inflammasome Signaling Affect Susceptibility and Outcome of Colorectal Cancer. Carcinogenesis 2012, 33, 2126–2134. [Google Scholar] [PubMed]
- Shao, L.; Oshima, S.; Duong, B.; Advincula, R.; Barrera, J.; Malynn, B.A.; Ma, A. A20 Restricts Wnt Signaling in Intestinal Epithelial Cells and Suppresses Colon Carcinogenesis. PLoS ONE 2013, 8, e62223. [Google Scholar]
- Wang, T.; Xu, X.; Xu, Q.; Ren, J.; Shen, S.; Fan, C.; Hou, Y. Mir-19a Promotes Colitis-Associated Colorectal Cancer by Regulating Tumor Necrosis Factor Alpha-Induced Protein 3-Nf-Kappab Feedback Loops. Oncogene 2017, 36, 3240–3251. [Google Scholar]
- Luo, M.; Wang, X.; Wu, S.; Yang, C.; Su, Q.; Huang, L.; Fu, K.; An, S.; Xie, F.; To, K.K.W.; et al. A20 Promotes Colorectal Cancer Immune Evasion by Upregulating Stc1 Expression to Block Eat-Me Signal. Signal Transduct. Target Ther. 2023, 8, 312. [Google Scholar] [CrossRef]
- Geismann, C.; Hauser, C.; Grohmann, F.; Schneeweis, C.; Bolter, N.; Gundlach, J.P.; Schneider, G.; Rocken, C.; Meinhardt, C.; Schafer, H.; et al. Nf-Kappab/Rela Controlled A20 Limits Trail-Induced Apoptosis in Pancreatic Cancer. Cell Death Dis. 2023, 14, 3. [Google Scholar]
- Lv, Q.; Xie, L.; Cheng, Y.; Shi, Y.; Shan, W.; Ning, C.; Xie, B.; Yang, B.; Luo, X.; He, Q.; et al. A20-Mediated Deubiquitination of Eralpha in the Microenvironment of Cd163(+) Macrophages Sensitizes Endometrial Cancer Cells to Estrogen. Cancer Lett. 2019, 442, 137–147. [Google Scholar] [CrossRef]
- Hantel, C.; Ozimek, A.; Lira, R.; Ragazzon, B.; Jackel, C.; Frantsev, R.; Reincke, M.; Bertherat, J.; Mussack, T.; Beuschlein, F. Tnf Alpha Signaling Is Associated with Therapeutic Responsiveness to Vascular Disrupting Agents in Endocrine Tumors. Mol. Cell Endocrinol. 2016, 423, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, M.; Zhou, Q.; Wang, H.; Wang, Z.; Zhong, X.; Zhang, L.; Tai, S.; Cui, Y. The Prognostic Role of Socs3 and A20 in Human Cholangiocarcinoma. PLoS ONE 2015, 10, e0141165. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, S. Bladder Polypoid Cystitis-Derived A20 Associates with Tumorigenesis. Cell Biochem. Biophys. 2013, 67, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hadisaputri, Y.E.; Miyazaki, T.; Yokobori, T.; Sohda, M.; Sakai, M.; Ozawa, D.; Hara, K.; Honjo, H.; Kumakura, Y.; Kuwano, H. Tnfaip3 Overexpression Is an Independent Factor for Poor Survival in Esophageal Squamous Cell Carcinoma. Int. J. Oncol. 2017, 50, 1002–1010. [Google Scholar] [CrossRef]
- Ghadban, T.; Schmidt-Yang, M.; Uzunoglu, F.G.; Perez, D.R.; El Gammal, A.T.; Miro, J.T.; Wellner, U.; Pantel, K.; Izbicki, J.R.; Vashist, Y.K. Evaluation of the Germline Single Nucleotide Polymorphism Rs583522 in the Tnfaip3 Gene as a Prognostic Marker in Esophageal Cancer. Cancer Genet. 2015, 208, 595–601. [Google Scholar] [CrossRef]
- Pitt, S.C.; Hernandez, R.A.; Nehs, M.A.; Gawande, A.A.; Moore, F.D., Jr.; Ruan, D.T.; Cho, N.L. Identification of Novel Oncogenic Mutations in Thyroid Cancer. J. Am. Coll Surg. 2016, 222, 1036–1043. [Google Scholar] [CrossRef]
- Huang, T.; Yin, L.; Wu, J.; Gu, J.J.; Wu, J.Z.; Chen, D.; Yu, H.L.; Ding, K.; Zhang, N.; Du, M.Y.; et al. Microrna-19b-3p Regulates Nasopharyngeal Carcinoma Radiosensitivity by Targeting Tnfaip3/Nf-Kappab Axis. J. Exp. Clin. Cancer Res. 2016, 35, 188. [Google Scholar] [CrossRef]
- Kato, M.; Sanada, M.; Kato, I.; Sato, Y.; Takita, J.; Takeuchi, K.; Niwa, A.; Chen, Y.; Nakazaki, K.; Nomoto, J.; et al. Frequent Inactivation of A20 in B-Cell Lymphomas. Nature 2009, 459, 712–716. [Google Scholar] [CrossRef]
- Honma, K.; Tsuzuki, S.; Nakagawa, M.; Tagawa, H.; Nakamura, S.; Morishima, Y.; Seto, M. Tnfaip3/A20 Functions as a Novel Tumor Suppressor Gene in Several Subtypes of Non-Hodgkin Lymphomas. Blood 2009, 114, 2467–2475. [Google Scholar] [CrossRef]
- Schmitz, R.; Hansmann, M.L.; Bohle, V.; Martin-Subero, J.I.; Hartmann, S.; Mechtersheimer, G.; Klapper, W.; Vater, I.; Giefing, M.; Gesk, S.; et al. Tnfaip3 (A20) Is a Tumor Suppressor Gene in Hodgkin Lymphoma and Primary Mediastinal B Cell Lymphoma. J. Exp. Med. 2009, 206, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Roschewski, M.; Staudt, L.M.; Wilson, W.H. Diffuse Large B-Cell Lymphoma-Treatment Approaches in the Molecular Era. Nat. Rev. Clin. Oncol. 2014, 11, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tatarczuch, M.; Waltham, M.; Shortt, J.; Polekhina, G.; Hawkes, E.A.; Ho, S.J.; Trotman, J.; Brasacchio, D.; Co, M.; Li, J.; et al. Molecular Associations of Response to the New-Generation Btk Inhibitor Zanubrutinib in Marginal Zone Lymphoma. Blood Adv. 2023, 7, 3531–3539. [Google Scholar] [PubMed]
- Frenzel, L.P.; Claus, R.; Plume, N.; Schwamb, J.; Konermann, C.; Pallasch, C.P.; Claasen, J.; Brinker, R.; Wollnik, B.; Plass, C.; et al. Sustained Nf-Kappab Activity in Chronic Lymphocytic Leukemia Is Independent of Genetic and Epigenetic Alterations in the Tnfaip3 (A20) Locus. Int. J. Cancer 2011, 128, 2495–2500. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, F.; Shen, Q.; Chen, S.; Wang, X.; Wang, L.; Yang, L.; Wu, X.; Huang, S.; Schmidt, C.A.; et al. Characteristics of A20 Gene Polymorphisms in T-Cell Acute Lymphocytic Leukemia. Hematology 2014, 19, 448–454. [Google Scholar]
- Chen, H.; Hu, L.; Luo, Z.; Zhang, J.; Zhang, C.; Qiu, B.; Dong, L.; Tan, Y.; Ding, J.; Tang, S.; et al. A20 Suppresses Hepatocellular Carcinoma Proliferation and Metastasis through Inhibition of Twist1 Expression. Mol. Cancer 2015, 14, 186. [Google Scholar]
- Catrysse, L.; Ghahremani, M.F.; Vereecke, L.; Youssef, S.A.; Guire, C.M.; Sze, M.; Weber, A.; Heikenwalder, M.; de Bruin, A.; Beyaert, R.; et al. A20 Prevents Chronic Liver Inflammation and Cancer by Protecting Hepatocytes from Death. Cell Death Dis. 2016, 7, e2250. [Google Scholar]
- Feng, Y.; Zhang, Y.; Cai, Y.; Liu, R.; Lu, M.; Li, T.; Fu, Y.; Guo, M.; Huang, H.; Ou, Y.; et al. A20 Targets Pfkl and Glycolysis to Inhibit the Progression of Hepatocellular Carcinoma. Cell Death Dis. 2020, 11, 89. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Zong, Z.; Xiao, Y.; Li, N.; Guo, C.; Zhang, L.; Shi, Y. A20 Inhibits the Motility of Hcc Cells Induced by Tnf-Alpha. Oncotarget 2016, 7, 14742–14754. [Google Scholar]
- Dimitrakopoulos, F.D.; Kottorou, A.E.; Kalofonou, M.; Kalofonos, H.P. The Fire Within: Nf-Kappab Involvement in Non-Small Cell Lung Cancer. Cancer Res. 2020, 80, 4025–4036. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, Q.; Willette-Brown, J.; Xi, S.; Zhu, F.; Burkett, S.; Back, T.; Song, N.Y.; Datla, M.; Sun, Z.; et al. The Pivotal Role of Ikkalpha in the Development of Spontaneous Lung Squamous Cell Carcinomas. Cancer Cell 2013, 23, 527–540. [Google Scholar] [CrossRef]
- Xia, Y.; Yeddula, N.; Leblanc, M.; Ke, E.; Zhang, Y.; Oldfield, E.; Shaw, R.J.; Verma, I.M. Reduced Cell Proliferation by Ikk2 Depletion in a Mouse Lung-Cancer Model. Nat. Cell Biol. 2012, 14, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Dooley, A.L.; Feldser, D.M.; Shen, L.; Turk, E.; Ouyang, C.; Jacks, T. Requirement for Nf-Kappab Signalling in a Mouse Model of Lung Adenocarcinoma. Nature 2009, 462, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Zaynagetdinov, R.; Stathopoulos, G.T.; Sherrill, T.P.; Cheng, D.S.; McLoed, A.G.; Ausborn, J.A.; Polosukhin, V.V.; Connelly, L.; Zhou, W.; Fingleton, B.; et al. Epithelial Nuclear Factor-Kappab Signaling Promotes Lung Carcinogenesis Via Recruitment of Regulatory T Lymphocytes. Oncogene 2012, 31, 3164–3176. [Google Scholar] [PubMed]
- Langsch, S.; Baumgartner, U.; Haemmig, S.; Schlup, C.; Schafer, S.C.; Berezowska, S.; Rieger, G.; Dorn, P.; Tschan, M.P.; Vassella, E. Mir-29b Mediates Nf-Kappab Signaling in Kras-Induced Non-Small Cell Lung Cancers. Cancer Res. 2016, 76, 4160–4169. [Google Scholar]
- Liao, Y.; Cao, L.; Wang, F.; Pang, R. Mir-605-5p Promotes Invasion and Proliferation by Targeting Tnfaip3 in Non-Small-Cell Lung Cancer. J. Cell Biochem. 2020, 121, 779–787. [Google Scholar] [CrossRef]
- Breitenecker, K.; Homolya, M.; Luca, A.C.; Lang, V.; Trenk, C.; Petroczi, G.; Mohrherr, J.; Horvath, J.; Moritsch, S.; Haas, L.; et al. Down-Regulation of A20 Promotes Immune Escape of Lung Adenocarcinomas. Sci. Transl. Med. 2021, 13, eabc3911. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, L.; Liu, Z.; Yin, J.; Jiang, X.; Lu, J. Expression of A20 Is Reduced in Pancreatic Cancer Tissues. J. Mol. Histol. 2012, 43, 319–325. [Google Scholar] [CrossRef]
- Xu, P.; Wang, X.; Qian, J.; Li, Z.; Yao, J.; Xu, A. The Prognostic Evaluation of Ca19-9, D-Dimer and Tnfaip3/A20 in Patients with Pancreatic Ductal Adenocarcinoma. Medicine (Baltimore) 2021, 100, e24651. [Google Scholar]
- Yao, J.; Li, Z.; Wang, X.; Xu, P.; Zhao, L.; Qian, J. Mir-125a Regulates Chemo-Sensitivity to Gemcitabine in Human Pancreatic Cancer Cells through Targeting A20. Acta Biochim. Et Biophys. Sin. 2016, 48, 202–208. [Google Scholar]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [PubMed]
- Deretic, V.; Levine, B. Autophagy Balances Inflammation in Innate Immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [PubMed]
- Jee, S.C.; Cheong, H. Autophagy/Mitophagy Regulated by Ubiquitination: A Promising Pathway in Cancer Therapeutics. Cancers 2023, 15, 1112. [Google Scholar] [CrossRef]
- Vessoni, A.T.; Filippi-Chiela, E.C.; Menck, C.F.; Lenz, G. Autophagy and Genomic Integrity. Cell Death Differ. 2013, 20, 1444–1454. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of Autophagy and Inhibition of Tumorigenesis by Beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an Autophagy Gene Essential for Early Embryonic Development, Is a Haploinsufficient Tumor Suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar]
- Funderburk, S.F.; Wang, Q.J.; Yue, Z. The Beclin 1-Vps34 Complex--at the Crossroads of Autophagy and Beyond. Trends Cell Biol. 2010, 20, 355–362. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in Mammalian Development and Differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar]
- Kang, M.R.; Kim, M.S.; Oh, J.E.; Kim, Y.R.; Song, S.Y.; Kim, S.S.; Ahn, C.H.; Yoo, N.J.; Lee, S.H. Frameshift Mutations of Autophagy-Related Genes Atg2b, Atg5, Atg9b and Atg12 in Gastric and Colorectal Cancers with Microsatellite Instability. J. Pathol. 2009, 217, 702–706. [Google Scholar]
- Barnard, R.A.; Regan, D.P.; Hansen, R.J.; Maycotte, P.; Thorburn, A.; Gustafson, D.L. Autophagy Inhibition Delays Early but Not Late-Stage Metastatic Disease. J. Pharmacol. Exp. Ther. 2016, 358, 282–293. [Google Scholar] [PubMed]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D.; et al. Autophagy Is Required for Glucose Homeostasis and Lung Tumor Maintenance. Cancer Discov. 2014, 4, 914–927. [Google Scholar] [PubMed]
- Yang, A.; Herter-Sprie, G.; Zhang, H.; Lin, E.Y.; Biancur, D.; Wang, X.; Deng, J.; Hai, J.; Yang, S.; Wong, K.K.; et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov. 2018, 8, 276–287. [Google Scholar]
- Pant, A.; Yao, X.; Lavedrine, A.; Viret, C.; Dockterman, J.; Chauhan, S.; Chong-Shan, S.; Manjithaya, R.; Cadwell, K.; Kufer, T.A.; et al. Interactions of Autophagy and the Immune System in Health and Diseases. Autophagy Rep. 2022, 1, 438–515. [Google Scholar]
- Shi, C.S.; Kehrl, J.H. Traf6 and A20 Regulate Lysine 63-Linked Ubiquitination of Beclin-1 to Control Tlr4-Induced Autophagy. Sci. Signal. 2010, 3, ra42. [Google Scholar]
- Inomata, M.; Niida, S.; Shibata, K.; Into, T. Regulation of Toll-Like Receptor Signaling by Ndp52-Mediated Selective Autophagy Is Normally Inactivated by A20. Cell Mol. Life Sci. 2012, 69, 963–979. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. Mtor Signaling Pathway and Mtor Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Oshima, S.; Takahara, M.; Maeyashiki, C.; Nemoto, Y.; Kobayashi, M.; Nibe, Y.; Nozaki, K.; Nagaishi, T.; Okamoto, R.; et al. Tnfaip3 Promotes Survival of Cd4 T Cells by Restricting Mtor and Promoting Autophagy. Autophagy 2015, 11, 1052–1062. [Google Scholar]
- Zhai, Y.; Lin, P.; Feng, Z.; Lu, H.; Han, Q.; Chen, J.; Zhang, Y.; He, Q.; Nan, G.; Luo, X.; et al. Tnfaip3-Deptor Complex Regulates Inflammasome Secretion through Autophagy in Ankylosing Spondylitis Monocytes. Autophagy 2018, 14, 1629–1643. [Google Scholar] [CrossRef]
- Kanayama, M.; Inoue, M.; Danzaki, K.; Hammer, G.; He, Y.W.; Shinohara, M.L. Autophagy Enhances Nfkappab Activity in Specific Tissue Macrophages by Sequestering A20 to Boost Antifungal Immunity. Nat. Commun. 2015, 6, 5779. [Google Scholar]
- Slowicka, K.; Serramito-Gómez, I.; Boada-Romero, E.; Martens, A.; Sze, M.; Petta, I.; Vikkula, H.K.; De Rycke, R.; Parthoens, E.; Lippens, S.; et al. Physical and Functional Interaction between A20 and Atg16l1-Wd40 Domain in the Control of Intestinal Homeostasis. Nat. Commun. 2019, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Merline, R.; Rödig, H.; Zeng-Brouwers, J.; Poluzzi, C.; Tascher, G.; Michaelis, J.; Lopez-Mosqueda, J.; Rhiner, A.; Huber, L.S.; Diehl, V.; et al. A20 Binding and Inhibitor of Nuclear Factor Kappa B (Nf-κb)-1 (Abin-1): A Novel Modulator of Mitochondrial Autophagy. Am. J. Physiol. Cell Physiol. 2023, 324, C339–C352. [Google Scholar] [PubMed]
- Zhang, Y.; Yi, W.; Xia, H.; Lan, H.; Chen, J.; Yang, Z.; Han, F.; Tang, P.; Liu, B. A20 Regulates Inflammation through Autophagy Mediated by Nf-κb Pathway in Human Nucleus Pulposus Cells and Ameliorates Disc Degeneration In vivo. Biochem. Biophys. Res. Commun. 2021, 549, 179–186. [Google Scholar] [PubMed]
- Ning, F.; Wang, H.; Liang, Z.; Lan, J. Tnfaip3 Overexpression Inhibits Diffuse Large B-Cell Lymphoma Progression by Promoting Autophagy through Tlr4/Myd88/Nf-Kappab Signaling Pathway. Discov. Med. 2024, 36, 1627–1640. [Google Scholar]

| Tumor Type | A20 Gene Alteration | Mechanism | References |
|---|---|---|---|
| breast cancer | increased | Suppressing TNF-α-induced apoptosis | [25,26] |
| Enhancing metastasis by multi-monoubiquitination of Snail1 | [25] | ||
| triple-negative breast cancer | overexpression | Activating the HSP70-mediated anti-apoptotic pathway | [26] |
| luminal (ER+) breast cancer | overexpression | Promoting EMT, increasing metastatic potential via Stat3 signaling, and recruiting granulocytic MDSCs | [26] |
| melanoma | increased | Enhancing cell proliferation, survival, and metastasis by stabilizing and activating key components of the Akt pathway | [29] |
| increased | Promoting immune evasion by regulating PD-L1 expression and decreasing the infiltration and activity of CD8 T cells | [10,11] | |
| gastric cancer | hypomethylation of A20 promoter | Not specifically stated | [31] |
| increased | Under Helicobacter pylori infection, inhibiting the NF-κB pathway and reducing expression level of anti-apoptotic genes | [32] | |
| colorectal cancer | genetic and epigenetic alteration | Not specifically stated | [33,34,35,36] |
| increased | Promoting tumor growth and immune evasion through a STC1-CRT-dependent pathway by reducing CRT on the tumor cell surface | [37] | |
| pancreatic cancer | Not stated | Contributing to the resistance of pancreatic cancer cells to TRAIL-mediated apoptosis | [38] |
| endometrial cancer | increased | Correlation of A20 expression with clinical parameters | [39] |
| adrenocortical carcinoma | increased | Correlation with therapeutic responsiveness | [40] |
| cholangiocarcinoma | increased | Correlations between A20 expression and patient outcomes | [41] |
| bladder cancer | increased | Not specifically stated | [42] |
| esophageal carcinoma | increased | Correlation of A20 expression with patient survival data | [43,44] |
| thyroid cancer | increased | Genomic analysis for mutation identification | [45] |
| nasopharyngeal carcinoma | increased | Reducing the inhibitory effects of mir-19b-3p on cell radiosensitivity | [46] |
| Tumor Type | A20 Gene Alteration | Mechanism | Reference |
|---|---|---|---|
| B-cell lymphoma | overexpression | Inducing apoptosis | [47,48,49] |
| silencing | Enhancing resistance to apoptosis and clonogenicity | [47,48,49] | |
| A20 depletion | Contributing to drug resistance and enhancing survival signals provided by NF-κB in cancer cells | [50] | |
| A20 mutation | Improving cancer cell survival in patients treated with BTK inhibitors | [51] | |
| HCC | decreased | Negative correlation between A20 expression level and tumor size | [54] |
| A20 depletion | Developing severe liver inflammation, fibrosis, and HCC | [56] | |
| A20 depletion | Inhibiting NF-κB-RIPK1-mediated necroptosis | [54,56] | |
| A20 depletion | Modulating NF-κB, RAC1, FAC signaling, and glycolysis metabolism | [56,57] | |
| non-small-cell lung cancer | A20 depletion | Enhancing NF-κB activity linked to increase in tumor development | [61] |
| A20 depletion | By targeting A20, miR-29b activates NF-κB signaling, thereby conferring resistance to apoptosis | [63] | |
| A20 depletion | miR-605-5p suppresses A20 expression, thereby promoting tumor cell invasion and proliferation | [64] | |
| A20 depletion | Impairing CD8+ T cell-mediated immune surveillance | [65] | |
| Hyperactivation of TBK1, along with increased expression and activation of STAT1, enhances sensitivity to IFN-γ and elevates PD-L1 levels, ultimately facilitating immune evasion | |||
| pancreatic cancer | decreased | A20 is suggested as a new biomarker | [66,67] |
| A20 depletion | miR-125a reduces gemcitabine sensitivity by directly targeting A20 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Cheong, H. The Role of A20 in Cancer: Friend or Foe? Cells 2025, 14, 544. https://doi.org/10.3390/cells14070544
Lee J, Cheong H. The Role of A20 in Cancer: Friend or Foe? Cells. 2025; 14(7):544. https://doi.org/10.3390/cells14070544
Chicago/Turabian StyleLee, Jinju, and Heesun Cheong. 2025. "The Role of A20 in Cancer: Friend or Foe?" Cells 14, no. 7: 544. https://doi.org/10.3390/cells14070544
APA StyleLee, J., & Cheong, H. (2025). The Role of A20 in Cancer: Friend or Foe? Cells, 14(7), 544. https://doi.org/10.3390/cells14070544







